Abstract
Efficient maintenance of memory CD8 T cells is central to long-term protective immunity. IL-7– and IL-15–driven homeostatic proliferation is essential for long-term memory CD8 T cell persistence after acute infections. During chronic infections, however, virus-specific CD8 T cells respond poorly to these cytokines. Yet, virus-specific CD8 T cells often persist for long periods of time during chronic infections. We have addressed this apparent paradox by examining the mechanism for maintaining virus-specific CD8 T cells during chronic infection. We find that homeostatic cytokines (e.g., IL-7/15), inflammatory signals, and priming of recent thymic emigrants are not sufficient to maintain virus-specific CD8 T cells over time during chronic infection. Rather, our results demonstrate that viral peptide is required for virus-specific CD8 T cell persistence during chronic infection. Moreover, this viral antigen-dependent maintenance results in a dramatically different type of T cell division than is normally observed during memory T cell homeostasis. Rather than undergoing slow, steady homeostatic turnover during chronic viral infection, CD8 T cells undergo extensive peptide-dependent division, yet cell numbers remain relatively stable. These results indicate that antigen-specific CD8 T cell responses during persisting infection are maintained by a mechanism distinct from that after acute infection.
A defining property of memory CD8 T cells generated after acute infection or vaccination is long-term antigen-independent persistence mediated by homeostatic turnover (1, 2). Slow, steady self-renewal of memory CD8 T cells is driven by IL-7 and IL-15 (3–7). This key memory CD8 T cell quality gradually develops as memory CD8 T cells differentiate from effector CD8 T cells in the absence of antigen (8, 9). During chronic infections, however, differentiation of antigen-specific CD8 T cells may occur differently. We and others have recently demonstrated that the expression of IL-7 and IL-15 receptors (CD127, CD122) can be suboptimal during chronic viral infections, and the ability to divide in response to IL-7 and IL-15 is compromised in vitro (10–14). In addition, when removed from chronically infected mice and adoptively transferred to naive mice, memory-phenotype, virus-specific CD8 T cells do not undergo homeostatic turnover and are not efficiently maintained (10). Thus, it is perhaps surprising that virus-specific CD8 T cell populations often persist for long periods of time during chronic viral infections.
Despite these previous studies, the pathway(s) used by antigen-experienced CD8 T cells for long-term persistence during chronic infection has not been determined. At least four mechanisms may account for the persistence of virus-specific CD8 T cells during chronic infections: (a) IL-7 and IL-15 expressed at different levels (or in a different manner) in the chronically infected hosts, (b) other cytokines/growth factors present during chronic infection, perhaps associated with persistent inflammation, (c) continual resupply of antigen-specific CD8 T cell populations from the naive T cell pool (e.g., recent thymic emigrants [RTEs]), and (d) a role for viral antigen in T cell maintenance. Determining which of these mechanisms controls virus-specific CD8 T cell persistence during chronic infections will have important implications for therapeutic manipulations designed to augment immunity to persisting pathogens.
In the current study, we have addressed this issue using the lymphocytic choriomeningitis virus (LCMV) model of chronic infection. Adoptive transfer approaches and a mutant strain of LCMV clone 13 that lacks one of the dominant CD8 T cell epitopes (Db/GP33) were used to examine the role of antigen versus environmental aspects of infection in the maintenance of virus-specific CD8 T cells in chronically infected mice. Our data indicate that antigenic peptide was a crucial signal necessary for virus-specific CD8 T cell maintenance during chronic infection. The inflammatory environment alone was not sufficient for persistence of virus-specific CD8 T cells generated during chronic infection. Further, congenic adoptive transfer approaches demonstrated that, in the presence of persisting antigen, virus-specific CD8 T cell populations were efficiently maintained without recruitment of new thymic emigrants. Finally, the mechanism for maintaining virus-specific CD8 T cells during chronic infection was via extensive antigen-dependent proliferation (>5–6 divisions in 1–3 wk) despite stable cell numbers. T cell maintenance associated with such extensive division during chronic viral infection contrasts with the slow, IL-7/IL-15–dependent homeostatic division observed for memory CD8 T cells generated after acute infection. These results define a novel mechanism of virus-specific CD8 T cell maintenance used during chronic infections.
RESULTS
CD122Lo and CD127Lo virus-specific CD8 T cells persist during chronic LCMV infection
To compare the persistence of virus-specific CD8 T cells generated after acute versus chronic infection, we used LCMV infection in mice. Infection of C57BL/6 mice with LCMV Armstrong (Arm) causes an acute infection and the virus is completely cleared within 8–10 d postinfection (p.i.) (15–17). In contrast, infection with LCMV clone 13, a variant of Arm that differs by only two amino acids and shares all T cell epitopes, causes a chronic infection with ∼2 mo of viremia (15, 16). Thereafter, clone 13 is cleared from most tissues, but persists in some reservoirs such as the kidneys for the life of the animal (15, 16). After both Arm (acute) and clone 13 (chronic) infection, Db/GP33 and Db/GP276-specific CD8 T cell populations are generated and these virus-specific CD8 T cell responses persist at relatively constant levels for months to years (Fig. 1 A and references 10, 16). After control of viremia (∼2–3 mo p.i.), LCMV-specific CD8 T cell populations from chronically infected mice were resting and phenotypically resembled memory CD8 T cells (e.g., CD44Hi, CD69Lo, CD25Lo, granzyme BLo, and CD62LLo; reference 10 and unpublished data). We and others have previously found that the stable number of Db/GP33-specific CD8 T cells in the chronically infected mice is maintained despite the low levels of CD122 and CD127 expressed by these cells in the spleen and blood (10, 12). It remained possible, however, that increased expression of these receptors outside of blood and spleen may account for T cell persistence. To test whether CD127 and CD122 expression might be elevated in other tissues, the levels of these cytokine receptors were examined on LCMV-specific CD8 T cells from spleen, PBMC, LN, BM, and liver at 2–4 mo after either Arm or clone 13 infection (Fig. 1 B). Both CD127 and CD122 levels were low in all tissues examined during clone 13 infection, whereas the expression of these cytokine receptors was high on virus-specific CD8 T cells from Arm immune mice (Fig. 1 B). Thus, after control of systemic viral replication, virus-specific memory phenotype CD8 T cells persist long term in chronically infected mice, but these cells express low levels of CD122 and CD127 in multiple tissues.
Figure 1.
Virus-specific CD8 T cells do not require IL-7 and IL-15 to persist in chronically infected hosts. (A) Longitudinal analysis of Db/GP33-specific CD8 T cells in the PBMCs after LCMV Arm or clone 13 infection. n = 3–9/time point. (B) Analysis of CD127 and CD122 expression on Db/GP276+ CD8 T cells from tissues of Arm immune (> day 30 p.i.) and clone 13 (chronic)–infected mice (∼2–4 mo p.i.). Shaded histograms represents clone 13–infected mice, and lines represent Arm immune mice. All histograms are gated on Db/GP276+ CD8 T cells. Similar results were obtained for Db/GP33+ CD8 T cells (not depicted). (C) IL-15−/− and WT mice were infected with LCMV Arm or clone 13. After 2–3 mo, the IL-15−/− mice were treated with 200 μg αIL-7Rα antibody (Ab) i.p every 2–3 d for 2 wk, and the maintenance of virus-specific CD8 T cells was compared with untreated WT mice. Left plot represents Arm-infected αIL-7Rα–treated IL-15−/− and untreated WT mice. Middle plot represents clone 13–infected αIL-7Rα–treated IL-15−/− and untreated WT mice. Right plot displays a direct comparison of the same Arm and clone 13–infected αIL-7Rα–treated IL-15−/− groups from the first two graphs. All plots show Db/GP33-specific CD8 T cell frequency in the blood as a percentage of the Db/GP33-specific CD8 T cell frequency on the first day of treatment. Graphs are representative of three independent experiments; n = 8–11/time point. *, P < 0.05 by unpaired two-tailed t test. (D) Absolute number of Db/GP33-specific CD8 T cells in the spleens of Arm immune and clone 13–infected WT untreated and IL-15−/− αIL-7Rα–treated mice at the end of treatment. Graphs are representative of two independent experiments; n = 8–10/group. *, P = 0.03 by unpaired two-tailed t test. Error bars represent standard error of the mean.
IL-7 and IL-15 are not required for virus-specific CD8 T cell maintenance during chronic LCMV infection
To test directly whether or not IL-7 and IL-15 were necessary for virus-specific CD8 T cell maintenance in vivo during chronic infection, IL-15−/− or WT mice were infected with either Arm or clone 13. 2–3 mo after infection, WT mice were left untreated, whereas IL-15−/− mice were treated with an antibody that blocks the interaction of IL-7 with the αIL-7Rα (A7R34). This approach has been used previously to block IL-7 signals in vivo including those essential for normal memory T cell homeostasis (6, 18–20). As expected, Db/GP33-specific memory CD8 T cell numbers significantly declined in the blood of Arm immune IL-15−/− mice upon initiation of IL-7Rα blockade compared to mice with intact IL-15 and IL-7 pathways (Fig. 1 C). The number of virus-specific CD8 T cells recovered from the spleens of αIL-7Rα–treated, IL-15−/− mice was also reduced compared with untreated WT mice (Fig. 1 D), consistent with previous studies (5–7). In contrast, Db/GP33-specific CD8 T cells were efficiently maintained in the blood of clone 13–infected IL-15−/− mice treated with αIL-7Rα antibody, and similar numbers of virus-specific CD8 T cells were found in the spleens of clone 13–infected WT and αIL-7Rα–treated IL-15−/− mice (Fig. 1, C and D). When compared directly, it was apparent that the loss of IL-15 and IL-7 signals had a substantially greater impact on the maintenance of virus-specific memory CD8 T cells from immune mice compared with virus-specific CD8 T cells in chronically infected mice (Fig. 1 C). Thus, unlike memory CD8 T cells that develop after acute infection, LCMV-specific CD8 T cells that persist in chronically infected mice do not appear to require IL-7 or IL-15 for efficient maintenance in vivo.
Chronic infection with a Db/GP33 variant virus
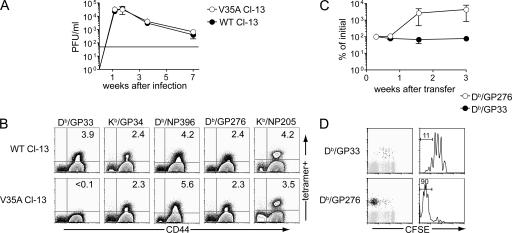
To examine the mechanism by which virus-specific CD8 T cells persist during chronic infection, we have used a strain of LCMV clone 13 with a mutation in the Db/GP33 epitope (Val→Ala at residue 35; V35A) that abolishes binding of the GP33 peptide to the Db MHC class I molecule (21). This V35A variant virus causes a chronic infection, and serum virus levels are indistinguishable between mice infected with V35A or WT clone 13 (Fig. 2 A). After infection with the V35A variant virus no Db/GP33 tetramer+ CD8 T cells were detected, whereas virus-specific CD8 T cell responses to four other epitopes were essentially unchanged compared with WT clone 13 infection (Fig. 2 B).
Figure 2.
The V35A clone 13 variant virus causes chronic infection similar to WT clone 13. C57BL/6 mice were infected with WT or the V35A variant clone 13. (A) Virus levels were determined in the blood of mice infected with WT and the V35A variant clone 13 strains. (B) Tetramer staining of splenocytes on day 8 p.i. Plots are gated on CD8 T cells; numbers in quadrants indicate percentages of CD8 T cells staining with the indicated tetramer. (C) CD8 T cells from Arm immune (> day 30 p.i.) were adoptively transferred into V35A-infected Ly5.1+ B6 mice (∼2–7 mo p.i.). Donor Ly5.2+ Db/GP276+ and Db/GP33+ CD8 T cells were monitored in the blood. Kinetics of expansion was normalized to numbers at day 5 after transfer. n = 3–4 mice per time point. Data are representative of two independent experiments. (D) CFSE profiles are shown for the donor (Ly5.2+) Db/GP33 and Db/GP276-specific CD8 T cells in V35A recipients 3 wk after transfer for the experiment in part C. Plots are gated on CD8+ T cells. Histograms are gated on tetramer+ CD8 T cells. The numbers over the gates in the histograms represent percentages of tetramer+ CD8 T cells that have fully diluted their CFSE. Data are representative of two independent experiments. Error bars represent standard error of the mean.
To determine whether the mutant V35A Db/GP33 epitope would activate memory CD8 T cells in vivo, CFSE-labeled memory CD8 T cells from Arm immune mice were adoptively transferred to mice persistently infected with V35A clone 13 after control of viremia. Db/GP33-specific memory CD8 T cells failed to expand when adoptively transferred to V35A-infected mice. Rather, the donor memory Db/GP33-specific CD8 T cells underwent homeostatic proliferation typical of antigen-independent memory CD8 T cells generated after acute infection (Fig. 2 D). (Note that slightly faster homeostatic proliferation is observed for memory CD8 T cells in V35A mice than in naive mice likely reflecting the known lymphopenia associated with chronic LCMV infection [22–24].) In contrast to the Db/GP33-specific population, donor Db/GP276-specific CD8 T cells from Arm immune mice underwent dramatic proliferative expansion in the same V35A-infected mice. These cells rapidly became CFSE negative (Fig. 2 D) consistent with a robust response of these memory CD8 T cells to the persisting antigen present in these mice.
Viral peptide is required to maintain virus-specific CD8 T cells during chronic LCMV infection.
To investigate whether viral peptide versus the chronically infected environment was required for virus-specific CD8 T cell maintenance during persisting infection, groups of congenic (Ly5.1+ or Ly5.2+) C57BL/6 mice were infected with either WT or the V35A clone 13. After control of viremia (∼2–3 mo p.i.), CD8 T cells were purified from the spleens of WT clone 13–infected Ly5.2+ mice, and these cells were adoptively transferred to congenic (Ly5.1+) mice that had been infected with either V35A or WT clone 13 (Fig. 3 A) for a similar duration as the donor mice. Db/GP33- and Db/GP276-specific donor (Ly5.2+) CD8 T cells were monitored in the blood of the recipient mice over time by tetramer staining (Fig. 3 B). This approach allowed two comparisons to be made. First, Db/GP33-specific donor CD8 T cells were enumerated in V35A versus WT clone 13–infected recipients over time. When adoptively transferred to WT clone 13–infected mice, donor Db/GP33-specific CD8 T cells were efficiently maintained for up to 10 wk (Fig. 3 C). In contrast, the donor Db/GP33-specific CD8 T cell population rapidly disappeared when adoptively transferred to chronically infected V35A recipients lacking the Db/GP33 epitope (Fig. 3 C). Second, donor Db/GP33 and Db/GP276-specific CD8 T cell populations were monitored in the same V35A-infected mice controlling for any possible variation between infected recipient groups. In this situation, despite the loss of the Db/GP33-specific population, the donor Db/GP276-specific CD8 T cells persisted efficiently in the same V35A-infected recipient mice (Fig. 3 D). The difference between the Db/GP33 and Db/GP276 populations was apparent not only in the blood but in both lymphoid and nonlymphoid tissues (Fig. 3 E). At the time of transfer, there were approximately twice as many donor Db/GP276-specific cells compared with donor Db/GP33-specific CD8 T cells (Fig. 3 B and not depicted). 5 wk after transfer nearly ten times more donor Db/GP276-specific CD8 T cells than Db/GP33-specific CD8 T cells were found in the blood (Fig. 3 B), and greater numbers of Db/GP276+ compared with Db/GP33+ CD8 T cells were also recovered from the spleen (approximately sixfold), liver (approximately fourfold), and BM (approximately fivefold) 1–2 mo after transfer (Fig. 3 E). Thus, even in the same chronically infected hosts, only those CD8 T cell specificities that could interact with cognate antigen were efficiently maintained.
Figure 3.
Virus-specific CD8 T cells from chronically infected mice do not persist without cognate antigen. (A) Schematic of experiments. Ly5.2+ C57BL/6 mice were infected with WT clone 13, and Ly5.1+ C57BL/6 were infected with either WT or V35A clone 13 on the same day. 2–3 mo later, after control of viremia, CD8 T cells were purified using magnetic beads from spleens of Ly5.2+ mice and equal numbers of Db/GP33+ CD8 T cells were transferred to Ly5.1+ WT or V35A clone 13–infected recipients. (B) Representative analysis of the Db/GP33+ and Db/GP276+ donor (Ly5.2+) CD8 T cells in the PBMCs of recipient mice. Numbers are the percentages of donor tetramer+ CD8 T cells. Plots are gated on Ly5.2+ CD8 T cells. (C) Frequency of Db/GP33+ donor cells in the blood of WT clone 13 and V35A clone 13–infected recipients over time. Difference between Db/GP33+ donor cells in WT clone 13 versus V35A clone 13 is significant by unpaired two-tailed t test at last time point (P = 0.008). Graph is representative of two independent experiments. (D) Frequency of Db/GP33+ and Db/GP276+ donor cells in the blood of V35A clone 13–infected recipients. Db/GP33+ CD8 T cells declined significantly in number over time (P < 0.05 by unpaired two-tailed t test). Difference between Db/GP33 and Db/GP276 frequency is also significant at all points after the initial bleed (P < 0.05 by unpaired two-tailed t test). Graph represents data from five independent experiments each with 2–3 mice/group. (E) Number of tetramer+ CD8 T cells in lymphoid and nonlymphoid tissues. BM represents two femurs. Graphs represent two to three independent experiments; n = 3–6/group. *, P < 0.04 by unpaired two-tailed t test. Difference between Db/GP33+ and Db/GP276+ populations in the bone marrow did not reach statistical significance (P = 0.09 by unpaired two-tailed t test). Dashed line represents limit of detection for parts C, D, and E. Error bars represent standard error of the mean.
Virus-specific CD8 T cells are maintained by extensive division during chronic infection
Normally, after acute infection or vaccination, memory CD8 T cell maintenance is accomplished by slow, steady homeostatic turnover leading to self-renewal (25). We next wanted to determine whether the LCMV-specific CD8 T cells in chronically infected mice were dividing in a similar manner. As a reference, CFSE-labeled memory CD8 T cells from LCMV Arm immune mice were adoptively transferred to congenic naive recipient mice. Over the course of 4 wk, the donor memory Db/GP33-specific CD8 T cells showed the expected slow rate of homeostatic turnover, and by week 4 after transfer, ∼50% of these cells had divided approximately one to three times (Fig. 4, A, C, and D). To examine the division profiles of virus-specific CD8 T cells during chronic infection, CD8 T cells from WT clone 13–infected mice were labeled with CFSE and adoptively transferred to congenic, infection-matched V35A-infected recipients. Division was monitored for donor virus–specific CD8 T cells with (Db/GP276) and without (Db/GP33) persisting viral antigen. Donor Db/GP33+ cells in the V35A clone 13–infected recipients did not divide, and the frequency and number of these cells declined in the blood and spleen over 4 wk (Fig. 4, A–D and Fig. 3). In contrast, donor Db/GP276-specific CD8 T cells in the same recipient mice not only divided, but did so at a rate much faster than was observed for normal homeostatic turnover of Arm immune CD8 T cells. By week 3 after transfer, a substantial population of extensively divided tetramer+ cells was present, and by week 4 after transfer, half or more of the Db/GP276-specific CD8 T cells had diluted their CFSE completely (Fig. 4, A–D). Similar division profiles were also observed in the spleen, LN, BM, and nonlymphoid tissues such as the liver (Fig. 4 B and not depicted). The difference between the slow, steady turnover of memory CD8 T cells and the extensive division of virus-specific CD8 T cells in the presence of antigen during chronic infection is summarized in Fig. 4 D, where the percentage of virus-specific CD8 T cells that were undivided, in divisions 1–5, or that had divided to CFSE negative (6+ divisions) is plotted. In ∼1 mo memory CD8 T cells from Arm immune mice either remained undivided or were found in divisions 1–5 (mainly divisions 1 and 2). In contrast, during chronic infection in the presence of persisting antigen, virus-specific CD8 T cells were either undivided or had divided to CFSE negative in the same time frame (Fig. 4, C and D). Thus, during chronic LCMV infection it appears that virus- specific CD8 T cell persistence is accompanied by extensive cell division (five or more divisions) in contrast to memory CD8 T cells that persist after acute infection.
Figure 4.
Virus-specific CD8 T cells are maintained by extensive proliferation during chronic infection. The experimental approach outlined in Fig. 3 A was used to monitor cell division history during chronic LCMV infection. (A) Proliferation patterns of donor virus-specific CD8 T cells in the blood. Left column indicates homeostatic proliferation of memory CD8 T cells from Arm immune mice (> day 30 p.i.) after adoptive transfer into naive mice. The middle column shows division of Db/GP33-specific CD8 T cells without antigen in the V35A-infected recipients, whereas the right column shows the division of the Db/GP276-specific CD8 T cell population with persisting antigen present in the same adoptive hosts. The donor CD8 T cells used for adoptive transferred were isolated from chronically infected donors 2–3 mo after infection. (B) Proliferation of WT clone 13–derived donor virus-specific CD8 T cells in the liver of V35A clone 13–infected recipients at 4 wk after transfer. (C) Histograms of donor tetramer+ CD8 T cells in the PBMCs at 4 wk after transfer. Numbers indicate the percentages of virus-specific CD8 T cells that have undergone no division (right gate), 1–5 divisions (middle gate), and 6+ divisions (left gate). All histograms are gated on Ly5.2+CD8+tetramer+ populations. (D) Graphs indicate the percentages of tetramer+ CD8 T cells that have undergone 0 divisions, 1–5 divisions, or 6+ divisions. Top two rows represents PBMCs, bottom row represents spleen, both at 4 wk after transfer. Panels are representative of two independent experiments for Arm immune and four independent experiments for chronic infection, each with n = 2–7 mice/group. A significantly greater percentage of Db/GP276+ CD8 T cells have undergone 6+ divisions in the spleen compared with Db/GP33+ CD8 T cells (P = 0.03 by unpaired two-tailed t test). The differences in the blood are also statistically significant with more Db/GP276+ CD8 T cells that have undergone 6+ divisions and fewer undivided compared with Db/GP33+ CD8 T cells (P = 0.03 and P = 0.02, respectively, by unpaired two-tailed t test). Error bars represent standard error of the mean.
DISCUSSION
During persisting infections virus-specific CD8 T cells often express low levels of IL-7 and IL-15 receptors and/or respond poorly to IL-7 and IL-15 in vitro (10–13). These previous studies indicate that virus-specific CD8 T cells generated during chronic infection may not develop the same maintenance characteristics as those memory CD8 T cells that persist after acute infection. However, virus-specific T cells after acute and chronic LCMV infection incorporate similar levels of BrdU during a 1-wk pulse suggesting ongoing cell division in vivo (26) notwithstanding the low levels of CD122 and CD127. Despite these observations, the mechanism for antigen-specific CD8 T cell maintenance during persisting infections remains poorly understood. In the present study, we have defined not only the mechanism of virus-specific CD8 T cell maintenance during chronic viral infection, but also uncovered a division pattern that was distinct from that normally observed for homeostatic turnover of memory T cells. Our studies indicated that viral antigen was necessary for long-term persistence of virus-specific CD8 T cells during chronic infection, and also that the environment of chronic infection, including any inflammatory signals or altered IL-7 and IL-15 expression, was not sufficient to maintain virus-specific CD8 T cells in chronically infected mice. It remains possible that environmental factors may play an accessory role, but they alone cannot sustain virus-specific CD8 T cells during chronic LCMV infection.
During chronic LCMV infection many virus-specific CD8 T cells had divided more than five to six times in ∼1 mo in the presence of viral antigen, yet the number of these virus-specific CD8 T cells numbers remained relatively stable. This observation suggests that either a very small subset of CFSEHi cells are recruited to divide or that the antigen-driven division of this CD8 T cell population is accompanied by extensive cell death. This pattern of division may help explain the immunodominance changes and the immune “inflation” that has been observed during several persisting infections (16, 27, 28). A difference between death and division rates during homeostatic proliferation after acute infection may lead to only a subtle change in cell numbers because the rate of division is slow. In contrast, during chronic infection, where the persisting virus-specific CD8 T cell population undergoes extensive division over the course of several weeks, a minor discrepancy between the rates of division and death could be amplified to a large change in cell numbers. Therapeutic manipulation of the rate of either recruitment into division or death may provide a novel means to modulate antigen-specific CD8 T cell responses to persisting viral antigen.
The CFSE profiles in the present study reveal populations of both extensively divided and completely undivided virus-specific CD8 T cells during persisting infection. It is unclear why some virus-specific CD8 T cells proliferated extensively during chronic infection, whereas others failed to divide. The transfer of memory CD8 T cells from Arm immune mice to chronically infected recipients results in complete division of all Db/GP276-specific donor cells (Fig. 2). This observation suggests that lack of antigen encounter is an unlikely explanation for the undivided population of virus-specific CD8 T cells from chronically infected mice. A second possibility is that only a subpopulation of the virus-specific CD8 T cells generated during chronic infection are capable of the peptide-dependent division. It will be important to determine whether heterogeneity exists in these T cell populations based on antigen-dependent maintenance potential, division history, or other factors.
A recent study suggested that ongoing thymic output can result in priming of new virus-specific CD8 T cells during persisting infections (29). In the current study, we used congenically marked donor populations and found that the number of tetramer+ CD8 T cells remains reasonably stable for ∼10 wk when antigen is present (Fig. 3). These results suggest that RTEs are not necessary to maintain a virus-specific CD8 T cell population during chronic LCMV infection. This observation is consistent with studies in thymectomized mice where virus-specific CD8 T cell populations were stable for nearly 3 mo during chronic LCMV infection (30). However, our results do not exclude a contribution from RTEs primed on persisting antigen, and this pathway may be quantitatively different depending on the nature of viral persistence (e.g., presence or absence of viral antigens in the thymus). It will also be interesting to determine if a subpopulation of recently primed CD8 T cells possesses distinct functional characteristics compared with the majority of the antigen-specific population that has been maintained since early during the initial infection.
The present study suggests that during chronic infection virus-specific CD8 T cells acquire a mechanism of maintenance distinct from naive CD8 T cells or effector and memory CD8 T cells generated after acute infection. During chronic infection virus-specific CD8 T cells respond poorly to IL-7 and IL-15 and rather appear to rely on TCR signals for persistence. However, unlike naive CD8 T cells, the TCR signal required by virus-specific CD8 T cells during chronic infection cannot be supplied by MHC and self-peptide alone. Instead, viral antigen is necessary for long-term persistence. Unlike memory CD8 T cells that undergo proliferative expansion in response to antigen, virus-specific CD8 T cells present during chronic infection undergo extensive division, but the population does not expand dramatically in number in response to low levels of persisting viral antigen (compare the Db/GP276 response in Fig. 2 C and Fig. 3 D). These observations are consistent with previous studies that found reduced proliferative potential of virus-specific T cells during chronic infection (10, 31, 32), but also now extend the implications of this proliferative difference to T cell maintenance during chronic infection. This altered responsiveness to homeostatic signals and to persisting viral antigen suggests that during chronic infections virus-specific CD8 T cells undergo a fundamentally different pattern of differentiation compared with memory CD8 T cells that develop in the absence of persisting antigen after acute infection. Thus, during chronic viral infection, antigen-specific CD8 T cells appear to be “tuned” to respond to low levels of antigen in a dramatically different manner than memory CD8 T cells generated after acute infections.
Antigen-dependent T cell maintenance by extensive division may have relevance to other infectious and noninfectious settings. Many human infections are characterized by prolonged viral replication followed by low-grade persistence. Indeed, CD8 T cells specific for EBV and CMV often express low levels of CD127 compared with influenza virus-specific memory CD8 T cells (33). In addition, HIV-specific CD8 T cells can have dramatic defects in CD127 expression (34–36), and HIV-specific CD8 T cells are poorly maintained when antigen is removed by epitope mutation or HAART (37–39). In addition to chronic viral infections, persisting bacterial and parasitic infections may also lead to the generation of T cells with altered maintenance properties (40–42). Oligoclonal expansions of memory phenotype CD8 T cells found in mice have recently been suggested to share some similarities with CD8 T cells generated during chronic viral infections (43). These CD8 T cells appear to depend on MHC molecules for their persistence in vivo rather than homeostatic cytokines (43). Whether T cells in these different scenarios divide in a homeostatic manner or rather by extensive division as described for the LCMV-specific CD8 T cells in this study remains to be determined.
In summary, the present work defines specific viral antigen, rather than IL-7 and IL-15, inflammatory cytokines, or new recruitment from RTEs, as an essential signal that governs the persistence of virus-specific CD8 T cells during chronic LCMV infection in mice. In addition, we have identified a new mechanism for this type of antigen-dependent, long-term persistence by extensive cellular division without dramatic changes in cell numbers. These observations may provide a framework to reevaluate a long-standing debate about the role of persisting antigen in the maintenance of immunological memory. Finally, the antigen-dependent maintenance mechanism described in this study may have implications for therapeutic interventions if either the rate of proliferation or cell death can be modulated in vivo to alter the size or quality of antigen-specific CD8 T cell populations during persisting infections.
MATERIALS AND METHODS
Animals and viruses.
4–6-wk-old female C57BL/6 mice were purchased from the Jackson Laboratories, and 4–6-wk-old female congenic B6-Ly5.2/Cr mice were purchased from National Cancer Institute. IL-15−/− mice were originally obtained from Michael Caligiuri (Ohio State University, Columbus, OH) (44). Mice were infected with 2 × 106 PFUs of LCMV clone 13 or the V35A clone 13 variant virus i.v. or 2 × 105 PFU of LCMV Arm i.p. as described (15, 16). The V35A variant of LCMV clone 13 was isolated after infection of C57BL/6 mice containing LCMV-specific TCR transgenic T cells (P14 cells). In brief, a small number of naive P14 splenocytes was adoptively transferred to C57BL/6 mice followed by LCMV clone 13 infection. Virus was isolated from viremic mice ∼1 mo after infection by plaque purification and sequenced through the GP33-41 encoding region. Several clones were identified with the same Val to Ala mutation at residue 35, a mutation that has been previously observed (21). Virus was grown and viral titers were determined by plaque assay as described (15, 16). The αIL-7Rα antibody was purified by the hybridoma core at the Wistar Institute from the supernatant of the A7R34 hybridoma and used as described (20). All mice were used in accordance with Institutional Animal Care and Use Committee procedures.
Lymphocyte isolation and flow cytometry.
Lymphocyte isolation from lymphoid and nonlymphoid tissues and surface and intracellular stains were performed as previously described (10, 16). All antibodies were purchased from BD Biosciences except for CD127 (eBioscience), Ly5.2 (eBioscience), and granzyme B (Caltag). MHC class I peptide tetramers were made and used as described previously (10, 16).
CFSE labeling and adoptive transfers.
After WT clone 13 or V35A clone 13–infected mice were no longer viremic (2–3 mo after infection), spleens were harvested from WT clone 13 donor mice. CD8 T cells were purified from splenocytes using magnetic beads (MACS beads; Miltenyi Biotec). In brief, splenocytes were labeled with magnetic beads specific for CD8α and then run through a MACS LS separation column (Miltenyi Biotec) according to the manufacturer's protocol. The purified CD8 T cells were then labeled with CFSE as described previously (10, 16). Purified CD8 T cells were adoptively transferred i.v. to each recipient mouse. Between 2–2.5 × 105 Db/GP33-specific CD8 T cells were transferred in the experiments shown. In each individual experiment identical numbers of Db/GP33 tetramer+ CD8 T cells were adoptively transferred to each separate recipient mouse. Donor populations were monitored in the peripheral blood by retroorbital blood collection as described previously (10).
Acknowledgments
We acknowledge Antonio Polley for excellent technical assistance. We also thank Steven L. Reiner, Dave Masopust, and Mike Betts for helpful discussion and for critically reading the manuscript, and Vaiva Vezys and Aron Lukacher for sharing unpublished data.
This work was supported by National Institutes of Health (grant AI071309 to E.J. Wherry), the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health (E.J. Wherry), and the Wistar Institute.
The authors have no conflicting financial interests.
Abbreviations used: Arm, Armstrong; LCMV, lymphocytic choriomeningitis virus; p.i., postinfection; RTE, recent thymic emigrant.
References
- 1.Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. [DOI] [PubMed] [Google Scholar]
- 2.Wherry, E.J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schluns, K.S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269–279. [DOI] [PubMed] [Google Scholar]
- 4.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 5.Becker, T.C., E.J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldrath, A.W., P.V. Sivakumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluns, K.S., K. Williams, A. Ma, X.X. Zheng, and L. Lefrancois. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831. [DOI] [PubMed] [Google Scholar]
- 8.Kaech, S.M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 111:837–851. [DOI] [PubMed] [Google Scholar]
- 9.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 10.Wherry, E.J., D.L. Barber, S.M. Kaech, J.N. Blattman, and R. Ahmed. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 101:16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang, K.S., M. Recher, A.A. Navarini, N.L. Harris, M. Lohning, T. Junt, H.C. Probst, H. Hengartner, and R.M. Zinkernagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738–745. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, M.J., D.A. Hildeman, S. Sabbaj, D.E. Gaddis, A.E. Tebo, L. Shang, P.A. Goepfert, and A.J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 174:5926–5930. [DOI] [PubMed] [Google Scholar]
- 13.Obar, J.J., S.G. Crist, E.K. Leung, and E.J. Usherwood. 2004. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J. Immunol. 173:2705–2714. [DOI] [PubMed] [Google Scholar]
- 14.Obar, J.J., S. Fuse, E.K. Leung, S.C. Bellfy, and E.J. Usherwood. 2006. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 80:8303–8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed, R., A. Salmi, L.D. Butler, J.M. Chiller, and M.B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau, L.L., B.D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature. 369:648–652. [DOI] [PubMed] [Google Scholar]
- 18.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, H. Yoshida, and S. Nishikawa. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondrack, R.M., J. Harbertson, J.T. Tan, M.E. McBreen, C.D. Surh, and L.M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaech, S.M., J.T. Tan, E.J. Wherry, B.T. Konieczny, C.D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. [DOI] [PubMed] [Google Scholar]
- 21.Puglielli, M.T., A.J. Zajac, R.G. van der Most, J.L. Dzuris, A. Sette, J.D. Altman, and R. Ahmed. 2001. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb. J. Virol. 75:5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broomhall, K.S., M. Morin, D.C. Pevear, and C.J. Pfau. 1987. Severe and transient pancytopenia associated with a chronic arenavirus infection. J. Exp. Pathol. 3:259–269. [PubMed] [Google Scholar]
- 23.Bro-Jorgensen, K., and M. Volkert. 1974. Defects in the immune system of mice infected with lymphocytic choriomeningitis virus. Infect. Immun. 9:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binder, D., J. Fehr, H. Hengartner, and R.M. Zinkernagel. 1997. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J. Exp. Med. 185:517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surh, C.D., O. Boyman, J.F. Purton, and J. Sprent. 2006. Homeostasis of memory T cells. Immunol. Rev. 211:154–163. [DOI] [PubMed] [Google Scholar]
- 26.Zajac, A.J., J.N. Blattman, K. Murali-Krishna, D.J. Sourdive, M. Suresh, J.D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Most, R.G., K. Murali-Krishna, J.G. Lanier, E.J. Wherry, M.T. Puglielli, J.N. Blattman, A. Sette, and R. Ahmed. 2003. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology. 315:93–102. [DOI] [PubMed] [Google Scholar]
- 28.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U.H. Koszinowski, R.E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022–2029. [DOI] [PubMed] [Google Scholar]
- 29.Vezys, V., D. Masopust, C. Kemball, D.L. Barber, L.A. O'Mara, C.P. Larsen, T.C. Pearson, R. Ahmed, and A.E. Lukacher. 2006. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 203:2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, N.E., J.R. Bonczyk, Y. Nakayama, and M. Suresh. 2005. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J. Virol. 79:9419–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migueles, S.A., A.C. Laborico, W.L. Shupert, M.S. Sabbaghian, R. Rabin, C.W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 32.Wherry, E.J., J.N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Leeuwen, E.M., G.J. de Bree, E.B. Remmerswaal, S.L. Yong, K. Tesselaar, I.J. ten Berge, and R.A. van Lier. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 106:2091–2098. [DOI] [PubMed] [Google Scholar]
- 34.Wherry, E.J., C.L. Day, R. Draenert, J.D. Miller, P. Kiepiela, T. Woodberry, C. Brander, M. Addo, P. Klenerman, R. Ahmed, and B.D. Walker. 2006. HIV-specific CD8 T cells express low levels of IL-7Ralpha: Implications for HIV-specific T cell memory. Virology. 53:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, et al. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900–2909. [DOI] [PubMed] [Google Scholar]
- 36.Boutboul, F., D. Puthier, V. Appay, O. Pelle, H. Ait-Mohand, B. Combadiere, G. Carcelain, C. Katlama, S.L. Rowland-Jones, P. Debre, et al. 2005. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 19:1981–1986. [DOI] [PubMed] [Google Scholar]
- 37.Alter, G., G. Hatzakis, C.M. Tsoukas, K. Pelley, D. Rouleau, R. LeBlanc, J.G. Baril, H. Dion, E. Lefebvre, R. Thomas, et al. 2003. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 171:477–488. [DOI] [PubMed] [Google Scholar]
- 38.Jamieson, B.D., O.O. Yang, L. Hultin, M.A. Hausner, P. Hultin, J. Matud, K. Kunstman, S. Killian, J. Altman, K. Kommander, et al. 2003. Epitope escape mutation and decay of human immunodeficiency virus type 1-specific CTL responses. J. Immunol. 171:5372–5379. [DOI] [PubMed] [Google Scholar]
- 39.Casazza, J.P., M.R. Betts, L.J. Picker, and R.A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaph, C., J. Uzonna, S.M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 10:1104–1110. [DOI] [PubMed] [Google Scholar]
- 41.Dudani, R., Y. Chapdelaine, H. Faassen Hv, D.K. Smith, H. Shen, L. Krishnan, and S. Sad. 2002. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J. Immunol. 168:5737–5745. [DOI] [PubMed] [Google Scholar]
- 42.Zaph, C., K.A. Rook, M. Goldschmidt, M. Mohrs, P. Scott, and D. Artis. 2006. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J. Immunol. 177:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyman, O., J.H. Cho, J.T. Tan, C.D. Surh, and J. Sprent. 2006. A major histocompatibility complex class I–dependent subset of memory phenotype CD8+ cells. J. Exp. Med. 203:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fehniger, T.A., K. Suzuki, A. Ponnappan, J.B. VanDeusen, M.A. Cooper, S.M. Florea, A.G. Freud, M.L. Robinson, J. Durbin, and M.A. Caligiuri. 2001. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]