Abstract
Presence and extent of bronchus-associated lymphoid tissue (BALT) is subject to considerable variations between species and is only occasionally observed in lungs of mice. Here we demonstrate that mice deficient for the chemokine receptor CCR7 regularly develop highly organized BALT. These structures were not present at birth but were detectable from day 5 onwards. Analyzing CCR7−/−/wild-type bone marrow chimeras, we demonstrate that the development of BALT is caused by alterations of the hematopoietic system in CCR7-deficient mice. These observations together with the finding that CCR7-deficient mice posses dramatically reduced numbers of regulatory T cells (T reg cells) in the lung-draining bronchial lymph node suggest that BALT formation might be caused by disabled in situ function of T reg cells. Indeed, although adoptive transfer of wild-type T reg cells to CCR7-deficient recipients resulted in a profound reduction of BALT formation, neither naive wild-type T cells nor T reg cells from CCR7−/− donors impair BALT generation. Furthermore, we provide evidence that CCR7-deficient T reg cells, although strongly impaired in homing to peripheral lymph nodes, are fully effective in vitro. Thus our data reveal a CCR7-dependent homing of T reg cells to peripheral lymph nodes in conjunction with a role for these cells in controlling BALT formation.
Bronchus-associated lymphoid tissue (BALT) is part of the integrated mucosal immune system and is characterized by an aggregation of lymphoid cells (1). These aggregations are clustered in follicle-like structures (2) and are composed of B cells surrounded by a parafollicular region of T cells. Lymphocytes enter the BALT from the blood via high endothelial venules (HEVs) and leave via lymphatics. The occurrence of BALT differs greatly between species, being regularly present, e.g., in rabbits (1). In humans, it is not found at birth but arises in children and adolescents. In adults, BALT is absent in healthy individuals, but its development is induced under various disease conditions such as diffuse panbronchiolitis and hypersensitivity pneumonitis (for review see reference 3). Furthermore, BALT has been found in lungs of cigarette smokers and it has been shown that rats exposed to cigarette smoke have enlarged BALT as well as larger areas of bronchial epithelium covering BALT (for review see reference 3). In mice, BALT is only occasionally present (4) but repetitive inhalations with heat-killed bacteria induced BALT development in this species (reference 5; unpublished data), demonstrating that BALT is inducible by infection or inflammation very much like in human.
A primary adaptive immune response is initiated in secondary lymphoid organs, such as LNs, Peyer's patches (PP), or spleen. In accordance with that, splenectomized lymphotoxin-α–deficient (LTα−/−) mice or alymphoplastic mice (aly/aly), which lack spleen, LNs, and Peyer's patches, are unable to generate primary immune responses to a variety of pathogens, antigens, and allografts (6–8). Surprisingly, splenectomized LTα−/− mice infected with viruses propagating in the respiratory tract are able to generate antigen-specific B and T cells, although with delayed kinetics (9, 10). As LTα−/− mice are known to develop BALT, this suggests that lymphocytes can also be primed at sites other than the “classical” lymphoid organs, in this case presumably in the BALT. Thus, it seems conceivable that induced BALT facilitates primary immune responses to respiratory infections. However, mechanisms that control the development of BALT are largely unknown.
In this study we show that mice deficient for the chemokine receptor CCR7 possess severely reduced numbers of regulatory T cells (T reg cells) in the lung-draining bronchial LN (brLN) and that these animals are prone to spontaneously develop BALT to a considerable degree without being exposed to an immunological insult. We demonstrate that this predisposition is of hematopoietic and not stromal origin and that CCR7-deficient mice start to develop BALT at day 5 after birth. Adoptive transfer of T reg cells from wild type but not CCR7−/− donors into CCR7-deficient recipients largely interferes with the development of BALT. Furthermore, we present data that show that the classical role of T reg cells, i.e., the suppression of effector T cells, requires their homing into lymphoid organs, which in turn is dependent on functional CCR7. Along this line, treatment of newborn C57BL/6 with a neutralizing anti–l-selectin antibody induced BALT within 1 wk.
RESULTS
CCR7-deficient mice spontaneously develop BALT
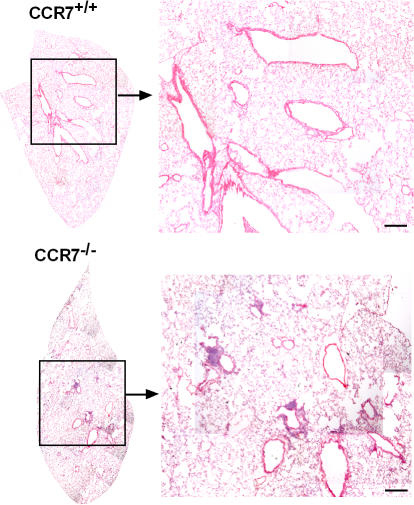
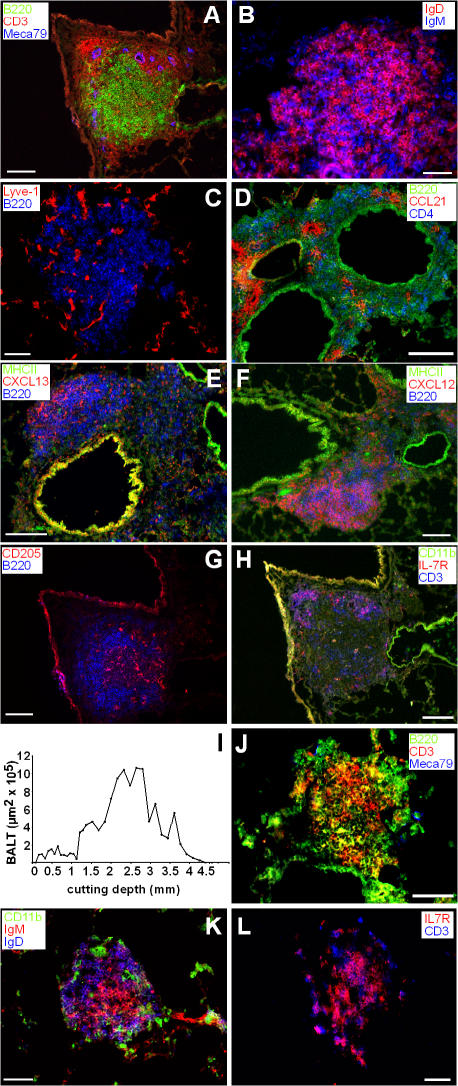
Upon analysis of the lungs of wild-type and CCR7-deficient mice by histology, we identified considerable amounts of perivascular and peribronchiolar infiltrates in CCR7-deficient mice that were rarely present in wild-type animals (Fig. 1). Immunohistology identified these infiltrates as archetypical BALT: B cell follicles encircled by a T cell–rich zone harboring HEVs (Fig. 2 A), which might support entry of naive lymphocytes. The B cell zone consists of IgD+IgM+ as well as IgD−IgM+ cells (Fig. 2 B) and contains lymphatic endothelium as revealed by anti–Lyve-1 staining (Fig. 2 C). Cells expressing the CCR7 ligand CCL21 primarily cluster throughout the BALT with some preference for perivascular areas, whereas only occasionally could CCL21 be detected on endothelial cells (Fig. 2 D). CXCL13 and CXCL12, ligands for CXCR5 and CXCR4, respectively, were primarily found in the B cell zone (Fig. 2, E and F). DCs (CD205+) were distributed all across the lymphoid structures (Fig. 2 G), whereas IL7-R+ cells were primarily found in the T cell area (Fig. 2 H). Furthermore, vascular cell adhesion molecule (VCAM) and c-kit (CD117) were also found to be expressed in the BALT (not depicted).
Figure 1.
Histological overview of lungs of wild-type and CCR7-deficient mice. Cryosections of adult lungs from 6–8-wk-old C57BL/6 mice or CCR7-deficient mice were stained with hematoxylin and eosin. BALT of CCR7-deficient mice is characterized by aggregations of lymphoid cells clustered in follicle-like structures. Sections shown are representative of 36 CCR7-deficient and 15 wild-type animals analyzed. Bars, 300 μm.
Figure 2.
Immunohistology of lungs of CCR7-deficient mice and positioning of BALT within the lung. (A–H) Cryosections of 6–8-wk-old CCR7-deficient mice were stained with antibodies as indicated. Shown are sections representative for 11 animals analyzed. (I) Lungs of CCR7-deficient mice were cut in 8-μm cryosections. Every 10th section was stained with hematoxylin and eosin. From all these sections overviews were achieved using automated image assemblies. Areas of BALT were measured. Shown is the BALT area per section in dependence of the dorsoventral cutting depth of one animal representative of six animals analyzed. (J–L) Cryosections of 6–10-wk-old gnotobiotic CCR7-deficient mice were stained with antibodies as indicated. Shown are sections representative of seven animals analyzed. Bars, 50 μm.
Subsequently, we examined the distribution of the BALT within the lung by investigating consecutive dorsoventral sections through the whole organ (Fig. 2 I). The size of all BALT structures identified was measured and monitored with regard to its position within the lung. In CCR7-deficient mice, BALT is positioned concentric around the main bronchi and arteries, whereas only little BALT is found in the periphery of the lung (Fig. 2 I).
As it is known that repetitive inhalations with heat-killed bacteria induce BALT in mice, we analyzed germ-free CCR7-deficient mice to exclude accidental formation of BALT caused by environmental bacterial challenge (Fig. 2, J–L). Similar to the structures observed in specific pathogen–free (SPF) mice, we found BALT in germ-free CCR7−/− mice, although less organized in the latter. B and T zones were not separated (Fig. 2 J) although the composition of the B cell population was similar to SPF mice (Fig. 2 K). In addition, IL7R-positive and -negative T cells were present in BALT of gnotobiotic CCR7-deficient mice (Fig. 2 L). Furthermore, we treated CCR7-deficient mice from the second trimester of pregnancy until 6 wk after delivery with antibiotics and analyzed the offspring for the occurrence of BALT. Here we failed to find any difference in treated mice compared with untreated control mice (unpublished data). Together, these data demonstrate that BALT formation in CCR7−/− mice is caused by endogenous factors and is independent of environmental pathogens, although subtle influences on microarchitectural features of the BALT are recognized.
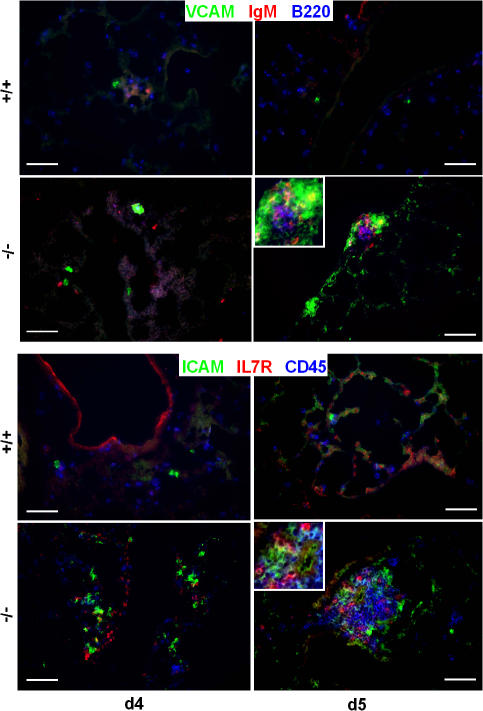
Further analysis revealed that BALT is not present in newborn CCR7-deficient animals (unpublished data). However, ICAM- and VCAM-positive clusters containing IL7R+ and ckit+ cells were readily detectable in CCR7−/− but not in wild-type lungs at day 4 (Fig. 3 and not depicted). From day 5 onwards, BALT was present during the entire life span of CCR7−/− mice (Fig. 3).
Figure 3.
BALT formation in newborn mice. Cryosections of 4- and 5-d-old (d4 and d5, respectively) lungs of wild-type (+/+) and CCR7-deficient (−/−) mice were stained with antibodies as indicated. Bars, 100 μm.
Cells that allow the spontaneous formation of BALT in CCR7-deficient mice are of hematopoietic origin
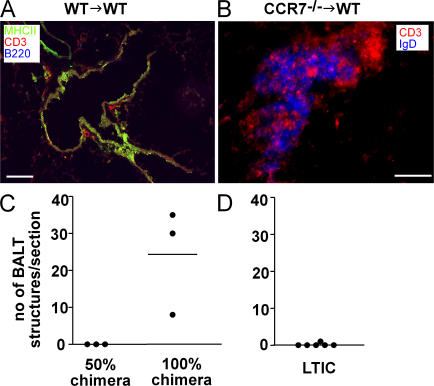
To address the cause (hematopoietic vs. nonhematopoietic) for the exceptional BALT development in CCR7-deficient mice, we created bone marrow chimeras. Chimeric mice were generated by transplantation of CCR7-deficient bone marrow into irradiated congenic C57BL/6 Ly5.1 wild-type recipients and vice versa. 11 to 12 wk later, chimeras were killed and lungs and blood were harvested. Analysis of the blood revealed >98% donor chimerism in the periphery (unpublished data). Similar to the phenotype observed in CCR7-deficient animals, lungs of wild-type mice reconstituted with CCR7-deficient bone marrow showed a marked network of BALT (Fig. 4 B). However, the structures in the reconstituted resembled those identified in germ-free mice. They were less organized than in SPF CCR7-deficient animals because no T cell ring surrounding the B cell area could be identified (Fig. 4 B and compare Fig. 2 A). As expected, transfer of wild-type bone marrow into wild-type animals did not result in BALT induction (Fig. 4 A). Interestingly, reconstitution of wild-type animals with a mixture of 50% CCR7−/− and 50% wild-type bone marrow, which resulted in an ∼50% peripheral chimerism after 11 wk, did not elicit BALT formation (Fig. 4 C), whereas reconstitution of wild-type recipients with 100% CCR7-deficient bone marrow consistently induced considerable amounts of BALT (Fig. 4 C). To examine a possible contribution of lymphoid tissue inducing cells (LTICs) in the formation of BALT, we transferred LTICs (CD4+CD3−) from CCR7-deficient donors into newborn wild-type recipients. However, this never resulted in BALT formation (Fig. 4 D). Together, these data indicate that a suppressive/regulatory cell population might be missing in CCR7−/− mice.
Figure 4.
Lack of CCR7 on hematopoietic cells causes spontaneous induction of BALT. (A and B) Stable bone marrow chimeras were prepared as described in Materials and methods using 6–8-wk-old recipients. Chimeras showed a peripheral blood donor chimerism >98% when analyzed 11–12 wk later. Lungs were subjected to immunohistology using mAbs as indicated. Transfer of CCR7-deficient bone marrow in irradiated congenic wild-type recipients resulted in the induction of BALT (B), which was not observed after the transfer of wild-type bone marrow (A). Figures shown are representative of three animals analyzed per group. Bars: A, 50 μm; B, 100 μm. (C) Lung sections of 50 and 100% chimeras were analyzed for the amount of BALT per central lung section (2,000–3,000-μm cutting depth; compare Fig. 2 I). (D) Lung sections of wild-type mice, which received lymphoid tissue-inducer cells of CCR7-deficient mice, were analyzed for the amount of BALT per lung section. Each dot represents one animal analyzed.
Lack of T reg cells in lung-draining brLNs of CCR7−/− mice
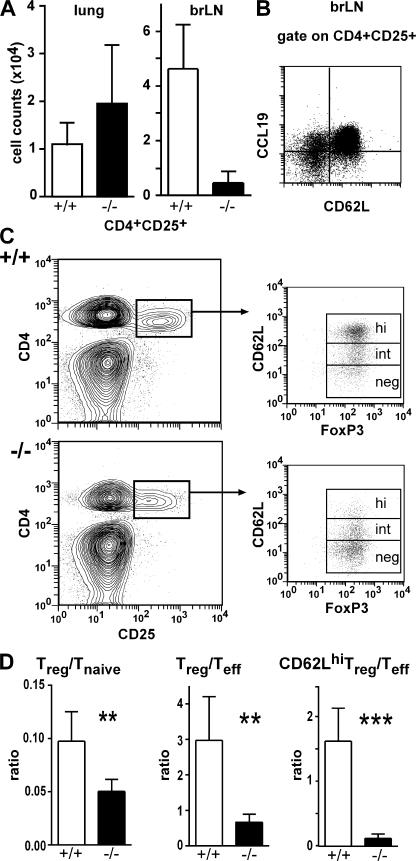
Because T reg cells are known to suppress several immune functions, we tested the hypothesis that a lack in CD4+CD25+ T reg cells could be involved in the spontaneous development of BALT in CCR7-deficient mice. Therefore, we analyzed the frequency of T reg cells in different organs of wild-type and CCR7-deficient mice. We found that CD4+CD25+ T cells are reduced to <10% in all skin-draining peripheral LNs but not in the spleen (unpublished data). In lungs of CCR7-deficient mice, the number of CD4+CD25+ T cells was slightly increased (Fig. 5 A). In contrast, in the brLN of CCR7-deficient animals the number of CD4+CD25+ cells was again reduced to ∼10% of that found in wild types (Fig. 5 A). Because these data suggested that T reg cells require CCR7 for LN homing, we analyzed the expression of CCR7 on CD4+CD25+ brLN cells. In agreement with previous data (11), we found that CCR7 is expressed by the vast majority of cells within this cell population (Fig. 5 B). Further characterization of the CD4+CD25+ population residing in the brLN of wild-type and CCR7-deficient mice revealed that >95% of them express FoxP3, a transcription factor thought to be specific for T reg cells. Interestingly, by analyzing the expression of l-selectin on CD4+CD25+FoxP3+ cells, we observed that in wild types >50% of these cells coexpressed high levels of l-selectin, whereas this cell population was nearly completely missing in the brLN of CCR7-deficient mice (Fig. 5 C).
Figure 5.
Distribution of T reg cells in lung and brLN in wild-type and CCR7-deficient mice. Leukocytes were isolated from lungs and brLN of 6–8-wk-old C57BL/6 and CCR7-deficient mice and were stained using anti-CD4 and anti-CD25 mAb. (A) Absolute cell counts of T reg cells present in the lung and the brLN of wild-type (+/+; open bars) and CCR7-deficient (−/−; closed bars) mice. (B) CD4+CD25+ cells from brLN were analyzed for their expression of CD62L and of CCR7 (CCL19-hIgG). (C) brLN CD4+CD25+ cells of wild type and CCR7−/− were further analyzed for the expression of surface CD62L and intracellular FoxP3. (D) Ratio of T reg cells/T naive cells (left), T reg cells/T effector cells (middle), and CD62Lhi T reg cells/T effector cells (right) present in the brLN of wild-type (open bars) and CCR7-deficient (closed bars) mice are shown (wt, n = 5; CCR7−/−, n = 4; mean + SD; **, P < 0.01). Similar results were obtained in eight wild-type and eight CCR7-deficent animals on the BALB/c background (not depicted).
In addition to the absolute reduction of T reg cell numbers in the brLN we also observed a relative reduction of this cell population among T cell subsets residing in the brLN of CCR7-deficient animals. The ratio between regulatory and naive T cells in the brLN (number of CD4+CD25+FoxP3+/number of CD4+CD25−FoxP3−CD62L+) shifted from 0.1 in wild-type animals to 0.05 in CCR7−/− mice (Fig. 5 D, left). Furthermore, the ratio between all T reg cells and effector T cells (CD4+CD25+FoxP3+/CD4+CD25+FoxP3−CD62L; Fig. 5 D, middle) decreased from 3.0 in wild type to 0.7 in CCR7-deficent animals and that of CD62Lhigh T reg cells/effector T cells from 1.6 to 0.12 (Fig. 5 D, right). Together these data demonstrate that CCR7-deficient mice carry both an absolute and a relative reduction of T reg cells in the brLN and that this deficiency might be responsible for the spontaneous formation of BALT in these mice.
Transfer of wild-type T reg cells into CCR7-deficient mice reduces BALT in CCR7−/− mice
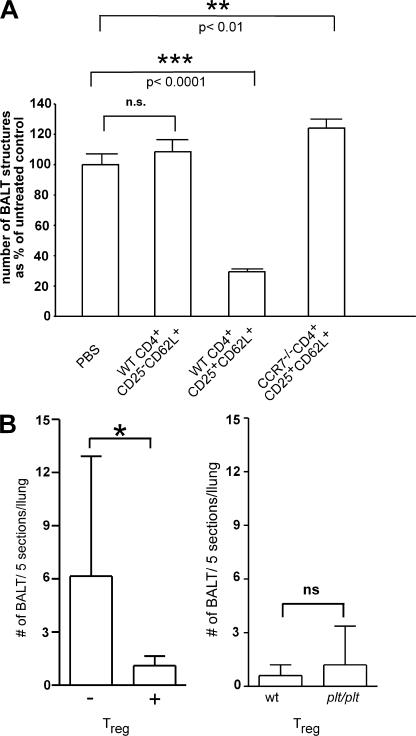
To investigate the role of CD4+ T reg cells in BALT development in CCR7-deficient mice, we adoptively transferred 0.5–1 × 106 T cells of different phenotype at intervals of 2 wk into CCR7−/− mice starting at 4–11 d old. Mice were killed 2 wk after the last transfer. Consecutive sections of whole lungs were stained with hematoxylin and eosin and the number of BALT structures were counted. Lungs of CCR7-deficient recipients receiving CD4+CD25+CD62L+ T reg cells from wild-type donors showed a significant decrease in the amount of BALT structures compared with lungs of mice treated with CD4+CD25−CD62L+ naive T cells derived from wild types or treated with PBS (P < 0.0001; Fig. 6 A). In contrast, treatments of CCR7- deficient recipients with T reg cells isolated from CCR7- deficient donors did not have an effect on BALT reduction (Fig. 6 A). This shows that wild-type, but not CCR7-deficient, T reg cells are able to interfere with BALT existence in CCR7−/− mice. To further delineate the effect of T reg cells on BALT induction, 1-d-old CCR7-deficient mice received either a single i.v. injection of PBS or were adoptively transferred with 5 × 105 CCR7-proficient T reg cells. 6 d after transfer, the number of BALT structures was analyzed. In untreated mice we found a mean of 6.2 BALT structures per 5 sections per lung, whereas this number decreased to 1.1 in animals that received T reg cells (Fig. 6 B, left).
Figure 6.
Transfer of wild-type T reg cells into CCR7-deficient mice diminishes BALT in CCR7−/− mice. 4–11-d-old CCR7−/− mice received PBS or 0.5–1 × 106 sorted cells as indicated three times at intervals of 2 wk. Mice were killed 2 wk after the last transfer. Consecutive sections of whole lungs were stained with hematoxylin and eosin and the number of BALT structures was counted. Data shown here were obtained and pooled from different experiments (PBS, n = 6; wild-type CD4+CD25−CD62L+, n = 6; wild-type CD4+CD25+CD62L+, n = 5; CCR7−/− CD4+CD25+CD62L+, n = 2; mean + SD). (B, left) 1-d-old CCR7-deficient mice received 5 × 105 CCR7-proficient CD4+CD25+ cells i.v. into the superficial temporal vein. Mice were killed 6 d after the transfer (day 7). From each recipient, five sections per lung were analyzed for the presence of BALT structures. Shown are results from animals receiving (+; n = 10) and animals not receiving (−; n = 13) T reg cells. (B, right) Number of BALT structures of CCR7-deficient recipients that received T reg cells from wild-type (wt) or plt/plt donors (mean + SD; n = 5).
Paucity of LN T cell (plt/plt) mice, a naturally occurring mutant that lacks expression of the CCR7 ligands CCL19 and CCL21, as well as CCR7-deficient mice show profound alterations in thymic T cell development (12). We wondered whether the inability of CCR7-deficient T reg cells to interfere with BALT development might be caused by developmental defects of T reg cells rather than by impaired homing. Because plt/plt and CCR7-deficient mice show similar defects with regard to thymic T cell development, we hypothesized that a potential intrinsic defect in T reg cell function would also be apparent in T reg cells derived from plt/plt mice. To test this hypothesis, we compared the efficiency of T reg cells of wild-type and plt/plt origin to interfere with BALT development in 1-d-old CCR7-deficient mice. Interestingly, in this experimental setup, wild-type and plt/plt T reg cells were equally potent in interfering with BALT development analyzing the number of BALT structures 6 d after transfer (Fig. 6 B, right). Because both wild-type and plt/plt-derived T reg cells express CCR7, these results suggest that the impaired homing rather than a developmental defect of CCR7-deficient T reg cells seems to be causally involved in BALT development in CCR7-deficient mice.
Adoptively transferred CCR7-deficient T reg cells fail to regulate in vivo and are impaired in LN homing
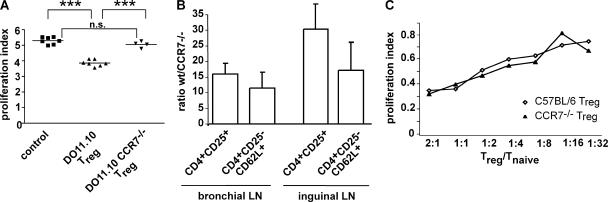
To clarify whether CCR7−/− T reg cells are able to fulfill their classical function in vivo, i.e., suppressing effector T cell function, we performed adoptive transfer assays using DO11.10 cells that carry a transgenic T cell receptor specific for OVA. To that end, CD4+ CD25− CFSE-labeled DO11.10 cells were given i.v. to BALB/c recipients together with equal numbers of CD4+CD25+ cells isolated either from DO11.10 or DO11.10 CCR7−/− donors. Control mice received CD4+ CFSE-labeled DO11.10 cells only. On the next day, mice were immunized intratracheally with OVA and 3 d later mice were killed and the brLN analyzed for the proliferation of CFSE-labeled CD4+ cells. Compared with mice that did not receive T reg cells, the proliferation of the CFSE-labeled reporter cells was significantly reduced in those recipients that also received T reg cells from DO11.10 donors (P < 0.001; Fig. 7 A). In contrast, adoptive transfer of T reg cells of DO11.10 CCR7−/− origin did not affect the T cell proliferation (Fig. 7 A). When we analyzed the brLN for the amount of transgenic T reg cells we found substantially fewer T reg cells from CCR7-deficient DO11.10 donors compared with those from wild-type DO11.10 donors (unpublished data). Although the analyses include cells that proliferated after the transfer, these data suggest a defect in homing. We therefore adoptively transferred wild-type and CCR7-deficient lymphocytes from spleen and LNs that were depleted for B220+ and CD8+ cells followed by labeling with the fluorescent dyes TAMRA (carboxytetramethylrhodamine) and DDAO (7-hydroxy-9H 1,3-dichloro-9, 9-dimethylacridin-2-one), respectively. After 15 h, recipients were killed and the number of naive and T reg cells that homed to the bronchial and peripheral LN was determined. Analyzing CD4+CD25+ T reg cells we found that CCR7-deficient cells homed 16 and 30 times less efficiently to the brLN and the inguinal LN, respectively, compared with wild-type cells (Fig. 7 B). For naive cells (CD4+CD25−CD62L+) the difference was less pronounced. Here, CCR7−/− cells homed 12 and 17 times less efficiently to the brLN and the inguinal LN, respectively, compared with wild-type cells (Fig. 7 B). These data demonstrate that CCR7-deficient T reg cells are exquisitely impaired in homing to the brLN, which as a consequence results in less controlled T cell activity.
Figure 7.
Adoptively transferred CCR7-deficient T reg cells fail to regulate in vivo, are impaired in LN homing, but suppress T cell proliferation in vitro. (A) 8–12-wk-old BALB/c recipients received equal numbers of CD4+ CD25− CFSE-labeled DO11.10 cells and either CD4+CD25+ DO11.10 or CD4+CD25+DO11.10 CCR7−/− cells. As controls, other mice received only CD4+ CFSE-labeled DO11.10 cells. 3 d after intratracheal application of OVA, mice were killed and the brLN analyzed for proliferation of CFSE-labeled CD4+ cells. The proliferation index was calculated as described in Materials and methods. Shown are data from two independent experiments with each symbol representing individual animals (***, P ≤ 0.0001). (B) Lymphocytes from spleen and LN of BALB/c and CCR7−/− were enriched for CD4+ cells by depleting B220+ and CD8+ cells. Cells from wild-type donors were labeled with DDAO and those from CCR7-deficient donors with TAMRA. Before transfer, cells were adjusted to contain equal amounts of CD4+CD25+ cells. 0.5–1 × 106 CD4+CD25+ of each genotype were i.v. transferred. 15 h after the transfer, recipients were killed and the brLN as well as the inguinal LN were analyzed for the presence of naive (CD4+CD25−CD62L+) and regulatory (CD4+CD25+) T cells (mean + SD; n = 5 recipients). (C) An in vitro test with DCs, naive T cells of OTII Ly5.1 mice, and T reg cells was set up as described in Materials and methods. T cells were cultured for 3 d and proliferation of OTII Ly5.1 T cells was measured by FACS analysis. Tests were set up in duplicates or triplicates. Shown are results from one of three independent experiments obtaining similar results.
T reg cells of wild-type and CCR7-deficient mice are equally functional in vitro
To dissect whether CCR7-deficient T reg cells are just impaired in homing to LN and thereby prevented from exerting regulation or posses an intrinsic defect, we performed in vitro assays similar to the in vivo assays described in the previous paragraph. OVA-loaded wild-type DCs were cultured together with CFSE-labeled CD4+CD25− Ly5.1 OTII cells in the presence of different numbers of CD 4+CD25+CD62L+ Ly5.2+ T reg cells isolated either from OTII or OTII-CCR7−/− donors. Analyzing the proliferation of CFSE-labeled cells on day 3 failed to reveal any difference in the regulating capability between the CCR7-deficient and wild-type T reg cells (Fig. 7 C). This suggests that CCR7-deficient T reg cells are functionally intact once they have access to the targets they regulate on.
Accumulation of FoxP3+ cells in the thymic medulla in wild-type and CCR7-deficient mice
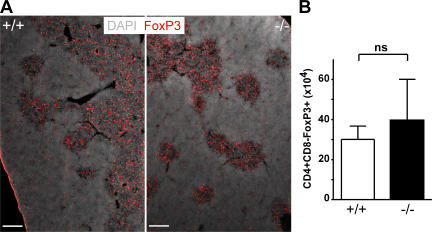
Several experiments of this study provide evidence that the homing of T reg cells to LN depends on CCR7 expression. However, because it has been suggested that CCR7 might be required for guiding single-positive thymocytes from the cortex into the medulla (13), we compared positioning and number of thymic T reg cells in wild-type and CCR7-deficient mice. Applying immunohistology on frozen thymic sections, we observed that in wild-type mice FoxP3+ cells locate to a large extent to the thymic medulla and only few FoxP3+ cells are present in the cortex (Fig. 8 A). Of interest, a very similar picture was obtained for CCR7−/− mice. Again, the vast majority of FoxP3+ cells locate to the medulla, whereas some disperse through the cortex (Fig. 8 A). Furthermore, we failed to observe any significant difference regarding the absolute number of CD4+FoxP3+ cells in the thymus between both strains (P > 0.05; Fig. 8 B). Together, these data argue against an essential role for CCR7 in the corticomedullary transit of positively selected FoxP3+ T cells and thus support the notion that wild-type and CCR7-deficient T reg cells might gain comparable intrinsic functionality.
Figure 8.
CCR7 deficiency does not affect localization of FoxP3+ cells in the thymus. (A) Cryosections of 8–12-wk-old wild-type B6 (+/+) and CCR7-deficient B6 mice (−/−) were stained with antibodies as indicated. Shown are sections representative of four animals analyzed. Bars, 200 μm. (B) Thymocytes were isolated from 8–12-wk-old C57BL/6 and CCR7-deficient mice and stained with antibodies against CD4, CD8, and FoxP3. Shown are the number of CD4+CD8−FoxP3+ cells (mean + SD; n = 3).
CCR7-deficient B cells are not impaired in egressing from BALT
It has been recently suggested that lymphocytes might require CCR7 expression to egress from nonlymphoid organs such as skin or lung (14, 15). To address the possibility that BALT might develop in CCR7-deficient animals because of impaired lymphocyte egress, we applied competitive transfer of wild-type and CCR7-deficient cells to CCR7-deficient recipients. Because B cells are the major cell population contributing to BALT in CCR7-deficient mice, we injected magnetic cell sorting–purified B cells from wild-type and CCR7-deficient donors labeled with TAMRA and DDAO, respectively, into CCR7-deficient recipients. In another series of experiments, wild-type and CCR7-deficient cells were labeled vice versa. 6 d after transfer recipients were killed and the number of wild-type and CCR7-deficient B cells present in the same BALT structures was determined by histology. These experiments revealed that significantly less adoptively transferred CCR7-deficient B cells than wild-type B cells were present in the BALT (P < 0.01; Fig. 9). These results strongly suggest that CCR7-deficent B cells are not impaired in leaving BALT in CCR7-deficient mice.
Figure 9.
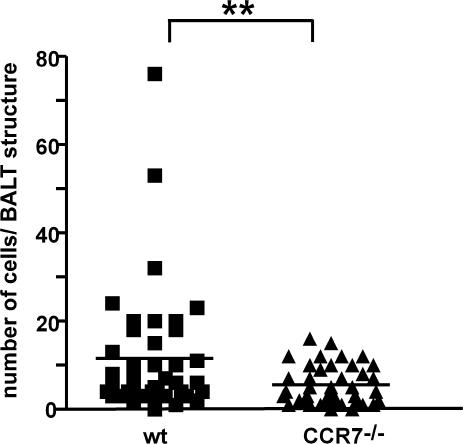
CCR7 deficiency does not cause accumulation of B cells in BALT. CCR7-deficient BALB/c mice received equal amounts of TAMRA- and DDAO-labeled wild-type or CCR7-deficient B220+ cells, respectively. Cryosections were analyzed for the number of DDAO+ and TAMRA+ cells present in BALT structures. In total, 41 BALT structures in four recipients were analyzed. Each symbol represents the number of cells identified in individual BALT structures (P < 0.01; paired Student's t test).
Induction of BALT in newborn mice by anti–l-selectin antibodies
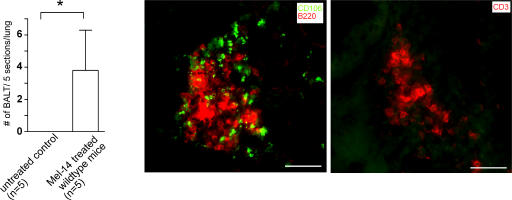
Because 7-d-old CCR7-deficient mice already exhibit pronounced BALT, which was substantially reduced after adoptive transfer of wild-type T reg cells, we hypothesized that it might be possible to induce BALT in wild-type mice by interfering with cell homing. Because both l-selectin and CCR7 are essentially required for lymphocyte homing to LN, we treated 3-d-old mice with a single injection of a neutralizing anti–l-selectin mAb (Mel-14) or PBS. Analyzing the lungs 1 wk later we found BALT structures in all (5/5) Mel-14–treated mice with a mean of 3.8 ± 2.5 (mean ± SD) BALT structures per 5 sections per lung, whereas not a single structure could be found in any of the 5 control mice treated with PBS (Fig. 10). Further histological analysis of Mel-14–induced BALT revealed the typical BALT architecture of CCR7-deficient mice at a comparable age: moderately organized clusters containing B, T, and VCAM+ (CD106+) cells (Fig. 10).
Figure 10.
Treatment of newborn wild types with anti-CD62L mAb induces BALT. 3-d-old wild-type mice were i.p. injected with 300 μg anti-CD62L mAb (Mel-14). 7 d later, mice were killed. The presence of BALT was analyzed as described for Fig. 6 B. Shown are the number of BALT structures per five sections per lung from five treated and five untreated mice (mean + SD; n = 5; *, P < 0.05, Wilcoxon test). Bars, 50 μm.
DISCUSSION
In this study we have investigated the correlation between the emergence of BALT and malfunction of T reg cells in CCR7-deficient mice. We have shown that CCR7-deficient mice exhibit atypical amounts of BALT. These structures harbor all morphological features characteristic for secondary lymphoid organs, including the presence of HEV and lymphatics. We demonstrate that spontaneous BALT formation originates in the hematopoietic system of CCR7-deficient mice, most likely from the aberrant function of distinct cell types derived thereof. Analyzing the distribution of T reg cells in different organs of CCR7−/− mice, we found an ∼90% reduction of this cell population in the brLN in CCR7 mutants. Furthermore, adoptive transfer of wild-type T reg cells into CCR7-deficient recipients reverted the occurrence of BALT substantially. We also showed that CCR7 deficiency is linked to a strongly impaired homing of T reg cells to LN, where they mediate some of their suppressive functions. In addition, a single injection of a neutralizing anti–l-selectin mAb in newborn C57BL/6 mice was sufficient to allow for BALT induction.
Little is known about the development of BALT. In mice, BALT does not usually occur regularly, but can be induced by inflammatory reactions such as aerosol treatment with heat-inactivated Propionibacterium acnes (reference 16; unpublished data). Although the development of secondary lymphoid organs such as LN and PP and the expression of CXCL13 and CCL21 in the spleen are typically dependent on LTα (17–19), data provided by Moyron-Quiroz et al. (4) show that the formation of BALT and the expression of CXCL13 and CCL21 in the lung occurs independently of LTα. Consequently, lungs of LTα−/− mice often feature BALT (20). The expression of LTα is influenced by inflammatory stimuli and treatment of mice with antibiotics suppressed the spontaneous appearance of BALT in LT-deficient mice. However, such treatment does not interfere with BALT development in CCR7-deficient mice. Furthermore, as we find BALT in lungs of germ-free CCR7-deficient mutants, the development of BALT in CCR7-deficient mice seems to occur independently of the presence of inflammatory stimuli such as pathogen-associated molecular patterns. Although BALT is clearly detectable as early as 5 d after birth we cannot formally exclude the possibility that in these mice noninfectious stimuli such as dust might contribute to BALT formation.
One of the early steps in secondary lymphoid organ development is the local accumulation of CD4+CD3− IL-7Rαhi LTICs that deliver LT α1β2 signals to resident stromal cells. LTICs are capable of inducing the formation of PP as well as nasal-associated lymphoid tissue (21, 22). A similar role for these cells as inducers of peripheral LNs is also generally accepted (23). CCR7-deficient mutants occasionally lack the inguinal LN, whereas CXCR5−/− mice miss the inguinal, brachial, and axillary LNs to varying degrees. Of interest, CCR7−/−CXCR5−/− mice lack all peripheral LN but carry the mesenteric LN. CD4+CD3− IL-7Rαhi cells express CXCR5 as well as CCR7, indicating that both receptors cooperate during an early step of secondary lymphoid organ development. Indeed, it has been demonstrated that the function of CXCR5 on CD4+CD3− IL-7Rαhi cells can be complemented by CCR7 (24, 25). As LTICs are known to induce secondary lymphoid organs, we investigated whether LTICs might be involved in triggering the development of BALT by their putative aberrant localization to peribronchial areas. Although we could never induce BALT by adoptively transferring CCR7−/− LTICs to wild-type animals, we cannot rule out that this might be caused by limitations of our experimental setup. Thus, it remains to be determined what mechanisms are involved in detail in BALT formation. Data obtained from CCR7-deficient and LTα−/− mice demonstrate that BALT develops spontaneously in the absence of CCR7 signaling. This is of particular interest because it has been shown that ectopic expression of CCL21 and to a lesser degree that of CCL19 induces ectopic secondary lymphoid tissue (26–28). Although it is currently unclear how these, on first sight, contradictory observations contribute to lymphoid tissue development it seems likely that they affect different stages of the process. Thus, it seems possible that, in a first step of ectopic lymphoid tissue induction, overexpression of CCR7 ligands might be required for the recruitment of LITC to locations such as the pancreatic islets, where they are not present under physiological conditions. In contrast, BALT in CCR7-deficient mice regularly develops at positions where it is also found in wild-type mice, which is consistent with inflammatory insults. Therefore, it seems possible that the peribronchiolar region represents a “preconditioned” area that potentially favors the development of “add on” lymphoid tissue once required to fight infection. In such a scenario distinct control mechanisms would be required to ensure that BALT does not form spontaneously. Here, we demonstrate that wild-type T reg cells are able to block BALT formation considerably in newborn/young CCR7-deficient animals, suggesting BALT manifestation is controlled by T reg cells. Because adoptively transferred T reg cells from plt/plt mice but not from CCR7-deficient mice efficiently suppressed BALT formation, it seems likely that unimpaired homing of T reg cells is required for mediating the control function. That we could induce BALT in newborn wild-type mice by injecting a neutralizing anti–l-selectin mAb supports this idea.
Another interesting observation obtained in this study relates to the degree of organization of BALT. Although BALT is highly organized in CCR7−/− mice kept at SPF conditions, structures present in adult germ-free mice as well as in bone marrow chimeras are less organized. These observations are reminiscent of those recently reported by Mebius et al. (23). In that experimental setup, s.c. injection of newborn LN cells induced ectopic lymphoid tissue, which showed little organization in adult mice, but segregated in T and B areas when induced in newborn mice. Interestingly, those disorganized aggregates induced in adult mice could be transformed into organized tertiary lymphoid tissue after transient immune activation (29). Thus it seems plausible that in CCR7−/− mice BALT is induced independent of inflammatory stimuli, whereas some type of immune activation could be required to induce segregation of cells.
A role of autoimmune-mediated insults in BALT formation has been recently suggested by Rangel-Moreno et al. (30). In that study it was shown that BALT occurs in patients suffering from pulmonary complications of autoimmune diseases, such as rheumatoid arthritis and Sjögren syndrome. Furthermore, they demonstrate that the presence of BALT correlates with lung damage in rheumatoid arthritis patients, suggesting a causal link between autoimmunity and BALT formation. Along this line, another study revealed that T reg cells from patients with rheumatoid arthritis possess reduced amounts of FoxP3 at both the messenger RNA and protein level and that these T reg cells have reduced suppressive activity in vitro (31). Because it has been reported that CCR7-deficient mice are prone to develop autoimmunity (32), it seems possible that, in addition to impaired T reg cells homing, self-reactive cells contribute to BALT formation in these animals.
It has been recently suggested that CCR7 might be involved in the egress of lymphocytes from nonlymphoid organs (14, 15). To test the hypothesis that impaired egress of lymphocytes from the lung might result in BALT, we competitively transferred B cells from wild-type and CCR7-deficient donors to CCR7−/− recipients. B cells were chosen because they are amply present in BALT. Interestingly, the frequency of transferred wild-type B cells present in BALT was considerably higher than that of CCR7−/− origin, rendering the hypothesis unlikely that impaired egress essentially contributes to BALT formation. Along this line, the anti–l-selectin mAb used in this study is well known for its ability to prevent the recruitment of fast moving cells from the blood stream to HEV, whereas there is no evidence available suggesting that l-selectin might contribute to lymphocyte egress.
The initial characterization of CCR7-deficient mice revealed impaired homing of naive T cells to LN and the splenic white pulp, impaired mobilization of DCs from the skin, and, as a consequence of both, an impaired functional organization of secondary lymphoid organs. Thus, when analyzing the early onset of induced immune reactions in these animals we observed a profoundly postponed humoral and cellular response (33), whereas the present study points to a role for CCR7 on T reg cells in controlling/dampening immune responses. CCR7-deficient mice are also distinguished by a disturbed thymic architecture, impaired T cell development, and impaired negative selection (12, 13, 32), rendering these animals potentially prone to develop autoimmunity. Indeed, it has been recently noticed that CCR7−/− and plt/plt mice manifest mild autoimmunity to the lachrymal and salivary glands. Although it has not been addressed experimentally in this study, it seems possible that impaired negative selection might contribute to BALT development and that the transfusion of wild-type T reg cells might overcome this defect.
Little is known about the migration pattern of T reg cells. Szanya et al. (34) addressed the trafficking behavior of two subsets of splenic T cells (CD4+CD25+CD62L+ and CD4+CD25+CD62L−). They showed that T reg cells (CD4+CD25+CD62L+) but not activated T cells (CD4+ CD25+CD62L−) express high levels of CCR7 messenger RNA. Because both CD62L and CCR7 are essential for the homing of T cells into LNs, these authors hypothesize that the characteristic profile of adhesion molecule and chemokine receptors allows CD4+CD25+CD62L+ T reg cells to efficiently enter LNs (34). Our data support this hypothesis, as we have shown that the expression of CCR7 is necessary for the entrance of T reg cells into peripheral LNs. Because of the lack of this receptor, T reg cells are strongly impaired in entering the lung-draining LN.
T reg cells are known to control immunological processes. The majority of naturally arising CD4+ T reg cells express CD25, the IL-2 receptor α-chain, and treatment of mice with a neutralizing anti–IL-2 mAb selectively reduces the number of T reg cells (35). The finding that IL-2–deficient mice lack CD4+CD25+ T reg cells and develop BALT spontaneously (36) links T reg cells to BALT formation. Data provided in this study put considerable weight to the hypothesis that T reg cells are involved in the control of BALT manifestation. We observed that T reg cells are reduced 10-fold in the bronchial and other peripheral LN of CCR7-deficient mice and that transfer of wild-type but not CCR7-deficient T reg cells into newborn CCR7-deficient recipients reduces the occurrence of BALT to a considerable degree.
In summary, the present study demonstrates that lack of CCR7 provokes the formation of BALT and provides evidence that T reg cells are important in controlling BALT formation.
MATERIALS AND METHODS
Mice.
CCR7−/− mice have been described previously (33). CCR7-deficient mice were backcrossed for at least seven generations to the C57BL/6 or BALB/c genetic background. Some histological stainings were also performed on CCR7-deficient mice on the original 129SV X BALB/c mixed background. C57BL/6Ly5.2, C57BL/6Ly5.1, and BALB/c mice were purchased from Charles River Laboratories. The MHCII-restricted T cell receptor transgenic OT-II mice and DO11.10 mice have been described previously (37, 38). CCR7-deficient mice were bred to DO11.10 mice to obtain chemokine receptor–deficient, T cell receptor–transgenic mice. All animals were maintained under SPF conditions and used at the age of 8–12 wk or as indicated in the figure legends. Some CCR7-deficient mice were developed and maintained at germ-free conditions at the animal facility of Hannover Medical School as described previously (39). All animal experiments were conducted in accordance with local and institutional guidelines.
Immunohistology.
For immunohistology, mice were anesthetized and bled to death from the retroorbital plexus. The thorax was opened and the lung was perfused with cold PBS via the right heart ventricle. The lung was removed, filled via the trachea with OCT Tissue-Tek (Sakura) diluted 1:4 in PBS, embedded in OCT, and frozen on dry ice. 7–8-μm cryosections were prepared, air dried, and fixed for 10 min in ice-cold acetone. Cryosections were blocked with 10% mouse or rat serum and stained with the following antibodies: B220-FITC, B220-Cy5 (clone TIB146), CD3-Cy3, CD3-Cy5 (17A2), IgM (HB88), IgD (250), CD205 (NLDC-145), and IL7-R (A7R34; all grown and labeled in our laboratory); Meca79, MHCII-FITC (AF6-120.1), CD11c-biotin (HL3; all from BD Biosciences), goat anti-CCL21, goat anti-CXCL13 (both from R&D Systems), goat anti-CCL19, goat anti-CXCL12 (both from PeproTech), CD11b-FITC (MAC-1), B220-biotin, streptavidin-Cy5 (all from Caltag), streptavidin-Cy3, mouse anti-goat biotin, mouse anti-rat Cy3 (all from The Jackson Laboratory), CD45.2-FITC (104; Chemicon), streptavidin Alexa-488 (Invitrogen), Lyve-1 (24–228; Acris), CD54-biotin (3E2; BD Biosciences), and CD106 (clone 429 [MVCAM.A]; BD Biosciences). Cryosections of thymi were blocked with 10% rat serum followed by staining with FoxP3-biotin (clone FJK-16s; eBioscience) and streptavidin-Cy3. Immunohistological analysis of the lung and thymus was done as described previously (12, 40) using a motorized microscope (Axiovert M200; Carl Zeiss MicroImaging, Inc.) and KS300 software (Carl Zeiss MicroImaging, Inc.).
Quantification of BALT.
BALT was analyzed in mice of different age. In adult mice, BALT was prominently developed and always localized around or adjacent to the bronchi and vessels (Figs. 1 and 2). The amount of BALT present in adult CCR7-deficient mice that adoptively received T cells or PBS three times (Fig. 6 A) was quantitated as follows: lungs were cut completely and every 10th section was stained with hematoxylin and eosin. Consequently, on 60–75 sections per mouse the number of BALT structures present was determined. Because the size of BALT varied considerably, the number of areas consisting of >100 cells was determined. For the analysis of lungs of bone marrow chimeras or of mice that received LTICs (Fig. 4) three sections were taken at a cutting depth of ∼1.5, 2.5, and 3.5 mm, respectively (compare Fig. 2 I). For the analysis of newborn and young mice (day 0 to 10) five sections per lung were analyzed. After slicing in a dorsoventral direction, the criterion for the first section to be used for analysis was approval of macroscopically visible bronchi. At distances of 400 μm each, four further sections were subjected to analysis. Each slide analyzed contained all five plains and was stained with anti–B220-Cy3. Lungs were then analyzed for the presence of BALT and clusters consisting at least 10 B cells, locating in close proximity to each other, were enumerated.
Treatment of pregnant mice with antibiotics.
CCR7-deficient mice were treated from the second trimester of pregnancy onwards until 6 wk after delivery. Mother and offspring were maintained on 1.25 mg/ml sulfamethoxazole and 0.25 mg/ml trimethoprim.
Flow cytometry.
For flow cytometry, lungs were cut into small pieces and digested for 1 h at 37°C in 0.5 mg/ml PBS/collagenase A (Roche)/ DNaseI (20 units/ml; Roche). To obtain single cell suspensions, lungs were minced through a nylon mesh, washed with PBS supplemented with 3% FCS followed by separation on Lympholyte M (Cedarlane), and counted using a Neubaur chamber. Cells were stained using the following antibodies: CD3-PE (17A2), CD4-PerCp (L3T4), CD11c-FITC (HL3), MHCII (IAd)-biotin (IgG3) GR1-PE (RB6-8C5; all from BD Biosciences), CD8-Cy5 (CT-CD8a), CD25–Alexa-488 (PC61 5.3), CD11b-PE (MAC-1), CD45-APC (30F11), CD62L-PE (MEL-14; all from Caltag), CD4 (clone RMCD4), B220- Cy5 (clone tip 146), CD19-biotin (6D5; Southern Biotechnology Associates, Inc.), and FoxP3-Pe (clone FJK-16s; eBioscience). Thymocytes were isolated as described previously (12) and stained using the following antibodies: CD25-Alexa 488 (PC61 5.3), CD4 (clone RMCD4), CD8-APC-Cy7 (clone 53–6.7; BD Biosciences), and FoxP3-PE. Four-color flow cytometry was done with a FACS Calibur or an LSRII (BD Biosciences).
Stable bone marrow chimeras.
Bone marrow was prepared from femurs and tibiae of CCR7-deficient (C57BL/6), C57BL/6-Ly5.2, or congenic C57BL/6-Ly5.1 mice followed by separation on Lympholyte M. Recipients for bone marrow transplantation were 6–8 wk-old sex-matched CCR7-deficient or congenic C57BL/6-Ly5.1 mice. Before transplantation, adult recipient mice were lethally irradiated (5 Gy) twice at an interval of 5 h. Approximately 1.5 × 107 bone marrow cells were transferred to irradiated recipients. For 50% chimeras, 50% wild-type and 50% CCR7−/− bone marrow were mixed before transplantation. 11–12 wk later, chimerism was determined by FACS of peripheral blood using congenic markers. Chimeric mice were killed, and lungs and blood were harvested and analyzed.
Isolation of LTICs.
Mesenteric LNs of CCR7-deficient C57BL/6 mice were isolated and minced through a nylon mesh. Cells were sorted by flow cytometry for CD3−CD4+ by negative sorting for B220, CD8, CD11b, CD11c, Ter119, GR1, and CD19, and then further for CD3−CD4+. Donors were between embryonic day 19 and 10 d old. Recipients were 0.5–5 d old. Before transplantation, recipients were irradiated (3.5 Gy) and sorted cells were given i.p. or i.v. (1.3 × 103 to 6 × 104 CD3−CD4+ cells). 6 wk after transplantation, recipients were killed, and lungs were harvested and analyzed for the presence of BALT.
Isolation of CD4+CD25+ and CD4+CD25+ T cells.
Spleen and peripheral LNs of C57BL/6 mice were harvested and minced through a nylon mesh. CD4+ cells were isolated with CD4 Macs Microbeads (L3T4) following the manufacturer's instructions (Miltenyi Biotec). CD4+ cells were blocked with rat serum and stained using the following antibodies: CD62L-PE, CD25–Alexa-488, and CD4-biotin followed by streptavidin-PECy7, B220-Cy5, and CD8-Cy5. CD4+CD25+CD62Lhigh and CD4+CD25−CD62Lhigh T cells were sorted using a FACS Aria (BD Biosciences).
In vitro analysis of T reg cells.
Bone marrow–derived DCs were prepared as described previously (40). In brief, bone marrow from femurs and tibiae were cultured in RPMI 1640, supplemented with 30 ng/ml GM-CSF, 10% FCS, and 50 μM β-mercaptoethanol. At day 1 and 3 of the culture, medium was changed and the cells were selected for adherence. Additional culture medium was added at day 5. At day 7, cells were harvested, washed, and reseeded in culture medium supplemented with 30 ng/ml TNF (R&D Systems) and 1μg PGE2 (Sigma-Aldrich) to induce maturation. Cells were incubated from day 7 with 1 μg/ml OVA peptide specific for the TCR expressed by OT-II cells (OVA323–339 ISQAVHAAHAEINEAGR). CD4+CD25−OTII Ly5.1 C57BL/6 cells were sorted using a FACS Aria and labeled with CFSE as described previously (41). 5 × 104 DCs were cultured in 96-well chamber plates with 5 × 104 CD4+CD25−OTII Ly5.1 and T reg cells in media containing GMCSF and 50 ng/ml rIL-2. Proliferation of the CFSE-labeled CD4+CD25−OTII Ly5.1 cells was measured on day 3 by four-color flow cytometry with a FACS Calibur.
Transfer of CD4+CD25+ and CD4+CD25+ T cells.
0.5−1 × 106 CD4+CD25+CD62Lhigh or 1–1.5 × 106 CD4+CD25−CD62Lhigh cells isolated from wild-type or CCR7-deficient mice were transferred three times at intervals of 2 wk to CCR7−/−B6 mice. Recipients were 4–11 d old at the beginning of the transfer and killed 2 wk after the last transfer. The first injection was given i.p. and the second and the third i.v. In other experiments, 5 × 105 CD4+CD25+ isolated from wild-type or plt/plt mice were transferred i.v. into newborn CCR7−/−B6 mice. Recipients were 1 d old at the beginning of the transfer and killed 6 d after the transfer (day 7). Results were tested for significance with the unpaired Student's t test.
In vivo functionality of T reg cells.
CD4+ cells of DO11.10 mice and CD4+CD25+ cells from DO11.10 or DO11.10 CCR7−/− mice were isolated from spleen and peripheral LNs using the regulatory T cell isolation kit following the manufacturer's instructions (Miltenyi Biotec). CD4+ cells of DO11.10 mice were labeled with CFSE as described previously (41). 1.6 × 106 CD4+ reporter T cells of DO11.10 mice alone or together with either CD4+CD25+ of DO11.10 or DO11.10CCR7−/− mice were given i.v. 1 d before intratracheal immunization with OVA into BALB/c mice (Worthington; 60 μg OVA in 60 μl H20; OVA contained ∼700 U of LPS/μg). 3 d after immunization, mice were killed and the brLN was analyzed by flow cytometry for the proliferation of CFSE-labeled CD4+DO11.10 cells and the presence of transferred T reg cells. The proliferation index was calculated as follows: gates were set around cells of each proliferation cycle. The number of percent-gated cells in each cycle was divided by 100 and multiplied by the number of proliferated cycles. Finally, the calculated values were added up.
Adoptive transfer of T reg cells.
Lymphocytes from spleen and LN of BALB/c and CCR7−/− were enriched for CD4+ cells by depleting B220+ and CD8+ cells (Dynal). Cells from wild-type donors were labeled with DDAO (1μM for 10 min at 37°C) and those from CCR7-deficient donors with 5(6)-TAMRA. Before transfer, cells were adjusted to contain equal amounts of CD4+CD25+ cells. 0.5–1 × 106 CD4+CD25+ of each genotype were i.v. transferred. 15 h after the transfer, recipients were killed and spleen, blood, and LNs were harvested. Using mAbs specific for CD4 and CD25, the homing of the regulatory as well as naive T cells was assessed by flow cytometry.
Egress of adoptively transferred cells from BALT.
B220+ cells of Balb/C mice and CCR7−/− Balb/C mice were isolated from spleen using MACS MicoBeads following the manufacturer's instructions (Miltenyi Biotec). B220+ cells were labeled with TAMRA or DDAO as described in the previous paragraph and mixed at a ratio of 1:1 before transfer. Wild-type cells were labeled with TAMRA, CCR7−/− cells with DDAO, and vice versa. 7 × 106 cells of each genotype were transferred i.v. into CCR7−/− BALB/c recipients. 6 d after the transfer, mice were killed and the BALT was analyzed by histology for the presence of TAMRA+ and DDAO+ cells.
In vivo induction of BALT in C57BL/6 mice by application of a neutralizing anti-CD62L mAb.
300 μg anti-CD62L mAb (clone Mel-14) was given i.p. into 3-d-old wild-type B6 mice. 7 d later (day 10), mice were killed and lungs were harvested and analyzed histologically for the presence of BALT.
Acknowledgments
We would like to thank Heike Danzer and Stefanie Willenzon for excellent technical assistance and Günter Bernhardt and Oliver Pabst for valuable suggestions on the manuscript.
This work was supported by a Deutsche Forschungsgemeinschaft grant (SFB587-B3) to R. Förster.
The authors have no conflicting financial interests.
Abbreviations used: BALT, bronchus-associated lymphoid tissue; brLN, bronchial LN; HEV, high endothelial venules; LTα, lymphotoxin α; LTIC, lymphoid tissue inducing cell; PP, Peyer's patches; SPF, specific pathogen–free; VCAM, vascular cell adhesion molecule.
References
- 1.Luhrmann, A., T. Tschernig, and R. Pabst. 2002. Stimulation of bronchus-associated lymphoid tissue in rats by repeated inhalation of aerosolized lipopeptide MALP-2. Pathobiology. 70:266–269. [DOI] [PubMed] [Google Scholar]
- 2.Tirouvanziam, R., I. Khazaal, V. N'Sonde, M.A. Peyrat, A. Lim, S. de Bentzmann, J.J. Fournie, M. Bonneville, and B. Peault. 2002. Ex vivo development of functional human lymph node and bronchus-associated lymphoid tissue. Blood. 99:2483–2489. [DOI] [PubMed] [Google Scholar]
- 3.Tschernig, T., and R. Pabst. 2000. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 68:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Moyron-Quiroz, J.E., J. Rangel-Moreno, K. Kusser, L. Hartson, F. Sprague, S. Goodrich, D.L. Woodland, F.E. Lund, and T.D. Randall. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10:927–934. [DOI] [PubMed] [Google Scholar]
- 5.Toyoshima, M., K. Chida, and A. Sato. 2000. Antigen uptake and subsequent cell kinetics in bronchus-associated lymphoid tissue. Respirology. 5:141–145. [DOI] [PubMed] [Google Scholar]
- 6.Lakkis, F.G., A. Arakelov, B.T. Konieczny, and Y. Inoue. 2000. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat. Med. 6:686–688. [DOI] [PubMed] [Google Scholar]
- 7.Karrer, U., A. Althage, B. Odermatt, C.W. Roberts, S.J. Korsmeyer, S. Miyawaki, H. Hengartner, and R.M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11 −/−) mutant mice. J. Exp. Med. 185:2157–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennert, P.D., P.S. Hochman, R.A. Flavell, D.D. Chaplin, S. Jayaraman, J.L. Browning, and Y.X. Fu. 2001. Essential role of lymph nodes in contact hypersensitivity revealed in lymphotoxin-α–deficient mice. J. Exp. Med. 193:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund, F.E., S. Partida-Sanchez, B.O. Lee, K.L. Kusser, L. Hartson, R.J. Hogan, D.L. Woodland, and T.D. Randall. 2002. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 169:5236–5243. [DOI] [PubMed] [Google Scholar]
- 10.Lee, B.J., S. Santee, S. Von Gesjen, C.F. Ware, and S.R. Sarawar. 2000. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J. Virol. 74:2786–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huehn, J., K. Siegmund, J.C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G.F. Debes, J. Lauber, O. Frey, G.K. Przybylski, et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misslitz, A., O. Pabst, G. Hintzen, L. Ohl, E. Kremmer, H.T. Petrie, and R. Forster. 2004. Thymic T cell development and progenitor localization depend on CCR7. J. Exp. Med. 200:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno, T., F. Saito, D.H. Gray, S. Kuse, K. Hieshima, H. Nakano, T. Kakiuchi, M. Lipp, R.L. Boyd, and Y. Takahama. 2004. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. J. Exp. Med. 200:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debes, G.F., C.N. Arnold, A.J. Young, S. Krautwald, M. Lipp, J.B. Hay, and E.C. Butcher. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bromley, S.K., S.Y. Thomas, and A.D. Luster. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895–901. [DOI] [PubMed] [Google Scholar]
- 16.Itakura, M., A. Tokuda, H. Kimura, S. Nagai, H. Yoneyama, N. Onai, S. Ishikawa, T. Kuriyama, and K. Matsushima. 2001. Blockade of secondary lymphoid tissue chemokine exacerbates Propionibacterium acnes-induced acute lung inflammation. J. Immunol. 166:2071–2079. [DOI] [PubMed] [Google Scholar]
- 17.De Togni, P., J. Goellner, N.H. Ruddle, P.R. Streeter, A. Fick, S. Mariathasan, S.C. Smith, R. Carlson, L.P. Shornick, J. Strauss-Schoenberger, et al. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 264:703–707. [DOI] [PubMed] [Google Scholar]
- 18.Ngo, V.N., H. Korner, M.D. Gunn, K.N. Schmidt, D.S. Riminton, M.D. Cooper, J.L. Browing, J.D. Sedgwick, and J.G. Cyster. 1999. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansel, K.M., V.N. Ngo, P.L. Hyman, S.A. Luther, R. Forster, J.D. Sedgwick, J.L. Browning, M. Lipp, and J.G. Cyster. 2000. A chemokine- driven positive feedback loop organizes lymphoid follicles. Nature. 406:309–314. [DOI] [PubMed] [Google Scholar]
- 20.Futterer, A., K. Mink, A. Luz, M.H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 9:59–70. [DOI] [PubMed] [Google Scholar]
- 21.Finke, D., H. Acha-Orbea, A. Mattis, M. Lipp, and J. Kraehenbuhl. 2002. CD4+CD3− cells induce Peyer's patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 17:363–373. [DOI] [PubMed] [Google Scholar]
- 22.Fukuyama, S., T. Hiroi, Y. Yokota, P.D. Rennert, M. Yanagita, N. Kinoshita, S. Terawaki, T. Shikina, M. Yamamoto, Y. Kurono, and H. Kiyono. 2002. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 17:31–40. [DOI] [PubMed] [Google Scholar]
- 23.Mebius, R.E., P. Rennert, and I.L. Weissman. 1997. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 7:493–504. [DOI] [PubMed] [Google Scholar]
- 24.Ohl, L., G. Henning, S. Krautwald, M. Lipp, S. Hardtke, G. Bernhardt, O. Pabst, and R. Forster. 2003. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther, S.A., K.M. Ansel, and J.G. Cyster. 2003. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J. Exp. Med. 197:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan, L., C.R. Reilly, Y. Luo, M.E. Dorf, and D. Lo. 2000. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J. Immunol. 164:3955–3959. [DOI] [PubMed] [Google Scholar]
- 27.Luther, S.A., A. Bidgol, D.C. Hargreaves, A. Schmidt, Y. Xu, J. Paniyadi, M. Matloubian, and J.G. Cyster. 2002. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 169:424–433. [DOI] [PubMed] [Google Scholar]
- 28.Chen, S.C., G. Vassileva, D. Kinsley, S. Holzmann, D. Manfra, M.T. Wiekowski, N. Romani, and S.A. Lira. 2002. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J. Immunol. 168:1001–1008. [DOI] [PubMed] [Google Scholar]
- 29.Cupedo, T., W. Jansen, G. Kraal, and R.E. Mebius. 2004. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 21:655–667. [DOI] [PubMed] [Google Scholar]
- 30.Rangel-Moreno, J., L. Hartson, C. Navarro, M. Gaxiola, M. Selman, and T.D. Randall. 2006. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Invest. 116:3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valencia, X., G. Stephens, R. Goldbach-Mansky, M. Wilson, E.M. Shevach, and P.E. Lipsky. 2006. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 108:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurobe, H., C. Liu, T. Ueno, F. Saito, I. Ohigashi, N. Seach, R. Arakaki, Y. Hayashi, T. Kitagawa, M. Lipp, et al. 2006. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 24:165–177. [DOI] [PubMed] [Google Scholar]
- 33.Förster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Müller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 34.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 35.Setoguchi, R., S. Hori, T. Takahashi, and S. Sakaguchi. 2005. Homeostatic maintenance of natural Foxp3 + CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201:723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Contractor, N.V., H. Bassiri, T. Reya, A.Y. Park, D.C. Baumgart, M.A. Wasik, S.G. Emerson, and S.R. Carding. 1998. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J. Immunol. 160:385–394. [PubMed] [Google Scholar]
- 37.Barnden, M.J., J. Allison, W.R. Heath, and F.R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34–40. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh, C.S., A.B. Heimberger, J.S. Gold, A. O'Garra, and K.M. Murphy. 1992. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA. 89:6065–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabst, O., H. Herbrand, M. Friedrichsen, S. Velaga, M. Dorsch, G. Bernhardt, T. Worbs, A.J. Macpherson, and R. Forster. 2006. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J. Immunol. 177:6824–6832. [DOI] [PubMed] [Google Scholar]
- 40.Ohl, L., M. Mohaupt, N. Czeloth, G. Hintzen, Z. Kiafard, J. Zwirner, T. Blankenstein, G. Henning, and R. Forster. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 21:279–288. [DOI] [PubMed] [Google Scholar]
- 41.Lyons, A.B., and C.R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 171:131–137. [DOI] [PubMed] [Google Scholar]