Abstract
CCR7-mediated migration of naive T cells into the secondary lymphoid organs is a prerequisite for their encounter with mature dendritic cells, the productive presentation of cognate antigen, and consequent T cell proliferation and effector differentiation. Therefore, CCR7 was suggested to play an important role in the initiation of adaptive immune responses. In this study, we show that primary immunity can also develop in the absence of CCR7. Moreover, CCR7-deficient knockout (KO) mice display augmented immune responses. Our data cumulatively suggest that enhanced immunity in CCR7 KO mice is caused by the defective lymph node (LN) positioning of FoxP3+ CD4+ CD25+ regulatory T cells (T reg cells) and the consequent impediment of their function. The FoxP3+ T reg cells express CCR7 and, after their adoptive transfer, migrate into the LNs of wild-type mice. Here, they proliferate in situ upon antigen stimulation and inhibit the generation of antigen-specific T cells. Conversely, transferred CCR7-deficient T reg cells fail to migrate into the LNs and suppress antigen-induced T cell responses. The transfer of combinations of naive and T reg cells from wild-type and CCR7 KO mice into syngeneic severe combined immunodeficient mice directly demonstrates that CCR7-deficient T reg cells are less effective than their wild-type counterparts in preventing the development of inflammatory bowel disease.
Efficient adaptive immunity necessitates the encounter of exogenous antigens and rare antigen-specific immune cells in specialized microenvironments within the secondary lymphoid organs (SLOs; reference 1). The antigens are classically carried into the SLOs from the periphery by APCs. The cells of the adaptive arm of the immune system, particularly naive T cells, continue to recirculate via blood through the SLOs until they encounter their cognate antigen (2). The recognition of a cognate antigen by a T cell receptor in the context of MHC and with appropriate costimulation induces profound changes in T cell behavior, which signify the initiation of the adaptive immune response.
According to the current paradigm, the two distinct migratory paths of APCs and T cells, which converge in the T cell zones of SLOs, are governed by the chemokine receptor CCR7 (3). CCR7 is highly expressed on mature DCs as well as on naive and central memory T cells (4), whereas its two chemokine ligands CCL19 and CCL21 are produced constitutively in the lymphoid organs and lymphatic vessels. The cardinal role of CCR7 in the homing and positioning of naive T cells and DCs in the SLOs and the ensuing effect on the immune response are illustrated by the profoundly disrupted cellular architecture of the SLOs in CCR7-deficient (CCR7 KO) mice. Consequently CCR7 KO mice were shown to have a reduced ability to mount primary immune responses (5). However, recent studies indicate that adaptive immunity may develop through alternative pathways that do not involve CCR7. Peripheral antigens can diffuse in soluble form into the SLOs, where after acquisition, processing, and presentation by the resident APCs, they can induce immune responses (6, 7). Also, alternative chemoattractant receptors may be substituting for CCR7 in inducing the homing of T cell and APC subsets into the SLOs (8–13).
Therefore, we initially explored the contribution of CCR7 to the development of T cell immunity by setting up skin contact hypersensitivity (CHS) reactions in CCR7 KO mice. We show that T cell–mediated immunity to contact antigens develops in the absence of CCR7; moreover, the responses in CCR7 KO mice exceed those seen in WT BALB/c mice. The exacerbated CHS response in CCR7 KO mice is ameliorated by the transfer of WT CD4+ CD25+ regulatory T cells (T reg cells). This incriminates insufficient T reg cell function in the enhanced CHS in CCR7 KO mice and suggests that T reg cells may require CCR7 for their in vivo suppressive activity. Indeed, FoxP3+ T reg cells express CCR7 and use it to home into LNs, where they expand upon antigen stimulation and suppress effector cell responses. CCR7 KO T reg cells fail to localize to LNs and to inhibit specific effector cell response. Additionally, CCR7 KO T reg cells have an approximately twofold reduced capacity to protect in a transfer model of inflammatory bowel disease (IBD). This demonstrates that on one hand, CCR7 can be bypassed for the induction of immunity, but its expression by T reg cells is required for their migration into the LNs and contributes to their suppressive function in vivo.
RESULTS
Augmented CHS in CCR7 KO mice
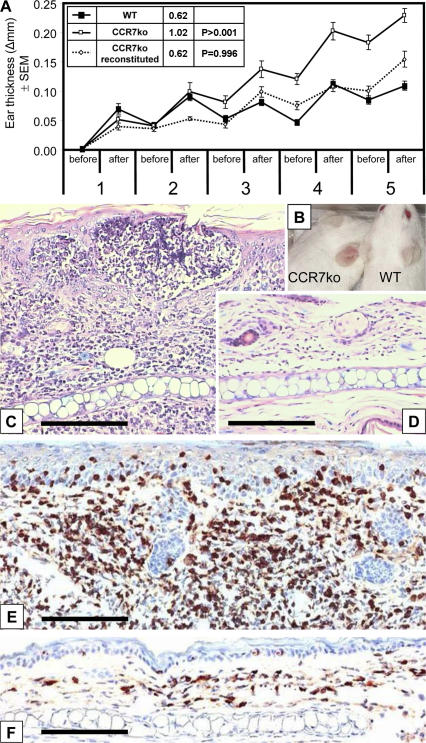
To evaluate the contribution of CCR7 to antigen sensitization and the subsequent immune response, we used a model for epicutaneous CHS in CCR7 KO mice. WT and CCR7 KO mice were painted with oxazolone on the shaved abdomen and challenged by applying this hapten onto the ears every second day for five times starting on day 7 after the sensitization. The CHS lesions observed in CCR7 KO mice were more severe than in WT mice. The difference in the ear thickness between CCR7 KO and WT mice became apparent after the third challenge (Fig. 1 A). After the last challenge, we measured an almost fivefold difference in auricular weights of the CCR7 KO (35.6 ± 3.5 mg) and WT mice (7.5 ± 4.0 mg), and an obvious skin flare was observed in CCR7 KO mice (Fig. 1 B). The histological evaluation revealed an augmented leukocyte infiltrate in the ears of CCR7 KO mice (Fig. 1 C) containing large numbers of CD3+ T cells (Fig. 1 E). The extent of T cell responses is known to be controlled by T reg cells. To investigate whether the enhanced CHS in CCR7 KO mice may be the result of impaired T reg cell function, we transferred WT CD4+ CD25+ T reg cells into CCR7 KO recipients 1 d before their sensitization. In mice reconstituted with WT T reg cells, we observed a significant reduction (P > 0.001) of ear thickness, resulting in lesions of similar overall severity as seen in WT mice (Fig. 1 A). To study whether CCR7 KO mice have a generally enhanced immune response, including to noncontact antigens, we compared tetanus toxoid–induced ex vivo splenocyte proliferation in CCR7 KO and WT mice after their immunization. We found that CCR7 KO mice have consistently higher spontaneous and tetanus toxoid–induced responses than WT mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061405/DC1).
Figure 1.
CCR7 KO mice show enhanced CHS reaction to oxazolone. (A) The ear thickness before and after five repeated oxazolone challenges in sensitized mice; data are expressed as mean differences between the hapten- and vehicle-challenged ears. From the third challenge onwards, CCR7 KO mice (white symbols; n = 19) have an enhanced ear swelling compared with WT mice (black symbols; n = 10). The augmented ear swelling in CCR7 KO was reversed by the transfer of 5 × 105 naive WT T reg cells (dotted line; n = 7). Inset table shows the area under the curve values and their statistical analysis (Student's t test). Error bars represent SEM. (B) After the last challenge, the difference between WT and CCR7 KO mice can be observed as an enhanced flare. (C and D) Microscopically, the CHS lesions contain a massive mononuclear infiltrate in CCR7 KO mice (C) in comparison with a moderate infiltrate in WT mice (D). (E and F) CD3 immunostaining of the CHS lesions in CCR7 KO (F) and WT mice (E) revealed that the infiltrate consists mostly of CD3+ cells. Bars, 50 μm.
CCR7 is expressed by FoxP3+ T reg cells and affects their tissue distribution
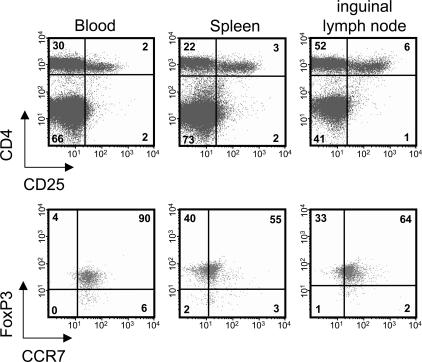
We used flow cytometry to study the surface expression of CCR7 by FoxP3+ CD4+ CD25+ T reg cells in different tissues of WT mice. As shown in Fig. 2, almost all T reg cells in the blood and a high proportion of them in SLOs bear CCR7. To examine whether CCR7 expression affects the tissue distribution of FoxP3+ T reg cells, their numbers in WT and CCR7 KO mice were compared. Single-cell suspensions from the blood, spleen, and peripheral LNs (inguinal and axillary) were immunostained, and cell numbers were assessed in flow cytometry.
Figure 2.
CCR7 is expressed by FoxP3+ CD4+ CD25+ T reg cells. Flow cytometric analysis of peripheral blood and single-cell suspensions of spleen and inguinal LNs of WT mice revealed that CD4+ CD25+ T reg cells coexpress FoxP3 and CCR7. Dot plots of FoxP3 and CCR7 staining (bottom) are gated on the CD4+ CD25+ T cells shown in the top panel. Numbers indicate the percentage of cells in each quadrant.
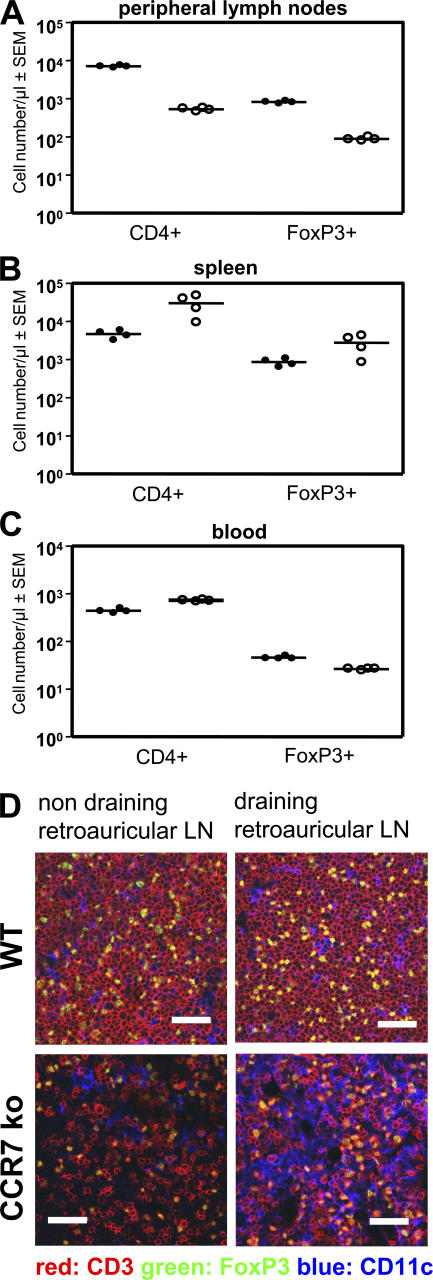
In comparison with WT mice, the LNs of CCR7 KO mice contained only very few CD4 T cells (5), including FoxP3+ T reg cells (Fig. 3 A). In contrast, the numbers of CD4+ and FoxP3+ T cells were increased in the spleen of CCR7 KO mice (Fig. 3 B), whereas in the blood, only CD4+ T cells were elevated (Fig. 3 C). This demonstrates a skewed distribution of FoxP3+ T cells between blood and the SLOs of CCR7 KO mice, including their relative frequency (Fig. S2 D, available at http://www.jem.org/cgi/content/full/jem.20061405/DC1). In comparison with WT mice, the percentage of T reg cells is slightly elevated in LNs of CCR7 KO mice. However, it is likely that it is not the percentage of T reg cells in LNs that determines their utility or lack of it but their ability to localize into functional microenvironments. Memory T cells (CD4+ CD44high CD62Lneg CD45RBlow) are relatively enriched in peripheral LNs of CCR7 KO mice (Fig. S2 E). Also, the proportion of CD4-activated cells (defined as CD4+ CD25+ CD44high FoxP3neg) is higher in both resting and CHS- draining LNs of CCR7 KO mice in comparison with their WT counterparts (Fig. S2 F).
Figure 3.
Numbers of CD4+ and FoxP3+ cells in the peripheral LN, blood, and spleen of WT and CCR7 KO mice. (A) Few CD4+ and FoxP3+ cells can be found in the peripheral LNs of CCR7 KO mice (white circles) compared with WT mice (black circles). (B and C) In contrast, both populations are increased in the spleen (B), whereas in blood, only the CD4+ cells are increased, and FoxP3+ cells are reduced (n = 4; C). Horizontal lines represent the mean values. (D) CD3+ and FoxP3+ T cells show altered distribution in retroauricular LNs of CCR7 KO mice compared with normal architecture in WT mice. Organized T cell zones of WT LNs are shown. This microarchitecture cannot be found in CCR7 KO mice, where diffuse T cell distribution is observed. Bars, 200 μm.
Immunofluorescent staining of LNs in CCR7 KO mice confirmed their disrupted architecture and altered cell distribution, also affecting FoxP3+ T cells (Fig. 3 D). Nevertheless, numerous contacts between T cells and DCs take place in the LNs of CCR7 KO mice, especially in the LNs draining CHS lesions (Fig. 3 D).
Because CCR7 may contribute to T cell selection in the thymus (14–16), we compared the thymic distribution of FoxP3+ T cells in CCR7 KO and WT mice. We could not observe any numerical differences in CCR7 KO mice; however, FoxP3+ cells appeared not only in the medulla but also in sparse foci in cortical areas (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20061405/DC1). In summary, CCR7 is highly expressed on FoxP3+ CD4+ CD25+ T reg cells and affects their localization in different organs.
CD4+ CD25+ T reg cells from CCR7 KO mice are unable to control the proliferation of CD4 T cells in vivo
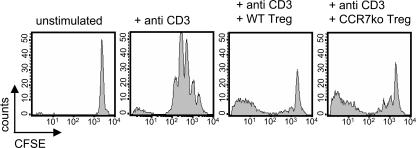
To investigate whether CCR7 on T reg cells is required for their regulatory function in vivo, we studied the responses of TCR transgenic T cells after their adoptive transfer. First, CD4+ CD25+ T reg cells were isolated from DO11.10 mice, CFSE labeled, and injected into WT mice. 24 h after the transfer, the animals were injected with OVA into the footpad. The draining and counterlateral popliteal LNs were excised 72 h after the OVA injection, and the fluorescence intensity of CFSE-labeled CD4+ CD25+ T reg cells was analyzed.
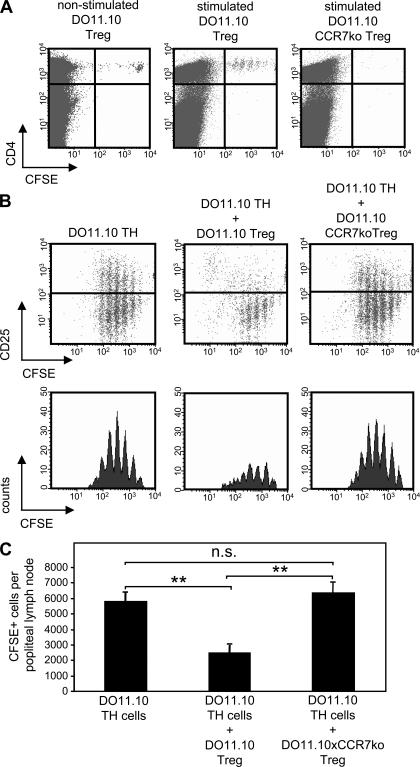
T reg cells from DO11.10 donors entered both LNs but expanded only in the draining LN as seen by their CFSE dilution profile (Fig. 4 A). In contrast, hardly any T reg cells from DO11.10 × CCR7 KO animals could be found in the draining LN (Fig. 4 A). The impact of impaired LN homing of CCR7 KO T reg cells was studied in the following cotransfer experiment. OVA TCR transgenic CD4+ T cells were CFSE labeled and injected i.v. either alone or in combination with unlabeled CD4+ CD25+ T reg cells from either DO11.10 mice or DO11.10 × CCR7 KO mice. The recipient mice were again challenged with OVA in the footpad and analyzed 72 h later. The transferred CD4+ T cells proliferated in response to their cognate antigen (Fig. 4 B) and up-regulated the expression of CD25 (Fig. 4 B). Cotransferred DO11.10 T reg cells but not DO11.10 × CCR7 KO T reg cells reduced the number of CD4+ T cells and blocked the CD25 expression of CD4+ T cells (Fig. 4 B). Mean numbers of CFSE-positive cells per draining popliteal LN (n = 4) are shown in Fig. 4 C. The overall number of CD4+ T cells in LNs regulated by WT T reg cells is significantly diminished (P > 0.01), which is consistent with their reduced proliferation. However, we cannot formally exclude the possibilities that WT T reg cells also induce the death of CD4+ T cells as suggested previously (17) or cause the reduction in their LN recruitment or the increase in their departure.
Figure 4.
Adoptively transferred CCR7 KO T reg cells cannot suppress the antigen-induced expansion of TCR transgenic T cells. (A) 5 × 105 transferred CFSE-labeled T reg cells from DO11.10 mice enter LNs and, after antigen stimulation, expand in draining popliteal LNs and not in counterlateral LNs. Only a few T reg cells from DO11.10 × CCR7 KO can be detected in the LNs after their transfer. (B) Antigen-induced expansion of 5 × 105 CFSE-labeled DO11.10 CD4+ Th cells; their concurrent CD25 up-regulation is suppressed by the cotransfer of DO11.10 T reg cells but not DO11.10 × CCR7 KO T reg cells. Th cells regulated by WT T reg cells divide approximately one generation less in comparison with two other groups (dot plots). In unregulated and CCR7 KO T reg cell–regulated groups, the majority of Th cells are in generations three, four, and five, whereas the majority of Th cells regulated by WT T reg cells are in generations two, three, and four; their overall numbers are also reduced (histograms). (C) Statistical evaluation of the data in B (n = 4). The cotransfer of T reg cells from DO11.10 mice reduced (**, P < 0.01) the mean total number of CFSE+ T cells in the draining popliteal LNs, whereas the cotransferred DO11.10 × CCR7 KO T reg cells showed no effect. Error bars represent SEM.
In conclusion, T reg cells from DO11.10 × CCR7 KO mice were unable to enter the draining LNs after antigenic challenge and, therefore, failed to suppress the expansion and effector differentiation of CD4+ T cells. However, CCR7 itself is not required for the suppressive function of T reg cells, as demonstrated by the classic in vitro suppression assay. Polyclonal CD4+ T cells from WT mice were stimulated in vitro with anti-CD3 mAb in the presence or absence of CD4+ CD25+ T reg cells from WT or CCR7 KO mice. After 72 h, the CFSE profile of the CD4+ responder cells was characterized. Both T reg cell populations demonstrated comparable suppression of CD4 T cell proliferation (Fig. 5).
Figure 5.
In vitro function of CD4+ CD25+ T reg cells is not dependent on CCR7 expression. Polyclonal CD4+ T cells were stained with CFSE and stimulated with anti-CD3 mAb for 72 h in the presence of CD4+ CD25+ T reg cells from either WT or CCR7 KO mice. Both T reg cell populations show similar in vitro suppression of T cell proliferation in a dose-dependent manner (a 1:1 ratio is shown).
CD4+ CD25+ T reg cells from CCR7 KO mice enter peripheral tissues
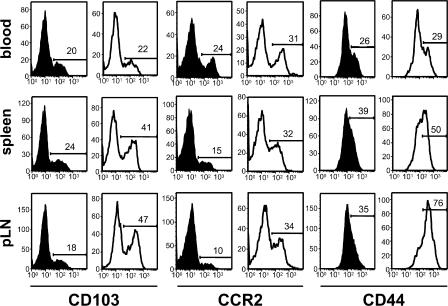
There are two potential sites where CD4+ CD25+ T reg cells can suppress immune reactions: the LNs, as shown in the previous section, and peripheral tissues. To examine whether CCR7 KO T reg cells are able to enter inflamed skin, we investigated the CHS ear lesions of CCR7 KO mice for the presence of FoxP3+ T reg cells. Unchallenged and challenged ears from both WT and CCR7 KO mice were stained with antibodies against CD3, FoxP3, and CD11c. Increased numbers of FoxP3+ T reg cells appeared within the inflamed ears of CCR7 KO mice in comparison with WT mice (Fig. 6). It was recently shown that a subpopulation of CD4+ CD25+ T reg cells that enters peripheral sites expresses high levels of the integrin marker CD103 (18). We compared the expression of CD103 on T reg cells in CCR7 KO and WT mice. A high proportion of the FoxP3+ CD4+ CD25+ T reg cells from CCR7 KO mice show CD103 expression in contrast to only a small subpopulation in WT mice (Fig. 7). In addition, we observed more CCR2+ FoxP3+ T cells and increased levels of CD44 on T reg cells from CCR7 KO mice (Fig. 7). Thus, we identified a pattern of adhesion molecule and chemokine receptor expression on CCR7 KO T reg cells, which may explain their ability to enter the CHS lesions. However, it is clear that their presence in these lesions is not sufficient to control the development of the pathological changes (Fig. 1), indicating that optimal regulation requires the entry of T reg cells into the LN.
Figure 6.
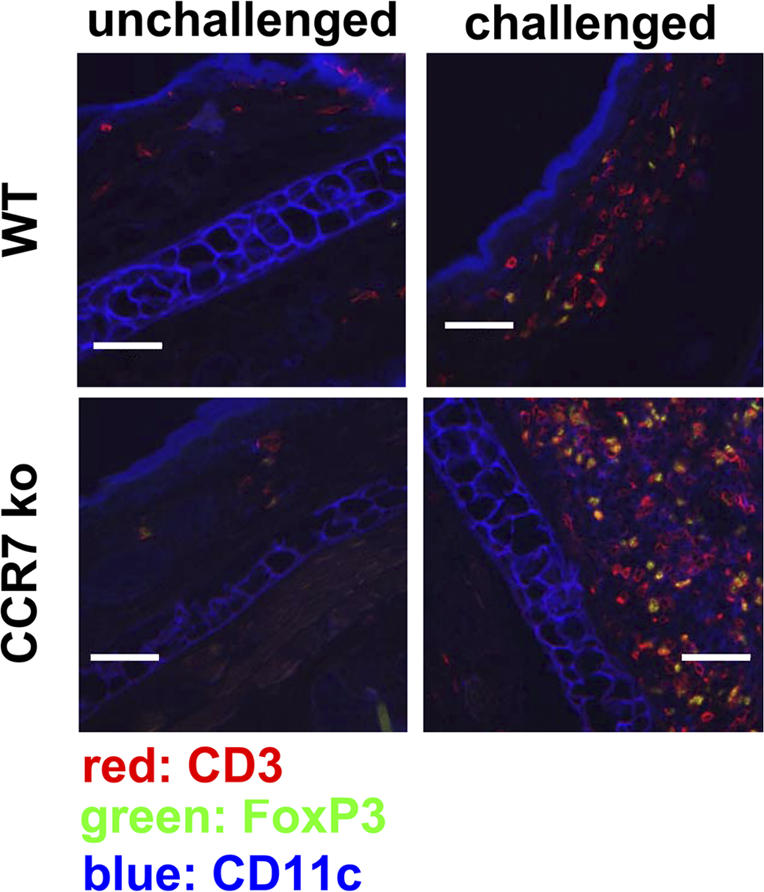
FoxP3+ T reg cells enter the CHS lesions of CCR7 KO mice. Immunofluorescence in the unchallenged (left) ears and oxazolon-challenged (right) ears of WT and CCR7 KO mice. The challenged ears of CCR7 KO mice contain an elevated number of CD3+ FoxP3+ T reg cells in comparison with WT mice. One representative ear sample out of 10 mice per group is shown. Bars, 200 μm.
Figure 7.
FoxP3+ T reg cells in CCR7 KO mice have a higher expression of CD44 and an increased frequency of CD103+ and CCR2+ subpopulations. Histograms of data acquired from the blood, spleen, and peripheral LNs are shown. 104 FoxP3+ T cells are depicted per histogram. In comparison with CCR7 KO mice (white histograms), WT mice (black histograms) have comparatively smaller subpopulations of CD103+ and CCR2+ T reg cells. CCR7 KO T reg cells also express higher levels of CD44. One out of four measurements on cells from different mice are shown. Numbers indicate the percentages of positive cells.
CCR7 KO T reg cells are inefficient in controlling IBD
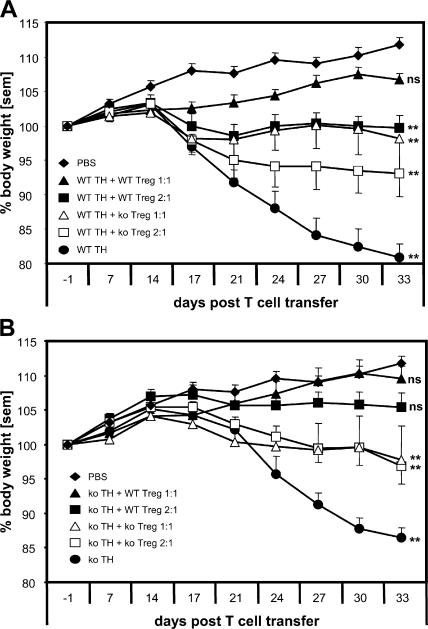
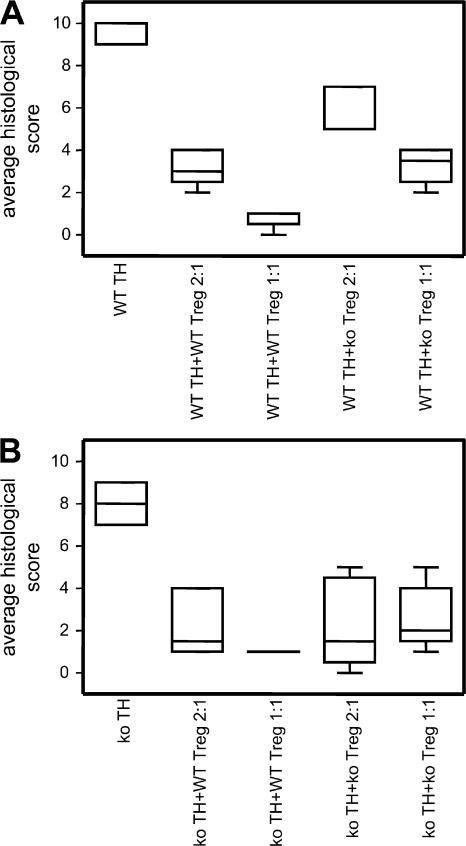
As T reg cells from CCR7 KO mice were unable to suppress the expansion of CD4+ T cells in the draining LNs, we explored their potential to control the induction of experimental IBD. CD4+ CD25neg T cells (T helper cell [Th cell]) from either WT or CCR7 KO mice were injected into SCID mice with or without WT or CCR7 KO CD4+ CD25+ T reg cells. The effect of two different ratios of T reg cells and Th cells was investigated (1:1 and 1:2). The transfer of WT and CCR7 KO Th cells lead to a comparable weight loss in SCID mice, albeit with a different time course. A delayed disease onset was observed after the transfer of CCR7 KO Th cells (Fig. 8). IBD induced by WT Th cells was prevented by WT T reg cells in both ratios studied, as shown by weight curves (Fig. 8 A) and histological evaluation of the lesions at the end of the experiment (Fig. 9 A and Fig. S4 A, available at http://www.jem.org/cgi/content/full/jem.20061405/DC1). CCR7 KO T reg cells in both ratios used were less potent in preventing the weight loss in comparison with their WT counterparts (Figs. 8 A, 9A, and S4 A). A cell dose dependency of the suppressive effect could be observed using both WT and CCR7 KO T reg cells. IBD induced by CCR7 KO Th cells was also prevented by WT T reg cells. Again, CCR7 KO T reg cells were less potent regulators (Figs. 8 B, 9B, and S4 B), but, for these cells, no dose dependency was observed. In conclusion, in the SCID transfer model of IBD, the induction of disease is not dependent on CCR7. However, CCR7 is required for the optimal regulation of IBD.
Figure 8.
Ineffective control of experimental IBD by CD4+ CD25+ CCR7 KO T reg cells. (A and B) The transfer of WT (A) or CCR7 KO (B) CD4 Th cells (black circles) into SCID mice induces weight loss, albeit with different kinetics. Disease induced by WT Th cells is prevented by the cotransfer of WT T reg cells in both ratios of 1:2 (black squares) and 1:1 (black triangles), whereas CCR7 KO T reg cells in ratios 1:2 (white squares) and 1:1 (white triangles) were less effective than their WT counterparts. The regulatory effect for both WT and CCR7 KO T reg cells was cell dose dependent (A). IBD induced by CCR7 KO Th cells developed with a 1-wk delay and was completely prevented by WT T reg cells and to a lesser extent by CCR7 KO T reg cells. In the latter case, no cell dose dependency could be observed (B). The last data points of the curves were analyzed with one-way analysis of variance and Dunnett's correction in comparison with untransferred control mice (**, P < 0.01). Error bars represent SEM.
Figure 9.
Mean histological scores of IBD lesions. Histological scores were obtained by evaluating sections prepared from the descending colon of individual mice. Epithelial cell damage, architectural changes, neutrophil, and mononuclear cell infiltrates as well as ulceration and granuloma formation were evaluated and scored blind independently of each other. Data are presented as box plots (n = 6 per group). Additionally, micrographs are shown in Fig. S4 (available at http://www.jem.org/cgi/content/full/jem.20061405/DC1). Data were analyzed with analysis of variance and Bonferroni correction to compare different groups. Apart from WT Th cells + WT T reg cells (2:1) compared with WT Th cells + KO T reg cells (1:1), all groups are significantly different (P < 0.001; A). In B, only KO Th cells are significantly different from other groups (P < 0.001). Error bars represent SEM.
DISCUSSION
Close encounters of APCs and naive T cells in the SLOs are essential for the efficient initiation of an immune response. CCR7 and its ligands CCL19 and CCL21 orchestrate the homing of mature DCs and T cell subsets into the T cell zones of the SLOs. Therefore, this chemokine pathway plays an important role in the effective induction of adaptive immunity. In this study, we show that immune responses can also develop in the absence of CCR7, including a CHS reaction to a hapten, a response to bacterial gut flora that manifests in an experimental IBD, and recall immunity to an injected T cell antigen.
These findings are rather surprising in light of a completely scrambled SLO architecture in CCR7 KO mice (5). In contrast to our current findings, the CCR7 KO mice were initially described to exhibit a mitigated CHS (5). This may be the result of the different haptens used and because the previous study evaluated the CHS lesions in CCR7 KO mice possibly too early to see a response (5). We show now that the development of immunity in these mice takes a retarded course. In complete agreement with our findings, a delayed time course but increased severity of CHS and augmented antigen-induced T cell proliferation have been seen in the mutant mouse strain plt (19). Plt mice do not express two out of the three known CCR7 ligands (CCL19 and CCL21-Ser) and, similar to CCR7 KO mice, lack the normal SLO organization because of a defect in DC and T cell homing (20, 21). However, until now, the ability of plt mice to mount immune responses and their apparent difference with the phenotype described for CCR7 KO mice (5, 19) have been attributed to the compensatory presence of one CCL21 gene, CCL21-Leu, which is expressed in the lymphatic endothelium of plt mice (22). Additionally, CCR7 was shown not to be essential for the establishment of functional T cell immunity to viral infection: CCR7 KO mice can clear lymphocytic choriomeningitis virus and develop cytotoxic and memory T cell responses against it (23).
So how is adaptive immunity induced in CCR7 KO mice? We can only speculate whether either DCs and T cells themselves or only their CCR7-mediated migratory paths are dispensable for immune responses. Recently, both epidermal DCs (Langerhans cells) and T cells were shown to be completely superfluous for the induction of CHS reactions (24, 25). Curiously, the absence of Langerhans cells leads even to an enhanced CHS. This is consistent with the notion that regulation of CHS but not its induction is dependent on antigen presentation by these cells (24). In the absence of T cells, the Ly49C-I+ NK cells can substitute for them completely in the induction of CHS to at least three different haptens, including oxazolone (25). In this study, an anti–L-selectin antibody (Mel-14) blocked the NK cell homing into the draining LN and impaired the CHS induced by these cells. This implies that for the induction of their antigen responsiveness, NK cells, just like T cells, respond to the antigens in the LN and may require antigen presentation by APCs. It is not clear whether NK cells use CCR7 or other chemokine receptors to home into the LNs.
Despite the lack of normal microanatomical organization, the SLOs of CCR7 KO mice still contain T cells and DCs, several of which are in close contact with each other, as shown here. This suggests that in the absence of CCR7, other migratory molecules may replace CCR7 to allow T cells and DCs to enter SLOs. T cell subsets appear to exhibit differential dependency on CCR7 for their homing into the SLOs (26). It was shown that central memory T cells may use CXCR4 to home into the LNs (8). APC homing into the draining LNs can be induced in addition to the canonical CCR7 (27) also via CCR2, CCR8, and CXCR3 (11–13). However, to induce immunity, antigens do not have to be necessarily carried into the draining LNs by migrating DCs. During the initial phase of a peripheral antigen challenge, free soluble antigens are channeled via lymphatics and LN conduits to LN-resident DCs (7, 28), which can efficiently process and present them (6). Additionally, massive antigenic stimulation may lead to the carry over of soluble antigen via blood into the spleen, where the necessary cellular players are present in sufficient numbers even in CCR7 KO mice.
Ultimately, we cannot answer the questions of how and where immunity is induced in CCR7 KO mice, but our results suggest a plausible explanation for why the immune responses in these mice take an exaggerated course. Our data unequivocally show that the counter-regulation of immunity by CD4+ CD25+ T reg cells is critically dependent on CCR7. Confirming previous results (29–32), we show that WT CD4+ CD25+ FoxP3+ T reg cells express CCR7. In addition, we demonstrate that WT T reg cells use it to home into the LN, where, upon antigen challenge, they proliferate and inhibit antigen-induced effector T cell responses, which is seen as a reduction in their numbers and CD25 expression. Unlike their WT counterparts, adoptively transferred CCR7 KO T reg cells are inefficient in homing to LNs and do not inhibit the antigen-induced generation of effector T cells. The contributions of CCR7 to T reg cell function were confirmed in our CHS model and also in our T cell transfer model of IBD. The latter has been classically used to study the immune regulation by T reg cells. In our studies, the cotransferred CCR7 KO T reg cells were approximately half as effective as WT T reg cells in suppressing the induction of IBD in SCID hosts.
Such ineffective T reg cell function may explain the findings that CCR7 KO mice spontaneously develop multiorgan autoimmunity, although not as severe as that observed in other susceptible strains (e.g., scurfy, NOD, and MLR/lpr). CCR7 KO mice have inflammatory infiltrates in the exocrine glands and stomach akin to those seen in Sjögren syndrome and Menetrier's gastritis, respectively, as well as an overt glomerular autoimmune disease (16, 33, 34). Peripheral tissue lesions of ongoing immune reactions are considered to be important sites of CD4+ CD25+ T reg cell activity (35). Accordingly, a key regulatory role has been ascribed to the CD103+ subpopulation of T reg cells. These cells express inflammatory chemokine receptors, particularly CCR2 (36), allowing their migration into peripheral sites (18, 37). We found that the CD103+ CCR2+ CD44high subpopulation is a predominant T reg cell subset in CCR7 KO mice. This may explain why the CHS lesions in CCR7 KO mice contain large numbers of T reg cells. At present, their potential contribution to the in situ regulation of CHS remains obscured. Their apparent inability to regulate in situ may be caused by the proinflammatory cytokine milieu present in the lesions. In particular, IL-6, which is overexpressed in CHS lesions of CCR7 KO mice (unpublished data), may directly inhibit the differentiation of T reg cells (38, 39) and convert the suppressive molecular arsenal of T reg cells into a stimulatory one. IL-6 in combination with TGF-β, the main suppressive cytokine of T reg cells, is a potent inducer of viciously proinflammatory Th17 cells (38–40).
There is an ongoing debate about whether different T reg cell subpopulations are separate cell lineages or just represent different maturation/activation stages of the same cell type (31, 41). In the latter case, it is possible that LN homing is part of the obligatory curriculum for all T reg cells and that the absence of CCR7 may hamper CD103+ T reg cell localization into functional LN microenvironments. Thus, sometime during their life span, CCR7-deficient CD103+ T reg cells may not be able to receive hypothetical maturation, activation, or armament signals from DCs. Subsequently, this may debilitate their regulatory function. CCR7 also orchestrates the migratory steps required for T cell maturation in the thymus (14–16). As naturally occurring CD4+ CD25+ T reg cells are selected in the thymus (42), it is possible that CCR7 deficiency compromises their thymic development. The dissimilar immunophenotype of T reg cells in CCR7 KO and WT mice may suggest that the lack of CCR7 impedes normal T reg cell development. However, the numbers of thymic FoxP3+ T reg cells are similar in WT and CCR7 KO mice, and, in both strains, T reg cell distribution in the medulla, the site of their selection (42), is similar too (Fig. S3 C). Immunostaining revealed sparse foci of FoxP3 cells in the cortical areas of the thymus in CCR7 KO mice but not in WT (Fig. S3 C). Because of their low numbers, these would hardly affect the overall thymic output of T reg cells but may reflect the requirement for CCR7 in the migratory step of T reg cells from the cortex to the medulla. Furthermore, we show that CCR7 KO T reg cells inhibit in vitro T cell proliferation to the same extent as their WT counterparts. This strongly suggests that the ineffective regulation by T reg cells observed in CCR7 KO mice is not caused by an intrinsic functional defect in these cells.
An additional factor may contribute to the enhanced cellularity of infiltrates in CHS lesions. CCR7 expression was shown to be required for the efficient departure of T cells from the peripheral sites (43, 44). We could confirm these findings (unpublished data) but argue that they may have only limited functional consequences because the lack of CCR7 can influence the tissue exit of only central memory cells (4) and a subpopulation of T reg cells (unpublished data). Among the lymphocyte subsets, these are the only cells known to express concurrently chemoattractant receptors that are required for tissue entry as well as CCR7.
The tightly regulated expression of various chemokines within the SLOs orchestrates the development and silencing of the immune responses by attracting and juxtapositioning functionally complementary cell types (45–48). Cumulatively, our data suggest that the CCR7-mediated positioning of T reg cells into the T cell zones is a prerequisite of their in vivo function. It also clearly indicates that at least in the models of immunity studied by us, LNs are the primary site of the suppressive activity of T reg cells. This concept is supported by several recent studies describing T reg cells entering LNs and establishing close contacts with DCs (49–52). It is also likely that T reg cell contacts with DCs are required not only for the antigen-mediated activation, armament, and expansion of T reg cells but may also influence the concurrent antigen presentation by DCs to effector T cells and the successive development of immunity (49, 50). In addition to inducing the LN homing of T reg cells into functional SLO microenvironments, CCR7 and its ligands may directly influence the quality of the synaptic interaction of T reg cells and DCs and bias its outcome. It was shown recently that DCs are decorated by CCR7 ligands, which can induce the tethering of T cells and influence their activation (53). Thus, the CCR7-mediated DC–T reg cell synapse may constitute an important facet of the direct suppressive activity of T reg cells. The expression of CCR7 by human T reg cells (30, 32, 54) indicates that in humans, this receptor may also contribute in a similar fashion to the suppression of immunity.
We conclude that CCR7 plays a major role in setting up the functional clustering of T reg cells and DCs within the LNs. This cell contact is essential for the antigen-dependent T reg cell expansion and, possibly, is a part of their suppressive function. Thus, CCR7 appears to be more important for the regulation of immunity than for its induction. Our findings question the utility of targeting CCR7 for the treatment of diseases with immune pathogenesis and suggest a potential use of CCR7 antagonists in breaking T reg cell–mediated immunosuppression.
MATERIALS AND METHODS
Animals.
CCR7-deficient mice that were described previously (5) have been crossed with BALB/c mice for 12 generations. The resulting strain, which is referred to as CCR7 KO, was crossed with DO11.10 mice to establish a TCR transgenic strain deficient for CCR7 (DO11.10 × CCR7 KO). The CCR7 KO, DO11.10, and DO11.10 × CCR7 KO mice were bred and housed under specific pathogen-free conditions at the animal facility of the Novartis Institutes for BioMedical Research (NIBR) and were used between the ages of 8 and 14 wk. Control BALB/c mice (referred to here as WT) and BALB/c SCID mice were purchased from Charles River Laboratories. All protocols were approved by both the NIBR and the municipal animal welfare committees. Experiments were performed in accordance with the laws of Austria.
CHS model.
Mice were sensitized with 2% oxazolone/acetone on the shaved abdomen on day 1 and painted with 0.02% oxazolone/acetone on the inner side of the right ears on days 7, 9, 11, 14, and 16. Left ears were challenged by vehicle only and served as controls. CHS was assessed by measuring ear swelling, which was determined before and 24 h after each challenge, and on day 17 by weighing the right and left ears (data expressed as Δ between challenged and control ears) and evaluating them by histology and immunostaining according to standard procedures. To study the effect of WT regulation on the development of CHS lesions in CCR7 KO mice, T reg cells from WT mice were prepared as described below in Cell isolations and adoptive transfer and were injected at 5 × 105 cells/mouse into a group of CCR7 KO mice 1 d before their sensitization.
Flow cytometry and cell quantification in FACS using counting beads.
All antibodies were purchased from BD Biosciences except FoxP3-FITC/PE (eBioscience) and KJ-126-APC (Caltag). MC-21 (anti–mouse CCR2) was a gift from M. Mack (University of Regensburg, Regensburg, Germany). Intracellular FoxP3 staining was performed according to the manufacturer's protocol. Samples were acquired on FACSCalibur (BD Biosciences) and analyzed with CellQuest software (BD Biosciences). Cell quantifications with counting beads (Caltag) were performed in duplicates of 50 μl of blood, spleen, and inguinal LNs. Samples were stained with CD4, CD25, and FoxP3. Erythrocytes were lysed, and the remaining cells were resuspended in 200 μl. After thoroughly mixing with 50 μl of counting beads, 15,000 beads were acquired in a FACSCalibur for each sample. The number of cells/microliter was calculated as [(number of acquired cells)/(number of acquired beads)] × (number of beads/microliter).
Immunofluorescence.
7-μm cryosections were prepared from peripheral LNs and ears of WT and CCR7 KO mice. Sections were fixed in acetone followed by 4% buffered paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS, and incubated first with rat anti–mouse FoxP3 (eBioscience) followed by donkey anti–rat AlexaFluor488. Sections were subsequently incubated with rat anti–mouse CD3 and hamster anti–mouse CD11c (both obtained from BD Biosciences), which were detected with goat anti–rat AlexaFlour546 and goat anti–hamster AlexaFluor633, respectively. All AlexaFluor conjugates were purchased from Invitrogen. Samples were washed after every antibody incubation step. At the end, sections were mounted with Vectashield containing DAPI (Vector Laboratories) and studied under a confocal microscope (Axiovert LSM; Carl Zeiss MicroImaging, Inc.).
Cell isolations and adoptive transfer.
Cell suspensions from spleens were produced with cell strainers (BD Biosciences) followed by erythrocyte lysis. CD4+ cells were isolated with the negative CD4 isolation kit (Miltenyi Biotec) according to the manufacturer's protocol. Afterward, CD4+ cells were stained with CD25-PE mAb (clone PC61; BD Biosciences) for 30 min, which was followed by incubation with anti-PE microbeads (Miltenyi Biotec). Labeled cells were then run over an LS column followed by an MS column, and the positive fraction was eluted. 90% purity was routinely achieved with this procedure. Cells were counted and labeled with CFSE (Invitrogen). 0.5 × 106 cells/population were injected i.v. into BALB/c mice.
In vitro suppression assays.
CD4+ T cells and CD4+ CD25+ T reg cells were isolated as described in the previous section. CD4+ T cells were labeled with CFSE, and 75,000 cells were incubated with 25,000, 50,000, or 75,000 T reg cells from either WT or CCR7 KO mice in a 96-well round-bottom plate. To induce the optimal proliferation of CD4+ T cells by 0.5 μg/ml anti-CD3 (clone 145-2c11; BD Biosciences), 20,000 B cells were added to each well.
IDB model.
Splenic CD4+ CD25neg (Th cells) and CD4+ CD25+ T cells (T reg cells) from WT and CCR7 KO mice were isolated by FACS (FACSAria; BD Biosciences). Sorted cells were >98% pure, and 2 × 105 cells of CD4+ and/or 105 (2:1 ratio) or 2 × 105 (1:1 ratio) CD4+ CD25+ cells were injected i.v. into recipient SCID mice. Body weight was monitored throughout the experiment. Studies were terminated 33 d after the T cell transfer. Descending colons of mice were fixed in 4% buffered paraformaldehyde, embedded in paraffin, and processed for histology. Individual sections were scored blind according to the following independent criteria: epithelial damage (0–2), architectural changes (0–2), neutrophil infiltration (0–2), mononuclear cell infiltrate (0–2), ulceration (0 and 1), and granuloma formation (0 and 1).
Statistics.
All data are presented as mean values ± SEM. The area under the curve was calculated and compared with a Student's t test. Last data points of IBD weight curves were analyzed using one-way analysis of variance with Dunnett's correction using data from normal nontransferred mice as a control. Histological scores were evaluated by one-way analysis of variance with Bonferroni correction for multiple comparisons.
Online supplemental material.
Fig. S1 demonstrates increased ex vivo antigen-induced T cell proliferation of CCR7 KO mice. Fig. S2 gives absolute numbers as well as ratios of different leukocyte populations within SLOs of WT and CCR7 KO mice. Fig. S3 shows that the numbers of FoxP3+ T reg cells in the thymus and their positioning in the medulla are similar in CCR7 KO and WT mice, whereas their distribution in the cortex is not. Fig. S4 depicts examples of the histological appearance of descending colons from different groups of the IBD model. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061405/DC1.
Supplemental Material
Acknowledgments
We are grateful to Liesbeth Mudde, Ernhilt Schwarzinger, Marion Zsák, Paula Bombosi, and Hermann Fahrngruber for excellent technical assistance and to José Carballido for helpful discussions and support. We are indebted to Michaela Hahn and Werner “Travniček” Höllriegl for mouse husbandry, Christina Schwab for cell sorting, and Matthias Mack for the anti–mouse CCR2 mAb.
M. Lipp and A. Rot are supported by the European Union's Sixth Framework Program collaborative grant INNOCHEM (LSHB-CT-2005-518167).
The authors have no conflicting financial interests.
Abbreviations used: CHS, contact hypersensitivity; IBD, inflammatory bowel disease; SLO, secondary lymphoid organ.
References
- 1.Itano, A.A., and M.K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Sanchez, N., L. Riol-Blanco, and J.L. Rodriguez-Fernandez. 2006. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J. Immunol. 176:5153–5159. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 5.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 6.Itano, A.A., S.J. McSorley, R.L. Reinhardt, B.D. Ehst, E. Ingulli, A.Y. Rudensky, and M.K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 19:47–57. [DOI] [PubMed] [Google Scholar]
- 7.Sixt, M., N. Kanazawa, M. Selg, T. Samson, G. Roos, D.P. Reinhardt, R. Pabst, M.B. Lutz, and L. Sorokin. 2005. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 22:19–29. [DOI] [PubMed] [Google Scholar]
- 8.Scimone, M.L., T.W. Felbinger, I.B. Mazo, J.V. Stein, U.H. Von Andrian, and W. Weninger. 2004. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J. Exp. Med. 199:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henning, G., L. Ohl, T. Junt, P. Reiterer, V. Brinkmann, H. Nakano, W. Hohenberger, M. Lipp, and R. Forster. 2001. CC chemokine receptor 7-dependent and -independent pathways for lymphocyte homing: modulation by FTY720. J. Exp. Med. 194:1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janatpour, M.J., S. Hudak, M. Sathe, J.D. Sedgwick, and L.M. McEvoy. 2001. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 194:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palframan, R.T., S. Jung, G. Cheng, W. Weninger, Y. Luo, M. Dorf, D.R. Littman, B.J. Rollins, H. Zweerink, A. Rot, and U.H. von Andrian. 2001. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu, C., E.W. Edwards, F. Tacke, V. Angeli, J. Llodra, G. Sanchez-Schmitz, A. Garin, N.S. Haque, W. Peters, N. van Rooijen, et al. 2004. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 200:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters, W., M. Dupuis, and I.F. Charo. 2000. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J. Immunol. 165:7072–7077. [DOI] [PubMed] [Google Scholar]
- 14.Misslitz, A., O. Pabst, G. Hintzen, L. Ohl, E. Kremmer, H.T. Petrie, and R. Forster. 2004. Thymic T cell development and progenitor localization depend on CCR7. J. Exp. Med. 200:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahama, Y. 2006. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 6:127–135. [DOI] [PubMed] [Google Scholar]
- 16.Kurobe, H., C. Liu, T. Ueno, F. Saito, I. Ohigashi, N. Seach, R. Arakaki, Y. Hayashi, T. Kitagawa, M. Lipp, et al. 2006. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 24:165–177. [DOI] [PubMed] [Google Scholar]
- 17.Shen, S., Y. Ding, C.E. Tadokoro, D. Olivares-Villagomez, M. Camps-Ramirez, M.A. Curotto de Lafaille, and J.J. Lafaille. 2005. Control of homeostatic proliferation by regulatory T cells. J. Clin. Invest. 115:3517–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegmund, K., M. Feuerer, C. Siewert, S. Ghani, U. Haubold, A. Dankof, V. Krenn, M.P. Schon, A. Scheffold, J.B. Lowe, et al. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 106:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori, S., H. Nakano, K. Aritomi, C.R. Wang, M.D. Gunn, and T. Kakiuchi. 2001. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 193:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano, H., S. Mori, H. Yonekawa, H. Nariuchi, A. Matsuzawa, and T. Kakiuchi. 1998. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 91:2886–2895. [PubMed] [Google Scholar]
- 21.Gunn, M.D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L.T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassileva, G., H. Soto, A. Zlotnik, H. Nakano, T. Kakiuchi, J.A. Hedrick, and S.A. Lira. 1999. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190:1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junt, T., E. Scandella, R. Forster, P. Krebs, S. Krautwald, M. Lipp, H. Hengartner, and B. Ludewig. 2004. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J. Immunol. 173:6684–6693. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan, D.H., M.C. Jenison, S. Saeland, W.D. Shlomchik, and M.J. Shlomchik. 2005. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 23:611–620. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary, J.G., M. Goodarzi, D.L. Drayton, and U.H. von Andrian. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7:507–516. [DOI] [PubMed] [Google Scholar]
- 26.Kursar, M., U.E. Hopken, M. Koch, A. Kohler, M. Lipp, S.H. Kaufmann, and H.W. Mittrucker. 2005. Differential requirements for the chemokine receptor CCR7 in T cell activation during Listeria monocytogenes infection. J. Exp. Med. 201:1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Fontecha, A., S. Sebastiani, U.E. Hopken, M. Uguccioni, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catron, D.M., A.A. Itano, K.A. Pape, D.L. Mueller, and M.K. Jenkins. 2004. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 21:341–347. [DOI] [PubMed] [Google Scholar]
- 29.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 30.Cavani, A., F. Nasorri, C. Ottaviani, S. Sebastiani, O. De Pita, and G. Girolomoni. 2003. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 171:5760–5768. [DOI] [PubMed] [Google Scholar]
- 31.Huehn, J., K. Siegmund, J.C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G.F. Debes, J. Lauber, O. Frey, G.K. Przybylski, et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valmori, D., A. Merlo, N.E. Souleimanian, C.S. Hesdorffer, and M. Ayyoub. 2005. A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Invest. 115:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davalos-Misslitz, A.C., J. Rieckenberg, S. Willenzon, T. Worbs, E. Kremmer, G. Bernhardt, and R. Forster. 2007. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur. J. Immunol. 37:613–622. [DOI] [PubMed] [Google Scholar]
- 34.Hopken, U.E., A.M. Wengner, C. Loddenkemper, H. Stein, M.M. Heimesaat, A. Rehm, and M. Lipp. 2007. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 109:886–895. [DOI] [PubMed] [Google Scholar]
- 35.Bluestone, J.A., and Q. Tang. 2005. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 17:638–642. [DOI] [PubMed] [Google Scholar]
- 36.Bruhl, H., J. Cihak, M.A. Schneider, J. Plachy, T. Rupp, I. Wenzel, M. Shakarami, S. Milz, J.W. Ellwart, M. Stangassinger, et al. 2004. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+ T cells. J. Immunol. 172:890–898. [DOI] [PubMed] [Google Scholar]
- 37.Huehn, J., and A. Hamann. 2005. Homing to suppress: address codes for Treg migration. Trends Immunol. 26:632–636. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 40.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 42.Klein, L., J. Emmerich, L. d'Cruz, K. Aschenbrenner, and K. Khazaie. 2005. Selection and behavior of CD4+ CD25+ T cells in vivo: lessons from T cell receptor transgenic models. Curr. Top. Microbiol. Immunol. 293:73–87. [DOI] [PubMed] [Google Scholar]
- 43.Debes, G.F., C.N. Arnold, A.J. Young, S. Krautwald, M. Lipp, J.B. Hay, and E.C. Butcher. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6:889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromley, S.K., S.Y. Thomas, and A.D. Luster. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895–901. [DOI] [PubMed] [Google Scholar]
- 45.Cyster, J.G. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23:127–159. [DOI] [PubMed] [Google Scholar]
- 46.Rot, A., and U.H. von Andrian. 2004. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22:891–928. [DOI] [PubMed] [Google Scholar]
- 47.Bystry, R.S., V. Aluvihare, K.A. Welch, M. Kallikourdis, and A.G. Betz. 2001. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2:1126–1132. [DOI] [PubMed] [Google Scholar]
- 48.Castellino, F., A.Y. Huang, G. Altan-Bonnet, S. Stoll, C. Scheinecker, and R.N. Germain. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 440:890–895. [DOI] [PubMed] [Google Scholar]
- 49.Tadokoro, C.E., G. Shakhar, S. Shen, Y. Ding, A.C. Lino, A. Maraver, J.J. Lafaille, and M.L. Dustin. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 203:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, Q., J.Y. Adams, A.J. Tooley, M. Bi, B.T. Fife, P. Serra, P. Santamaria, R.M. Locksley, M.F. Krummel, and J.A. Bluestone. 2006. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 7:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochando, J.C., A.C. Yopp, Y. Yang, A. Garin, Y. Li, P. Boros, J. Llodra, Y. Ding, S.A. Lira, N.R. Krieger, and J.S. Bromberg. 2005. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J. Immunol. 174:6993–7005. [DOI] [PubMed] [Google Scholar]
- 52.Ochando, J.C., C. Homma, Y. Yang, A. Hidalgo, A. Garin, F. Tacke, V. Angeli, Y. Li, P. Boros, Y. Ding, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662. [DOI] [PubMed] [Google Scholar]
- 53.Friedman, R.S., J. Jacobelli, and M.F. Krummel. 2006. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 7:1101–1108. [DOI] [PubMed] [Google Scholar]
- 54.Lim, H.W., H.E. Broxmeyer, and C.H. Kim. 2006. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J. Immunol. 177:840–851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.