Abstract
Intrarectal infection between men who have sex with men represents a predominant form of human immunodeficiency virus (HIV) transmission in developed countries. Currently there are no adequate small animal models that recapitulate intrarectal HIV transmission. Here we demonstrate that human lymphocytes generated in situ from hematopoietic stem cells reconstitute the gastrointestinal tract of humanized mice with human CD4+ T cells rendering them susceptible to intrarectal HIV transmission. HIV infection after a single intrarectal inoculation results in systemic infection with depletion of CD4+ T cells in gut-associated lymphoid tissue and other pathologic sequela that closely mimics those observed in HIV infected humans. This novel model provides the basis for the development and evaluation of novel approaches aimed at immune reconstitution of human gut-associated lymphoid tissue and for the development, testing, and implementation of microbicides to prevent intrarectal HIV-1 transmission.
Intrarectal infection by HIV, the causative agent of AIDS, is an important mode of HIV transmission currently accounting for over 400,000 cases in the United States. Presently, rhesus macaques (1) inoculated intrarectally with simian immunodeficiency virus (SIV) or SIV/HIV chimeric viruses (SHIV) are the principal surrogate animal model to study intrarectal HIV infection. There are no small animal models for this mode of HIV transmission or to study infection in the gastrointestinal (GI) tract, which in the early stages of HIV infection of humans, is a major site of virus replication. Subsequent depletion of CD4+ T cells in gut-associated lymphoid tissue (GALT) is a defining event in the course and outcome of disease (2, 3). With the aim of elucidating the molecular determinants of HIV transmission via the rectum and to establish a small animal model in which the efficacy of early drug prophylaxis, microbicides, and vaccines could be tested, we determined the susceptibility of bone marrow/liver/thymus (BLT) humanized mice (4) to intrarectal infection with HIV-1. In BLT mice, the bone marrow is reconstituted with human hematopoietic stem cells producing all human lymphoid lineages resulting in systemic repopulation. In addition, bone marrow–derived T cell progenitors are produced that repopulate an implanted human organoid consisting of a piece of autologous fetal liver and thymus resulting in human MHC-restricted functional T cells (4).
In this report, we demonstrate the in vivo repopulation of the mouse GALT with human hematopoietic cells derived from transplanted human stem cells. Based on this observation, we also provide the first demonstration of the exquisite susceptibility of BLT mice to intrarectal infection by cell-free HIV-1 that results in systemic infection including plasma viremia, systemic CD4+ T cell depletion, and the production of HIV-specific human antibodies. Intrarectal infection of BLT mice represents a significant advance that will facilitate the implementation of microbicides and prophylactics to prevent HIV transmission.
RESULTS AND DISCUSSION
Reconstitution of the BLT GALT with human lymphocytes
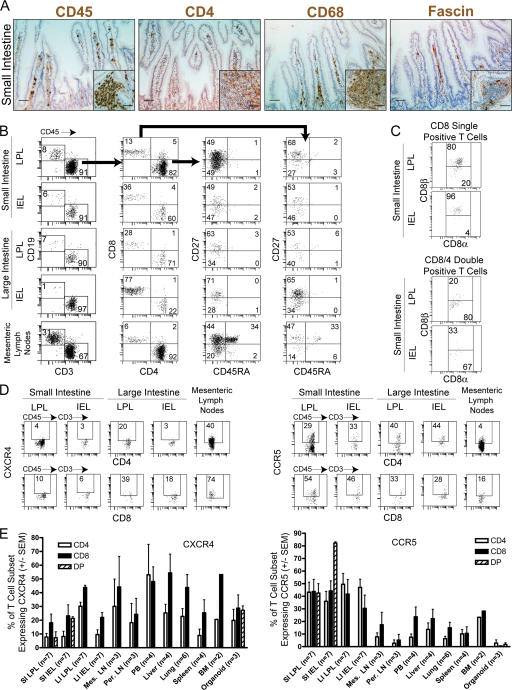
NOD/SCID mice are devoid of endogenous B and T cells, and therefore, the mouse component to mucosal immunity is limited to myeloid cells. To determine if human lymphoid cells encounter adequate signals to migrate into and repopulate the mouse GI tract, we first harvested the small intestine of humanized BLT mice (n = 7) and determined the presence of human hematopoieitic cells ∼24 wk after transplant. Histological analysis indicated an abundant accumulation of CD45+ hematopoietic cells that included human CD4+ cells, monocyte/macrophages, and DCs (Fig. 1 A and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062411/DC1). We then characterized the human hematopoietic cells in different parts of the GI tract from humanized BLT mice by isolating lamina propria lymphocytes (LPLs) and intraepithelial lymphocytes (IELs) from large and small intestine. In all cases, the gating strategy for flow cytometric analysis first identified human lymphoid cells based on their expression of the human leukocyte common marker CD45 and then by the appropriate lineage marker (i.e., CD3 or CD19) followed by the specific subset (i.e., CD4 and/or CD8). All portions of the mouse GALT were reconstituted with human hematopoietic cells, albeit at different levels (Fig. 1 B). Human CD19+ B and CD3+ T cells were identified in all fractions with single positive CD4 and CD8 human T cells representing the majority of human lymphoid cells in all portions of the reconstituted GALT. Substantial numbers of intraepithelial (IE) and lamina propria (LP) CD4+CD8+ double positive human T cells were found in the small intestine (Fig. 1 B). The CD8 molecule expressed on human double positive T cells present in the LP was predominantly the CD8α chain (Fig. 1 C). In contrast, the CD8 single positive cells in the LP expressed both the CD8α and CD8β chains (Fig. 1 C). These observations are fully consistent with what has been described for human gut (5, 6). Also consistent with what has been found in human gut, DCs represent a small proportion of the human lymphoid cells present, and lineage negative HLA-DRbright CD11c+ DC were most prominent in the small intestine IEL and LPL fractions (Fig. S1) (7). Phenotypic characterization of the T cells present in the different GALT fractions indicated that the majority of both CD4+ and CD8+ human T cells were CD27+CD45RA− memory cells. Because the mesenteric LNs are the secondary lymphatic tissues that drain the GI tract and play a major role in virus dissemination after intrarectal HIV infection, we determined that these important lymphoid tissues were also reconstituted with human T and B cells. In contrast to LPL and IEL, mesenteric LN contained a clear population of naive (CD45RA+CD27+) T cells that were absent from the GALT.
Figure 1.
Reconstitution of the GI tract of humanized BLT mice. (A) Immunohistochemical analysis of the small intestine of a BLT mouse for the presence of human hematopoietic cells. Histological analysis was performed on fixed/paraffin-embedded tissue sections for human CD45 (pan lymphocyte marker), CD4 (T cells, monocytes, and macrophages), CD68 (monocytes and macrophages), and fascin (dendritic cells) as previously described (4). Panels are small intestine lamina propria (effector site), and inserts are follicular lymphoid aggregates (inductive site). Bars, 50 μm. Sections stained with isotype-matched control antibodies did not show staining in these tissues (not depicted). In addition, staining for human CD11c and HLA-DR of a BLT mouse together with staining of a nonhumanized mouse with the same primary and secondary antibodies as an additional control are shown in Fig. S1. (B) Reconstitution of the GI tract from humanized mice. The entire GI tract was harvested ∼24 wk after transplant and separated into mesenteric LNs and large and small intestine. IELs and LPLs were isolated from both large and small intestines. Human hematopoietic cells were identified with anti–human CD45 antibodies and characterized for expression of lineage and differentiation specific markers as indicated in the different panels. Human T cells in this analysis were sequentially gated as CD45+→CD3+ or CD19+. CD45+CD3+ cells were analyzed for CD4+ or CD8+ expression (indicated with longer arrow between first and second panels). CD4+ or CD8+ cells were further analyzed for CD27 and CD45RA expression (indicated by the shorter arrow between the second and third row of panels for CD4+ cells and a large arrow between the second and fourth row of panels for CD8+ cells). In all cases individual gates were set using isotype-matched controls (not depicted). (C) Small intestine IEL and LPL CD8+ and CD4+CD8+ cells were further analyzed for their differential expression of CD8α and CD8β chains using the same gating strategy as for B. D shows a comparison of the levels of HIV coreceptor expression in human CD4+ and CD8+ T cells isolated from the large and small intestine and the mesenteric LNs of BLT mice. CD45+CD3+CD4+ or CD45+CD3+CD8+ cells from a representative mouse were analyzed for expression of CXCR4 or CCR5. (E) Distribution of CCR5 and CXCR4 coreceptor expression in human T cell subsets in gut, lymphoid, and nonlymphoid tissues of humanized BLT mice. Shown are averages of the indicated replicates with their respective error bars (±SEM). Gating was performed in the same manner as indicated in D. DP, double positive.
Differential expression of CCR5 and CXCR4 in the BLT GALT
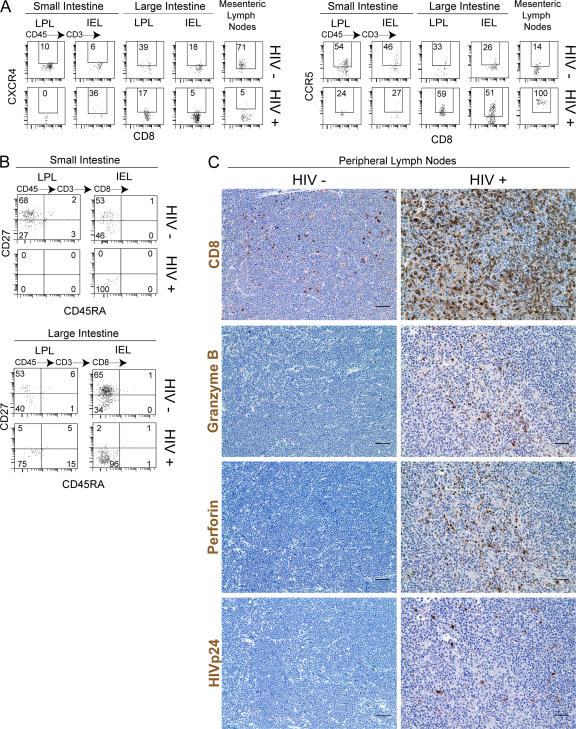
Coreceptor expression in human CD4+ cells is the major determinant of HIV tropism in vivo (8). Analysis of both CD4+ and CD8+ T cells present in the GALT of BLT mice indicated differential expression of CXCR4 and CCR5. The proportion of cells expressing CXCR4 and their respective expression levels were generally low in all fractions of the GALT except the mesenteric LN (Fig. 1 D for one representative BLT mouse and Fig. 1 E for the average [±SEM] for seven BLT mice). In contrast, the levels of expression and the proportion of cells expressing CCR5 were significantly higher in all fractions of the GALT (Fig. 1, D and E). Analysis of CCR5 expression on T cells in the gut with those from other lymphoid and nonlymphoid tissues demonstrated that the highest proportion of CD4+ CCR5+ cells were found in the GALT (Fig. 1 E). These results are consistent with what has been previously observed in human and macaque GALT (9–11) and extend our understanding to other organs like the liver and spleen. Collectively, these data establish the reconstitution of the GALT of humanized BLT mice with all of the human hematopoietic cells relevant to mucosal HIV transmission. Furthermore, phenotypic analysis of GI tract and mesenteric LN demonstrates that they are reconstituted in a manner that closely resembles that of normal human and macaque tissues.
Intrarectal infection of BLT mice with HIV-1
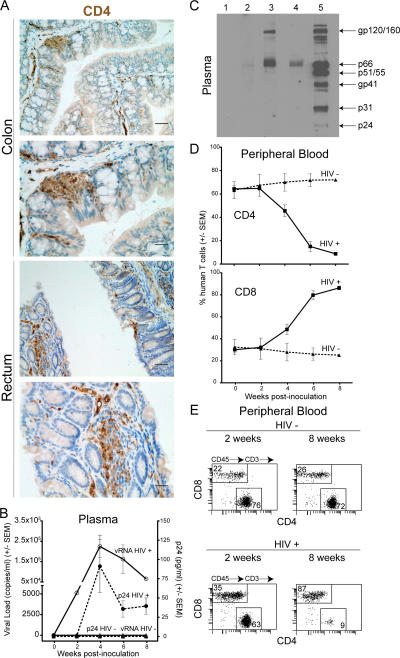
Because the anus, rectum, and colon are the most likely anatomical sites of initial infection after intrarectal exposure, we determined that these regions of the BLT mouse intestinal tract were repopulated with human CD4+ T cells (Fig. 2 A). CD4+ cells were found in abundant clusters within the follicular aggregates and throughout the lamina propria of both colon and rectum. Because we found that humanized BLT mice had human CD4+ T cells throughout their GALT, we were encouraged to determine the susceptibility of the BLT mice to infection by HIV-1 administered intrarectally. Both nonhumanized control (n = 4) and humanized BLT mice (repopulated with human cells, n = 7) were inoculated intrarectally with a single dose of cell-free HIV-1 (LAI CXCR4 strain, 200 ng p24 in PBS) and subsequently monitored for evidence of infection by assays for viral load and HIV antigenemia in the plasma. None of the HIV inoculated nonhumanized control mice showed any evidence of infection either in the plasma or in the tissues upon necropsy (not depicted). Similarly, none of the uninfected humanized BLT control mice showed evidence of infection (n = 4). In contrast, viral RNA and HIV antigenemia (capsid p24) were evident in the plasma of six out of seven HIV-inoculated BLT mice (Fig. 2 B). Furthermore, human IgG anti-HIV–specific antibodies were found in the plasma of three out of four infected BLT mice tested (Fig. 2 C).
Figure 2.
Intrarectal HIV infection of humanized BLT mice. (A) Immunohistochemical analysis of the colon and rectum of humanized BLT mice for the presence of human lymphocytes expressing CD4. Histological analysis was performed on fixed/paraffin-embedded tissue sections as previously described (4). Shown are two magnifications of each tissue (top and bottom) where bars indicate 25 μm and 12.5 μm, respectively. Sections stained with isotype-matched control antibodies did not show staining in these tissues and no cross-reactivity was observed with NOD/SCID mice tissue stained with anti–human CD4-specific antibodies (not depicted and Fig. S1). (B) Peripheral blood from intrarectally infected BLT (filled circles) or mock-infected control BLT mice (triangles) was analyzed for RNA viral load (copies per ml plasma, open symbols) and virus antigenemia (pg of p24 per ml plasma, filled symbols). (C) Western blot analysis of plasma from infected mice for the presence of human IgG antibodies specific for HIV proteins. Lane 1, plasma from a control mouse; lanes 2–4, plasma samples from three HIV-infected mice; lane 5, plasma from an HIV-infected individual. The position of the different HIV proteins in the gel is indicated on the right. (D) Specific depletion of human CD4+ cells from the peripheral blood of HIV-infected (squares) or control (triangles) humanized BLT mice. (E) Human T cell flow cytometry profile of the peripheral blood from a representative infected humanized BLT mouse 2 and 8 wk after intrarectal inoculation with cell-free HIV showing the depletion of CD4+ human T cells.
The appearance of viral RNA in the plasma of infected BLT mice (Fig. 2 B) preceded or coincided with a decline in peripheral blood human CD4+ T cells (Fig. 2 D). This decline eventually resulted in an almost complete depletion of human CD4+ T cells from peripheral blood (Fig. 2, D and E). Parallel to the decline of CD4+ T cells, there was an increase in the percentage of human CD8+ T cells that by 8 wk after inoculation represented the vast majority of the human T cells in the periphery of infected humanized BLT mice (Fig. 2 D). These results demonstrate the striking susceptibility of BLT mice to infection by HIV administered intrarectally.
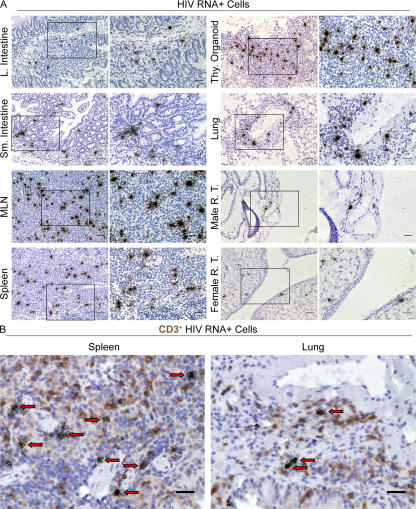
Peripheral blood serves as the most accessible site to monitor HIV replication, but the lymphatic tissues are the principal sites of virus production, persistence, CD4+ T cell depletion, and pathology. We therefore harvested the different internal organs from infected mice 8 wk after inoculation and investigated the disseminated systemic infection of lymphatic tissues after intrarectal challenge. We found productively infected (HIV RNA+) cells in LNs, spleen, and human thymic tissue (Fig. 3 A). In addition, we also documented the presence of productively infected cells in lung, large and small intestine, and the male and female reproductive tracts (Fig. 3 A). Consistent with what has been reported in humans and SIV-infected rhesus macaques, we found that most productively infected HIV RNA+ cells in humanized BLT mice were in fact human T cells (Fig. 3 B). Furthermore, replication-competent HIV was recovered from cells isolated from tissues obtained from infected mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20062411/DC1). These results highlight the disseminated infection that results after intrarectal exposure and the striking parallels between BLT mouse and human HIV infection in the most relevant tissue compartments.
Figure 3.
Disseminated HIV infection of humanized BLT mice. (A) In situ hybridization analysis of different tissues from infected humanized BLT mice for the presence of cells productively infected with HIV. Autoradiograph images were obtained using brightfield microscopy. Boxes indicate areas shown at higher magnification on the right where bars indicate 25 μm and 12.5 μm, respectively. (B) In situ hybridization of HIV-infected cells and immunocytochemical analysis for human CD3 expression in spleen and lung of humanized BLT mice. Arrows indicate productively infected CD3+ human T cells. Bars, 12.5 μm. Productive infection with replication competent virus was confirmed by rescue of infectious virus from spleen, human thymic tissue, and bone marrow of infected humanized BLT mice by coculture with activated human PBMCs (Fig. S2).
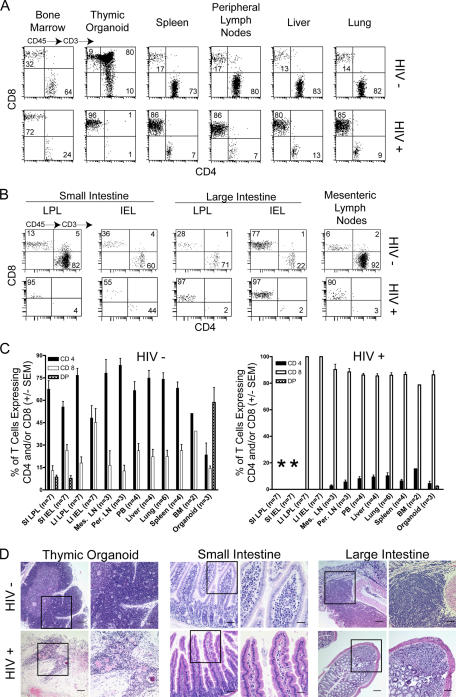
Systemic depletion of human CD4+ T cells in HIV-1–infected BLT mice
We documented a dramatic depletion of human CD4+ T cells in bone marrow, human thymic tissue, spleen, and LNs of infected BLT mice (Fig. 4 A–C). In addition, there was extensive depletion of human CD4+ T cells from lung and liver (Fig. 4, A and C). Furthermore, compared with uninfected BLT mice, there was a dramatic reduction in the levels of human CD4+ T cells in the large and small intestine and in the mesenteric LN of HIV-infected mice (Fig. 4 B). Histological examination of the human thymic tissue and mouse GI tract of infected animals revealed striking pathology (Fig. 4 D and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20062411/DC1), most likely caused by loss of thymocytes and stromal collapse. The histological images show the dramatic hypocellularity within the large and small intestine that is consistent with our flow cytometry analysis showing a dramatic loss of CD4+ T cells within this compartment (Fig. 4 B) (3, 12).
Figure 4.
Depletion of human CD4+ T cells from the GALT of HIV-infected humanized BLT mice. (A) Flow cytometry analysis of human T cells isolated from the indicated organs from a control and an HIV-infected humanized BLT mouse. Tissue samples were collected 8 wk after inoculation, and mononuclear cells were obtained for flow cytometry analysis as indicated in Materials and methods. The gating strategy was human CD45+→CD3+ and then CD4 and CD8. (B) Depletion of human CD4+ T cells from the small and large intestine and mesenteric LN of an HIV-infected BLT mouse. (C) Comparison of the percentages of human CD4+ T cells between control and HIV-infected BLT mice. Bars represent averages of the indicated number of mice (±SEM). Asterisks in C serve to indicate the virtual absence of human cells in the small intestine after HIV infection. (D) Comparison of thymic and gut tissues of control and HIV-infected BLT mice showing the considerable damage inflicted by the viral infection. Boxes indicate areas of the image shown at higher magnification on the right where bars indicate 50 μm and 25 μm, respectively. Larger images showing greater detail for thymic organoid and large intestine are included in Fig. S3. DP, double positive.
Because depletion of CD4+ T cells from the GALT is a major consequence of HIV infection, we characterized the human lymphoid cells present in both small and large intestine after infection. Compared with uninfected BLT mice, there was a dramatic reduction in the levels of human CD4+ T cells in the large and small intestine and in the mesenteric LN of HIV-infected mice (Fig. 4 B). Decrease in CD4+ T cells coincided with reciprocal increases in the percentage of CD8+ T cells. Surprisingly, very few human CD8+ T cells were found in the small intestine LPL and IEL fractions of HIV-infected mice (Fig. 4, B and C). The proportion of CD8+ T cells expressing CXCR4 in mesenteric LN and large intestine significantly decreased, whereas the levels of CD8+CCR5+ human T cells increased after HIV infection compared with uninfected BLT mice (Fig. 5 A). Importantly, the most dramatic change in coreceptor expression was noted in the mesenteric LNs, which contained very high levels of HIV-infected cells (Fig. 3 A) and where virtually all CD8+ human T cells expressed high levels of CCR5 (Fig. 5 A). Consistent with what is seen in human infections (3), the human T cells remaining in the BLT mouse GI tract after infection (mostly CD8+; Fig. 4 B) had an effector memory phenotype (CD27− CD45RA−) (Fig. 5 B) (13). Finally, we characterized the frequency of CD8+ cells present in the lymph node before and after HIV infection for further evidence of a general effector T cell response. Consistent with their role as a major site of ongoing viral replication and a principle immune inductive site, we demonstrated a dramatic infiltration of human CD8+, granzyme+, and perforin+ cells within the LNs of HIV-infected BLT mice (Fig. 5 C).
Figure 5.
Phenotypic analysis of human CD8+ T cells before and after HIV infection. (A) Coreceptor expression in human CD8+ T cells after HIV infection. Cells were isolated from gut tissue 8 wk after infection and from a noninfected control BLT mouse. Cells were then analyzed for CXCR4 and CCR5 expression. The gating strategy was CD45+CD3+CD8+ and then CXCR4 or CCR5. (B) Analysis of CD27 and CD45RA expression in human CD8+ T cells from an infected humanized BLT mouse. The gating strategy was the same as in A but instead of coreceptor expression, CD27 and CD45RA expression were analyzed. (C) Analysis of the effector T cell response to HIV in LNs. Shown are images of lymph node tissue from a control (not infected) BLT mouse and an HIV-infected BLT mouse stained for CD8, granzyme B, perforin, and HIVp24. Note the dramatic infiltration by human CD8+ cells and the increase in the numbers of cells expressing granzyme B and perforin in the tissue from the infected animal. Bars, 25 μm.
Lymphocyte migration into effector tissues like the gut is the result of a series of poorly understood but highly complex interactions between cell adhesion molecules, integrins, chemokines, and chemokine receptors. Our results demonstrate the high degree of compatibility between the mouse and human systems that results in the appropriate repopulation of the mouse gut tissue with human lymphoid cells. For the most part, the reconstituted BLT gut clearly resembles human gut descriptions in the literature (5, 6). Perhaps the most telling features were the presence of human CD4CD8αα cells, a T cell subset known only to exist in GALT, and the presence of abundant Peyer's patches in the small intestine and lymphoid follicular aggregates in the large intestine localized with human lymphocytes (T and B), macrophages, and DCs. In addition, human lymphocytes (T and B), macrophages, and DCs were found distributed throughout the effector lamina propria in humanized BLT mice suggesting that these mice have largely “normal human” GALT. However, some differences were also noted. For example, there was heterogeneity in the repopulation of human cells within the lamina propria of each humanized BLT mice, with some portions of the gut demonstrating lower repopulation. In addition, there were somewhat higher percentages of CD4+ T cells in BLT small intestine IELs compared with normal human gut, and no γδT cells were identified. Despite these differences and based on the remarkable similarities observed between the GALT of BLT mice and humans, we tested the susceptibility of humanized BLT mice to mucosal HIV transmission. A clear understanding of the molecular basis of mucosal HIV transmission has been hindered to a significant extent by the lack of adequate systems that recapitulate the events taking place during human infection. Our results show remarkable similarities between intrarectally HIV-infected humanized mice, HIV-infected humans, and SIV-infected macaques including the devastating effects to the GALT. One notable difference was a delay in peak viremia compared with infected macaques. The 2-wk delay in peak of viremia is probably because of the chimeric nature of the model, with a lower density of viral targets in humanized BLT mice compared with macaques, which also could explain the relatively lower magnitude of viremia compared with infected macaques. Therefore, from the context of HIV transmissibility, our observations represent a remarkable finding. The fact that either very low levels of viral replication takes place in regions of low target cell densities or the preservation of virus infectivity until localized into tissues with suitable numbers of target cells implies that this virus has incredible resilience that has not been fully appreciated before.
Because of the limited species tropism of HIV, there are few models where potential clinical interventions can be evaluated and where pathogenesis can be studied. Mice represent an outstanding model to study a variety of aspects related to numerous virus infections. However, mice are refractory to HIV infection and cannot support HIV replication (14–16). Thus, the susceptibility of humanized BLT mice to one intrarectal HIV exposure points to the great potential of this system to study mucosal HIV transmission and prevention. In addition, the CD4+ T cell depletion and disease-defining pathology observed in our system demonstrate the utility of this small animal model to address key questions regarding HIV-induced pathogenesis. Furthermore, the AIDS-like pathology and depletion of the BLT GALT after HIV infection highlights its usefulness to evaluate therapeutic interventions aimed at GALT reconstitution that might result in the possible reestablishment of a functional immune system in humans. Therefore, the BLT humanized mouse model has great potential to evaluate novel approaches for the prevention and treatment of HIV infection, from preclinical evaluation of microbicides to preexposure prophylactic regimens aimed at preventing the spread of AIDS.
MATERIALS AND METHODS
Preparation of humanized BLT mice and isolation of tissue lymphocytes for flow cytometry analysis.
Humanized mice were prepared essentially as we have previously described (4) and maintained under specific pathogen-free conditions at the University of Texas Southwestern Medical Center, at Dallas under protocols approved by the Animal Research Committee. In brief, NOD/SCID-hu mice (17) were transplanted with autologous human fetal liver CD34+ cells 3 wk after implantation and monitored for human reconstitution in peripheral blood by flow cytometry every 2–4 wk starting 6 wk after transplant as we have previously described (4). Peripheral blood analysis by flowcytometry was performed using anti–human CD45 pan-leukocyte antibodies to identify human hematopoietic cells. Using this gate, all other human lineages and subsets were analyzed. Animals were anesthetized with sodium pentobarbital and then killed via cardiac puncture and exsanguinations ∼24 wk after transplant. Tissues for immunohistochemical analysis and for in situ hybridization analysis were fixed immediately upon harvest and shipped to Dr. A.T. Haase in Minnesota for processing and analysis within 48 h of harvest. Immunohistochemical stainings and in situ hybridization analysis were performed essentially as previously described (4, 12, 18). For controls, tissues were stained with isotype match nonspecific antibodies. The specificity of each stain was also confirmed by staining tissue from NOD/SCID control animals with the same antibodies to human antigens. Tissue and blood samples were processed immediately for cell isolation and flow cytometry analysis. The gating strategy for each subset of cells included first human CD45 to identify human cells and to exclude mouse cells from the analysis. Human cells were then analyzed for the specific human leukocyte antigens indicated in Figs. 1, 2, 4, 5, and S1. Flow cytometry data were collected on the same day of harvest using a FACSCanto instrument with Diva software. Isolation of cells from the gut took ∼5 h. In brief, single cell suspensions for analysis were prepared essentially as we have previously described (4) except for GALT. GALT cells were prepared as previously described (19, 20) with modifications. Small and large intestine were flushed with ice cold PBS, cut into ∼1-inch segments, and inverted to expose the intraepithelial surface. These segments were washed five times by vigorous shaking in cold PBS, allowed to settle by gravity, then resuspended in 15–25 ml IEL extraction medium (freshly made; 3% FBS, 1 mM DTT, 1 mM EDTA in PBS). These mixtures were shaken gently for 30 min on a rotating platform at 37°C, then vigorously for 2 min at RT. The IEL supernatants were collected through a 70-μm cell strainer (Falcon). The intestinal segments were thoroughly washed and incubated for 60 min on a rotating platform at 37°C in 15–25 ml LPL digestion medium (freshly made; 60 μg/ml collagenase D and 10 U/ml DNaseI in RPMI-1640). During this incubation, the IEL supernatants were passed over IEL extraction medium–equilibrated columns of 0.5 g of dimethyldichlorosilane-treated glass wool fiber (Fisher) in 10-ml syringes. The column flow-through fractions were washed at 200 g for 5 min at 4°C, resuspended in solution B (0.5% BSA, 1 μg/ml penicillin G, 1 U/ml streptomycin, 1% citrate phosphate dextrose solution [CPD; Baxter], 25 mM KH2PO4, and 25 mM K2HPO4), and placed on ice. After the LPL digestion, the suspensions were shaken mildly for 1 min at RT. The LPL supernatants were collected through a 70-μm cell strainer, washed at 200 g for 5 min at 4°C, resuspended in solution B, and placed on ice. Live cells from both the IEL and LPL fractions of the small and large intestines were then counted using trypan blue exclusion and used for flow cytometry analysis.
Intrarectal infection of humanized mice with HIV.
Virus stocks were prepared and titrated and p24 content was determined as we have previously described (21, 22). Before inoculation, mice were anesthetized with sodium nembutal. Intrarectal inoculations were performed essentially as previously described using a total volume of 60–80 μL of virus (200 ng p24, ∼9 × 104 TCIU) (23).
Analysis of HIV infection of humanized mice.
Infection of BLT mice with HIV was monitored in peripheral blood every 2 wk by determining viral load (Amplicore; Roche), levels of viral antigenemia (ELISA p24; Beckman Coulter), and the levels of human CD4+ and CD8+ T cells in peripheral blood (flow cytometry), and by determining the presence of human antibodies to HIV proteins in plasma essentially as we have previously described (4, 21). Analysis of systemic infection was performed 8 wk after infection by in situ hybridization, immunohistochemistry, and flow cytometry also as we have previously described (4, 12). Rescue of infectious virus from different tissues was performed by coculture with PHA-activated PBMC from HIV seronegative donors, and viral spread was monitored by determining p24 levels in the culture supernatant. Human CD4+ T cell depletion was monitored in all tissues indicated essentially as we have previously described except that the six-color flow cytometry analysis also included determination of CCR5 and CXCR4 expression (4).
Online supplemental material.
Fig. S1 shows immunohistochemical analysis for human CD11c and HLA-DR–positive cells in the gut of BLT mice and flow cytometry analysis for the presence of human DC and NK cells. In addition, it demonstrates the lack of cross-reactivity of the human-specific antibodies used with mouse antigens. Fig. S2 demonstrates the rescue of replication-competent HIV from the spleen, thymic organoid, and bone marrow of infected BLT mice. Fig. S3 presents high resolution images showing the histopathological changes observed in the thymic organoid and large intestine during HIV infection. Figs. S1–S3 available at http://www.jem.org/cgi/content/full/jem.20062411/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Debora Payne and Jeannelle Vaughn (Veripath laboratories) for performing the viral load analysis; Donald Sodora for providing HIV+ plasma samples; Lishan Su and the members of his laboratory for technical advice regarding HIV infection of humanized mice; David Phillips and Kristin Sudol for sharing their expertise in intrarectal inoculations; Lora Hooper, Anisa Ismail, and Cassie Behrendt for sharing their expertise in the isolation of lymphocytes from the GI tract; Daniel Powell for technical support; and Beth Levine and Lora Hooper for advice and critical reading of the manuscript.
This work was supported in part by National Institutes of Health grants R37 AI028246 (A.T. Haase), CA82055 and AI39416 (J.V. Garcia), and training grant T32 AI07421 (J.D. Estes).
The authors have no conflicting financial interests.
Z. Sun and P.W. Denton contributed equally to this work.
References
- 1.Pauza, C.D., D. Horejsh, and M. Wallace. 1998. Mucosal transmission of virulent and avirulent lentiviruses in macaques. AIDS Res. Hum. Retroviruses. 14:S83–S87. [PubMed] [Google Scholar]
- 2.Veazey, R.S., and A.A. Lackner. 2005. HIV swiftly guts the immune system. Getting to the guts of HIV pathogenesis. Nat. Med. 11:469–470. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J.M., T.W. Schacker, L.E. Ruff, D.A. Price, J.H. Taylor, G.J. Beilman, P.L. Nguyen, A. Khoruts, M. Larson, A.T. Haase, and D.C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melkus, M.W., J.D. Estes, A. Padgett-Thomas, J. Gatlin, P.W. Denton, F. Othieno, A.K. Wege, A.T. Hasse, and J.V. Garcia. 2006. Humanized mice mount specific adaptive and innate immune response to EBV and TSST-1. Nat. Med. 12:1316–1322. [DOI] [PubMed] [Google Scholar]
- 5.Abuzakouk, M., J. Carton, C. Feighery, D.P. O'Donoghue, D.G. Weir, and C. O'Farrelly. 1998. CD4+ CD8+ and CD8alpha+ beta− T lymphocytes in human small intestinal lamina propria. Eur. J. Gastroenterol. Hepatol. 10:325–329. [DOI] [PubMed] [Google Scholar]
- 6.Carton, J., B. Byrne, L. Madrigal-Estebas, D.P. O'Donoghue, and C. O'Farrelly. 2004. CD4+CD8+ human small intestinal T cells are decreased in coeliac patients, with CD8 expression downregulated on intra-epithelial T cells in the active disease. Eur. J. Gastroenterol. Hepatol. 16:961–968. [DOI] [PubMed] [Google Scholar]
- 7.Bell, S.J., R. Rigby, N. English, S.D. Mann, S.C. Knight, M.A. Kamm, and A.J. Stagg. 2001. Migration and maturation of human colonic dendritic cells. J. Immunol. 166:4958–4967. [DOI] [PubMed] [Google Scholar]
- 8.Ray, N., and R.W. Doms. 2006. HIV-1 coreceptors and their inhibitors. Curr. Top. Microbiol. Immunol. 303:97–120. [DOI] [PubMed] [Google Scholar]
- 9.Veazey, R.S., K.G. Mansfield, I.C. Tham, A.C. Carville, D.E. Shvetz, A.E. Forand, and A.A. Lackner. 2000. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton, P.A., J. Elliott, M.A. Poles, I.M. McGowan, J. Matud, L.E. Hultin, K. Grovit-Ferbas, C.R. Mackay, I.S.Y. Chen, and J.V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 14:1761–1765. [DOI] [PubMed] [Google Scholar]
- 11.Cranston, R.D., P.A. Anton, I.M. McGowan, J. Elliott, M.A. Poles, J. Matud, L.E. Hultin, K. Grovit-Ferbas, C.R. Mackay, I.S.Y. Chen, and J.V. Giorgi. 2000. Gastrointestinal mucosal biopsy in HIV disease and AIDS. Gastrointest. Endosc. Clin. N. Am. 10:637–667. [PubMed] [Google Scholar]
- 12.Li, Q., L. Duan, J.D. Estes, Z.M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C.J. Miller, and A.T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 434:1148–1152. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa, S.C., L.A. Herzenberg, and M. Roederer. 2001. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat. Med. 7:245–248. [DOI] [PubMed] [Google Scholar]
- 14.Sun, J., T. Soos, V.N. Kewalramani, K. Osiecki, J.H. Zheng, L. Falkin, L. Santambrogio, D.R. Littman, and H. Goldstein. 2006. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80:1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariani, R., G. Rutter, M.E. Harris, T.J. Hope, H.-G. Krausslich, and N.R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieniasz, P.D., and B.R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868–9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCune, J.M., R. Namikawa, H. Kaneshima, L.D. Shultz, M. Lieberman, and I.L. Weissman. 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 241:1632–1639. [DOI] [PubMed] [Google Scholar]
- 18.Estes, J.D., Q. Li, M.R. Reynolds, S. Wietgrefe, L. Duan, T. Schacker, L.J. Picker, D.I. Watkins, J.D. Lifson, C. Reilly, J. Carlis, and A.T. Haase. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193:703–712. [DOI] [PubMed] [Google Scholar]
- 19.Das, G., M.M. Augustine, J. Das, K. Bottomly, P. Ray, and A. Ray. 2003. An important regulatory role for CD4+CD8alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 100:5324–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mysorekar, I.U., R.G. Lorenz, and J.I. Gordon. 2002. A gnotobiotic transgenic mouse model for studying interactions between small intestinal enterocytes and intraepithelial lymphocytes. J. Biol. Chem. 277:37811–37819. [DOI] [PubMed] [Google Scholar]
- 21.Wei, B.L., P.W. Denton, E. O'Neill, T. Luo, J.L. Foster, and J.V. Garcia. 2005. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J. Virol. 79:5705–5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredericksen, B.L., B.L. Wei, J. Yao, T. Luo, and J.V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitlin, L., T.E. Hoen, S.L. Achilles, T.A. Hegarty, A.E. Jerse, J.W. Kreider, S.S. Olmsted, K.J. Whaley, R.A. Cone, and T.R. Moench. 2001. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex. Transm. Dis. 28:417–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.