Abstract
We describe a mouse model of fetal loss in factor V Leiden (FvL) mothers in which fetal loss is triggered when the maternal prothrombotic state coincides with fetal gene defects that reduce activation of the protein C anticoagulant pathway within the placenta. Fetal loss is caused by disruption of placental morphogenesis at the stage of labyrinth layer formation and occurs in the absence of overt placental thrombosis, infarction, or perfusion defects. Platelet depletion or elimination of protease-activated receptor 4 (Par4) from the mother allows normal placentation and prevents fetal loss. These findings establish a cause–effect relationship for the observed epidemiologic association between maternal FvL status and fetal loss and identify fetal gene defects as risk modifiers of pregnancy failure in prothrombotic mothers. Pregnancy failure is mediated by Par4-dependent activation of maternal platelets at the fetomaternal interface and likely involves a pathogenic pathway independent of occlusive thrombosis. Our results further demonstrate that the interaction of two given thrombosis risk factors produces markedly disparate consequences on disease manifestation (i.e., thrombosis or pregnancy loss), depending on the vascular bed in which this interaction occurs.
Recurrent pregnancy loss, defined as two or more spontaneous abortions, affects ∼5% of all women of reproductive age. Epidemiological studies suggest that inherited and acquired thrombophilia of the mother, such as that caused by the Leiden polymorphism in blood coagulation factor V, contributes to the pathogenesis of fetal loss, as well as other adverse pregnancy outcomes (1–3). These studies also demonstrate a high study-to-study variability of association strength, suggesting the existence of as yet uncharacterized risk modifiers. The pathogenesis of thrombophilia-associated fetal loss remains to be established. Evaluation of placental pathologies has not revealed a clear correlation between the prothrombotic status of the mother and the presence of villous infarcts, blood clots, or fibrin deposits in the placenta (4, 5), raising the question of whether thrombotic processes are indeed causative in thrombophilia-associated fetal loss or constitute an epiphenomenon. On the other hand, clinical trials suggest that heparin anticoagulation may indeed mitigate pregnancy failure in prothrombotic women (6–9). In view of the uncertain etiology of thrombophilia-associated pregnancy failure and the lack of established criteria for a more precise risk stratification enabling identification of truly at-risk pregnancies, the prophylactic anticoagulation of asymptomatic women and the risk-to-benefit balance of anticoagulation therapy during pregnancy are subjects of intense debate (10–12).
Factor V Leiden (FvL) polymorphism, which renders coagulation factor V refractory to inactivating proteolysis by activated protein C, has been associated with both early (first trimester) and late fetal loss in women (2). In experimental mouse models homozygous status for the FvL polymorphism elicits pronounced thrombophilia but is not associated with intrauterine fetal loss or impaired fecundity (13). In contrast, observations in animals lacking the receptor components of the protein C anticoagulant pathway, thrombomodulin (Thbd) and the endothelial protein C receptor (Procr), demonstrate that the integrity of this pathway within the placenta is essential for the maintenance of pregnancy (14–16). Thbd- or Procr-knockout mice die in utero because of a placental malfunction that results from a lack of these receptors on zygote-derived trophoblast cells that line the passage of maternal circulation in the placenta. Importantly, fetal loss in these animal models occurs in the absence of maternal thrombophilia, and the relevance of these knockout models for fetal loss in thrombophilic mothers remains unclear.
In this paper, we test the hypothesis that the hemostatic balance in the placental vasculature is determined by the combination of maternal and fetal factors, which cooperatively regulate the activity of the coagulation system at the interface of maternal blood and fetal trophoblasts. We test and demonstrate this effect in a mouse model of pregnancy disorder in FvL mothers in which fetal loss is triggered by a combination of maternal and fetal prothrombotic defects. We exploit this model to identify risk modifiers of fetal loss associated with maternal FvL status and to characterize the pathogenic mechanism underlying pregnancy failure.
RESULTS
Development of an animal model of fetal loss associated with maternal thrombophilia
Two genetically altered strains of mice were used: mice with APC resistance caused by the FvL polymorphism (13) and Thbd Pro mice with a reduced ability to activate protein C zymogen (17). Both strains are viable in the homozygous state for the mutated allele and exhibit biochemical evidence of chronic, low-grade thrombin generation leading to enhanced fibrin deposition in multiple tissues but do not develop spontaneous thrombosis on a C57BL/6 genetic background. Homozygous FvQQ and ThbdProPro mice exhibit normal fertility and fecundity (13, 17). These two strains of mice were intercrossed to produce FvQQThbdPro+ females in which we could assess pregnancy outcome as a function of Thbd expression on fetal trophoblast cells.
Offspring of FvQQThbdPro+ intercrosses showed a complete absence of FvQQThbdProPro animals and a significant underrepresentation of FvQQTMPro+ pups (Table I, row 1). FvQQThbdPro+ females mated to ThbdProPro males, likewise, did not support intrauterine development of FvQ+ThbdProPro embryos (Table I, row 2). In contrast, in the reverse genetic cross (i.e., ThbdProPro females mated with FvQQThbdPro+ males; Table I, row 3), viable FvQ+ThbdProPro pups were born (P = 0.009 compared with FvQQThbdPro+ females mated to ThbdProPro males). Thus, FvQQThbdPro+ females do not support uterine development of FvQ+ThbdProPro embryos. Of note, viable FvQQThbdProPro offspring were obtained from intercrosses of FvQ+ThbdProPro animals (Table I, row 4), showing that compound homozygosity of FvL and TMPro alleles in the embryo is compatible with normal development. The compound homozygous animals did not develop thrombosis over a 1-yr observation period.
Table I.
Pregnancy outcome in FvL mothers
| Parental genotype
|
Genotype of offspring
|
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Fv++
ThbdPro+ |
Fv++
ThbdProPro |
FvQ+
Thbd++ |
FvQ+
ThbdPro+ |
FvQ+
ThbdProPro |
FvQQ
Thbd++ |
FvQQ
ThbdPro+ |
FvQQ
ThbdProPro |
n |
| FvQQ ThbdPro+ | FvQQ ThbdPro+ | – | – | – | – | – | 27 | 19* | 0* | 46 |
| FvQQ ThbdPro+ | Fv++ ThbdProPro | – | – | – | 15 | 0* | – | – | – | 15 |
| Fv++ ThbdProPro | FvQQ ThbdPro+ | – | – | – | 22 | 10** | – | – | – | 32 |
| FvQ+ ThbdProPro | FvQ+ ThbdProPro | – | 12 | – | – | 30 | – | – | 9 | 51 |
| Fv++ ThbdProPro | Fv++ ThbdPro+ | 15 | 4** | – | – | – | – | – | – | 19 |
| Fv++ ThbdPro+ | Fv++ ThbdProPro | 14 | 12 | – | – | – | – | – | – | 26 |
| FvQQ Thbd++ | Fv++ ThbdPro+ | – | – | 41 | 22# | – | – | – | – | 63 |
p-values were calculated by χ2 analysis using the expected numbers based on Mendelian inheritance. *, P < 0.0003; **, P < 0.04; #, P < 0.02. n, total number of pups analyzed.
Although the data in the previous paragraph clearly demonstrates that ThbdProPro embryos are aborted in FvQQThbdPro+ females but can survive in ThbdProPro females, we noted partial fetal loss of ThbdProPro embryos in the latter, which is reflected in a lower frequency of this genotype than predicted by Mendelian inheritance (P = 0.003; Table I, row 3). Partial loss of ThbdProPro embryos was also observed when the ThbdProPro females were mated to ThbdPro/+ males (Table I, compare rows 5 and 6), demonstrating synergy between fetal and maternal Thbd deficiency in mediating fetal loss. To unequivocally demonstrate the effect of maternal FvL status on fetal loss in the absence of the ThbdPro mutation in the mother, we assessed the survival of ThbdPro+ embryos in FvQQ mothers with normal Thbd expression. Significantly reduced numbers of FvQ+ThbdPro+ mice were observed in the offspring of FvQQ females mated with ThbdPro+ males (Table I, row 7), demonstrating the loss of ThbdPro+ embryos in FvQQ mothers.
These results show that (a) fetal loss occurs in female mice with a latent prothrombotic state caused by homozygous status for either the FvQ or the ThbdPro allele; (b) the penetrance of fetal loss phenotype in these prothrombotic mothers is increased by reduced fetal Thbd function; and (c) the combination of the same risk factors within vascular compartments other than the placenta is compatible with normal development and hemostasis.
Disparate consequences of thrombosis risk factor interactions in the placenta and nonplacental vascular beds
To extend our findings on Thbd function in pregnancy to additional, putative risk modifiers of fetal loss, we investigated how reduced tissue factor pathway inhibitor (Tfpi) and Procr function on fetal trophoblast cells affect pregnancy outcomes in FvL mothers. Genetic suppression of tissue factor–mediated procoagulant activity expressed by trophoblast cells prevents the intrauterine loss of Thbd−/−, as well as Procr−/− embryos, suggesting that the level of tissue factor inhibition by Tfpi on trophoblast cells may be a critical modulator of the risk of fetal loss in prothrombotic mothers (15, 16). The combination of heterozygous Tfpi deficiency with homozygous carrier status for the FvL mutation in the systemic vasculature causes fatal thrombosis shortly after birth in FvQQTfpi+/− animals (18).
An analysis of crosses between Tfpi+/− males and FvQQ females showed a trend of reduced survival of Tfpi+/− embryos in FvQQ mothers (62 FvQ+ Tfpi+/− vs. 80 FvQ+ Tfpi+/+), but the skewing of genotype distribution did not reach statistical significance. No intrauterine loss of Tfpi+/− embryos was observed in FvQQThbdPro+ mothers mated to Tfpi+/− males (5 FvQ+Thbd+/+Tfpi+/+, 6 FvQ+ThbdPro/+Tfpi+/+, 7 FvQ+Thbd+/+Tfpi+/−, and 3 FvQ+ThbdPro/+Tfpi+/−; n = 21). In conclusion, reduced Tfpi expression at the blood– trophoblast interface within the placental vascular bed does not significantly increase the risk of pregnancy failure in FvL mothers.
Procr, like Tfpi, is coexpressed with Thbd by fetal trophoblast cells (19) and facilitates Thbd-mediated activation of protein C. To examine whether reduced Procr expression by trophoblast cells causes pregnancy failure in FvL mothers, we used “low Procr” mice (i.e., homozygous Procrδδ). These mice exhibit severely reduced Procr expression yet develop and reproduce normally (20). FvQ+Procrδδ pups were significantly underrepresented in the offspring of FvQQProcrδ+ females mated to Procrδδ males (5 FvQ+Procrδδ vs. 18 FvQ+Procrδ+; n = 23; P < 0.007). In contrast, intercrosses of FvQ+Procrδδ mice yielded normal frequencies of FvQ+Procrδδ and FvQQProcrδδ pups (12 FvQ+Procrδδ, 5 FvQQProcrδδ, and 4 Fv++Procrδδ; n = 21), which remained completely viable and normal as adults. Therefore, akin to the ThbdPro mutation, reduced fetal Procr expression increases the risk of pregnancy failure in homozygous FvL mothers, whereas the same interaction does not produce a thrombotic pathology within the systemic vasculature.
Placental pathology of pregnancy failure in FvL females
Progeny from FvQQThbdPro+ intercrosses were analyzed at E10.5, E12.5, and E17.5 to determine the time of pregnancy failure and the associated pathology of FvQQThbdProPro embryos (Table II). Seven out of nine FvQQThbdProPro embryos were resorbed or severely developmentally retarded by E10.5; only one ThbdProPro embryo was present at E12.5, and none were at E17.5 (Table II). In timed pregnancies of FvQQThbdPro+ females mated to ThbdProPro males (Table II), FvQ+ThbdProPro embryos were present in normal numbers at E9.5, but some of these were already smaller or delayed in developmental progress at this time (Fig. 1, a and b). By E11.5, all FvQ+ThbdProPro embryos were in a stage of advanced decay (Fig. 1, c and d). These findings indicate that, in FvQQThbdPro+ mothers, ThbdProPro embryos exhibit growth defects as early as E9.5 and that fetal loss is observed as early as E10.5. Although some embryos appear normal at this time, eventually all ThbdProPro embryos in FvQQThbdPro+ mothers are eliminated before birth, and this occurs irrespective of the FvL status (homozygous or heterozygous) of the embryo. In contrast to ThbdProPro embryos, ThbdPro+ embryos are present in normal numbers and appearance at E10.5 but are under-represented at E12.5.
Table II.
Intrauterine survival of ThbdProPro embryos in FvQQThbdPro+ mothers
| Breeding pairs
|
|
Genotype of embryos
|
|
|||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Stage of embryonic development |
FvQQ
Thbd++ |
FvQQ
ThbdPro+ |
FvQQ
ThbdProPro |
FvQ+
ThbdPro+ |
FvQ+
ThbdProPro |
n |
| FvQQ ThbdPro+ | FvQQ ThbdPro+ | E10.5 | 10 | 23 | 2* | – | – | 35 |
| E12.5 | 18 | 13* | 1* | – | – | 32 | ||
| E17.5 | 9 | 11 | 0* | – | – | 20 | ||
| FvQQ ThbdPro+ | Fv++ ThbdProPro | E9.5 | – | – | – | 5 | 7 | 12 |
| E11.5 | – | – | – | 12 | 0* | 12 | ||
p-values were calculated by χ2 analysis using the expected numbers based on Mendelian inheritance. *, P < 0.004. Embryos in advanced stages of degradation were excluded from the analysis. n, total number of pups analyzed.
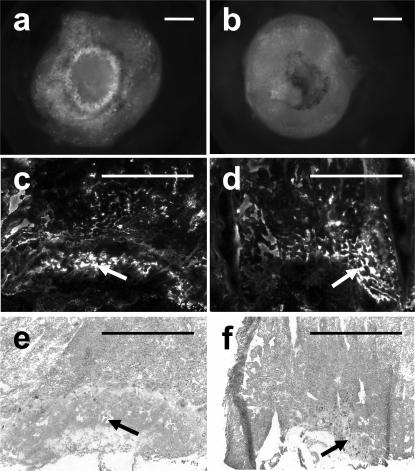
Figure 1.
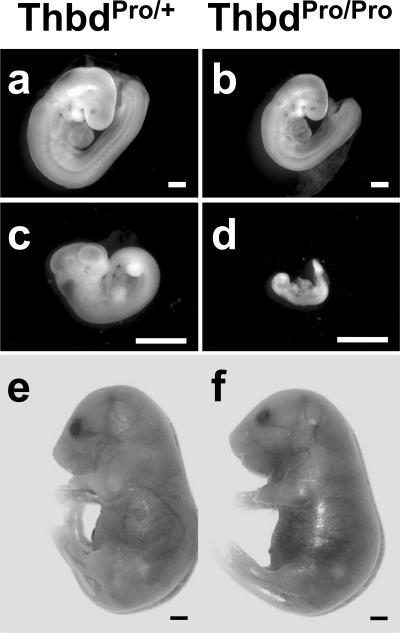
Phenotype of ThbdProPro embryos in FVQQThbdPro+ mothers. (a, c, and e) ThbdPro+ littermates at E9.5, E12.5, and E18.5, respectively, of ThbdProPro embryos (b, d, and f). At E9.5, some ThbdProPro embryos are smaller (b). By E11.5, most ThbdProPro embryos are in an advanced stage of decay (d). Platelet depletion of the mother with anti-GP1bα antibody treatment (e and f) results in normal-appearing ThbdProPro embryos. Pictures were taken at different magnifications. Bars, 1 mm.
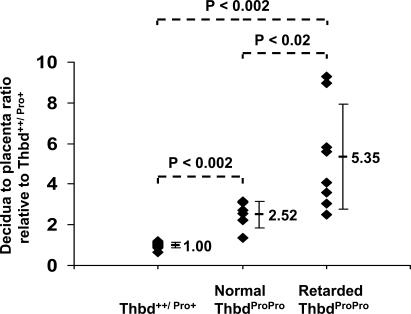
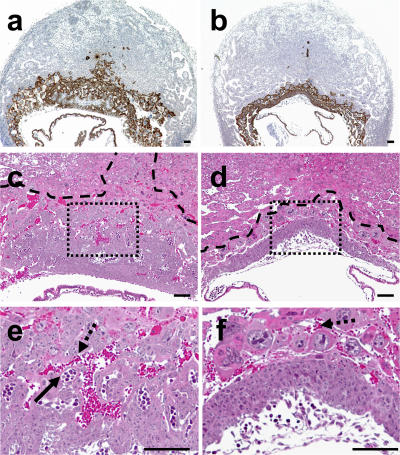
At E9.5, uteroplacental units corresponding to FvQ+ThbdProPro embryos tended to be smaller in comparison with littermate controls. Morphometric analysis of histological sections through these units showed a significantly higher decidual/placental ratio in units corresponding to apparently normal ThbdProPro embryos compared with ThbdPro+ and Thbd+/+ littermates, and this difference was more pronounced in units corresponding to retarded ThbdProPro embryos (Fig. 2 and Fig. 3, a and b). The smaller placental size could be attributed to a failure to form or expand the labyrinth layer of the placenta (Fig. 3, c–f). At E9.5, the placenta of all retarded ThbdProPro embryos (n = 8) had undergone chorioallantoic fusion, but the chorioallantoic surface had remained flat with little or no branching and an absence of fetal and maternal vessel in-growths, resulting in a distinct lack of a well-formed labyrinth layer. Of note, the same defect in labyrinth formation was also observed in three out of six placentas of apparently normal ThbdProPro embryos, indicating that the observed placental pathology precedes embryonic growth retardation. The placentas of other normal-appearing ThbdProPro embryos had begun branching morphogenesis to form a labyrinth and appeared similar to placentas of ThbdPro+ littermates. This observation, together with the observed survival of occasional ThbdProPro embryos to a late developmental stage (E12.5), suggests that the defect may be in progression rather than in the initiation of chorioallantoic morphogenesis.
Figure 2.
Placental size of ThbdProPro embryos relative to controls. Each symbol represents a measurement from a single fetoplacental unit. All litters were analyzed at E9.5. Mean ratios and SDs are shown.
Figure 3.
Placental phenotype of ThbdProPro embryos and littermate controls in FvQQThbdPro+ mothers. Histological sections through placentas of ThbdPro+ (a, c, and e) and a phenotypically normal ThbdProPro (b, d, and f) embryo at E9.5. a and b show cytokeratin-expressing trophoblast cells (brown staining). The placenta of ThbdProPro embryos (stained area) is smaller than that of ThbdPro+ embryos. c and d are higher magnification images of hematoxylin and eosin–stained sections adjacent to a and b, respectively. e and f correspond to boxes in c and d, respectively. Dashed lines in c and d mark the border of the placenta and decidua, as determined by cytokeratin staining. ThbdPro+ placentas (c and e) have formed a labyrinth characterized by maternal (bright red enucleated maternal red blood cells; dashed arrows) and fetal (purple hematoxylin-stained nucleated fetal red blood cells; continuous arrow) blood spaces. The labyrinth is distinctly absent in the placentas of littermate ThbdProPro embryos (d and f). Bars, 0.1 mm.
Fetal loss is not associated with placental thrombosis
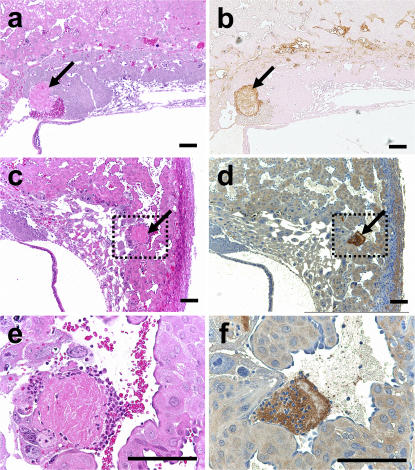
Plasma levels of thrombin–antithrombin (TAT) complexes and D-dimer were only mildly altered in FVQQTMPro+ mice and were not predictive of pregnancy failure (respective values: TAT, 4.52 ± 2.07 vs. 4.8 ± 0.92 μgapparent/l [wild type vs. FvQQThbdPro+; mean ± SD; P = 0.74]; D-dimer, 7.95 ± 4.02 vs. 10.12 ± 3.47 μgapparent/l [mean ± SD; P = 0.28]). TAT levels of pregnant FvQQThbdPro+ animals at 9.5 days post coitum (dpc) were also similar to gestation stage–matched controls (respective values: 8.21 ± 2.44 vs. 8.46 ± 6.98 μgapparent/l [wild type vs. FvQQThbdPro+; mean ± SD; P = 0.95]). The numbers, sizes, location, and general appearance of thrombi were similar in uteroplacental units corresponding to Thbd++, normal ThbdProPro, and retarded ThbdProPro embryos (Thbd++, 12 ± 12 clots; normal ThbdProPro, 13 ± 7 clots; retarded ThbdProPro, 7 ± 5 clots [all mean ± SD]). Thrombi identified by hematoxylin and eosin staining were validated by immunostaining with fibrin(ogen) and with p-selectin antibodies identifying platelet aggregates (Fig. 4). Spiral arteries within the deciduas were free of thrombi in wild-type and mutant fetoplacental units (not depicted). To detect blood perfusion defects secondary to potential blood clots in vessels not included in our survey and to rule out other causes of impaired blood supply to the placenta, FITC-dextran was injected in the maternal circulation. The fluorescent tracer accumulated in the placenta of wild-type pregnancies in a characteristic ring-like structure formed by maternal blood in the labyrinth. Congruent with the developmental retardation and anatomical absence of labyrinth, this structure was absent in the placenta of ThbdProPro embryos (Fig. 5). FITC-dextran could, however, be readily visualized in decidual and placental blood spaces in FVQQThbdPro+ mothers irrespective of embryonic genotype. These data support the notion that fetal loss in the described mouse model cannot be attributed to occlusive thrombosis and impaired perfusion of the placenta.
Figure 4.
Placental thrombosis in FvL mice. The number of placental thrombi was similar in placentas of wild-type and ThbdProPro embryos (see Results). Thrombi were identified on serial sections by hematoxylin and eosin staining (a, c, and e), and immunostaining with antifibrinogen antibodies (b) and anti–p-selectin antibodies (d and f). e and f are enlarged views of the boxed areas in c and d. Arrows indicate clots. Bars, 0.1 mm.
Figure 5.
Assessment of decidual and placental vascular patency by FITC-dextran infusion. Whole-mount (a and b) and sections (c–f) of decidual-placental units corresponding to a wild-type embryo in a wild-type mother (a, c, and e) and a FvQ+ThbdProPro embryo in a FvQQThbdPro+ mother (b, d, and f) are shown. From the fetal aspect of the placenta, a characteristic ring of maternal placental vasculature, marked by FITC-dextran, is observed in wild-type pregnancies but not in placenta of a FvQ+ThbdProPro embryo in a FvQQThbdPro+ mother (a and b). Decidual and placental regions in both types of pregnancies are perfused with FITC-dextran (c and d). Hematoxylin and eosin–stained sections adjacent to c and d are shown in e and f. Arrows show maternal blood spaces in the placenta. Bars, 1 mm.
Fetal loss in FvL mice is mediated by the protease-activated receptor 4 (Par4) on maternal platelets
We examined the role of platelets in the pregnancy disorder of FvL mothers by immunodepleting maternal platelets with anti-GP1bα antibodies. Platelet depletion initiated at E7.5 restored normal development of ThbdProPro embryos in FvQQ ThbdPro+ mothers mated to ThbdProPro males (13 ThbdPro+ and 18 ThbdProPro determined at E11.5 or later; n = 31 from four pregnancies). Treatment with nonimmune IgG did not prevent loss of ThbdProPro embryos (9 ThbdPro+ and 1 ThbdProPro; n = 10 from three pregnancies; P = 0.011 indicates a significant difference from the numbers expected by Mendelian inheritance). We observed that loss of ThbdProPro embryos could not be prevented when platelet depletion was initiated at E9.5 (17 ThbdPro+ and 2 ThbdProPro; n = 19 from four pregnancies; P = 0.0006 indicates a significant difference from the numbers expected by Mendelian inheritance). These data demonstrate that maternal platelets mediate fetal loss of ThbdProPro mice in FvQQ ThbdPro+ mothers and suggest that the initial platelet-mediated insult to the placenta occurs as early as E7.5.
Par4 is required for thrombin-mediated activation of mouse platelets. Using triallelic crosses, we generated FvQQPar4−/−ThbdPro+ animals to assess the role of Par4 in mediating pregnancy disorder. FvQQPar4−/−ThbdPro+ females mated with ThbdProPro males produced viable FvQ+Par4+/− ThbdProPro term embryos (15 ThbdProPro and 15 ThbdPro+; n = 30) that resembled their ThbdPro+ littermates in appearance and size. These data demonstrate that maternal Par4 deficiency overcomes the developmental block of ThbdProPro embryos in FvQQThbdPro+ mothers.
DISCUSSION
Our findings reveal several new aspects of the association between maternal thrombophilia and fetal loss. First, it lends direct experimental support to the notion that the FvL carrier status of the mother increases the risk of pregnancy failure, thereby establishing a cause–effect relationship for the epidemiologic association. Second, it demonstrates that hemostatic regulators on fetal trophoblast cells are dominant risk modifiers of fetal loss in FvL carriers. Thbd and Procr, allelic variants of which are suspected risk modifiers in human thrombophilia (21–25), are experimentally validated in the current mouse model as modulators of pregnancy success in FvL mothers. The experimental demonstration of a synergistic adverse effect of maternal and fetal prothrombotic mutations in causing pregnancy failure has direct implications for the risk stratification of patient populations. It also validates the approach taken in the “NOHA first” study (26), which systematically includes analysis of the paternal (in lieu of the fetal) genome to more accurately interpret the epidemiological association between maternal thrombophilia and fetal loss. Third, it establishes a novel and unique animal model of fetal loss associated with maternal inherited thrombophilia. Using this animal model, we have identified the Par4 receptor on maternal platelets as a key component of the pathogenic mechanism underlying fetal loss in FvL mice and have determined that, at least in mice, fetal loss occurs in the absence of overt thrombosis. The relevance of our observations for human FvL carriers remains to be determined.
Based on placental pathology, the pathogenesis of FvL-associated fetal loss in mice appears distinct from the complement-driven mechanism operating in fetal loss induced by antiphospholipid antibodies (27). Consistent with this notion, platelet depletion did not ameliorate fetal loss in the antiphospholipid model, and anti-C5 antibody treatment was insufficient to rescue fetal loss in the FvL model (unpublished data).
In striking contrast to fetal loss caused by reduced function or expression of Thbd or Procr on trophoblast cells in FvL mothers, the combination of the same risk factors does not cause acute thrombosis in the systemic vasculature. The reverse is true for Tfpi, where lethal thrombosis ensues when heterozygous Tfpi deficiency is combined with FvL homozygosity in the same animal (18) but is inconsequential with respect to pregnancy outcome. The absence of a measurable consequence of embryonic Tfpi heterozygosity on pregnancy outcome in FvL mothers may reflect a substantial contribution of maternally derived plasma Tfpi in local hemostasis at the fetomaternal interface. Collectively, these observations demonstrate a marked discrepancy of consequences (i.e., thrombosis vs. pregnancy loss) triggered by the interaction of thrombosis risk factors in different vascular beds and suggest that the effect of a given risk modifier on venous or arterial thrombosis is not necessarily predictive of pregnancy outcome.
The placental abnormalities of embryos with reduced Thbd activity carried by FvL mothers and those associated with Thbd-null embryos (15) share characteristic features suggestive of a common underlying pathogenic mechanism. Both occur in the absence of overt thrombosis. Embryos with reduced Thbd activity in FvL mothers can be rescued by platelet depletion at E7.5 but not at E9.5, indicating that the pathogenic insult has already occurred by E9.5. Growth retardation of Thbd-null embryos is observed as early as E8.5, suggesting a similar time of pathogenic insult. In both cases, placental morphogenesis is disrupted at the time of labyrinth layer formation. The combined results from the analysis of fetal loss in these two models suggest that the expression of tissue factor on placental trophoblast cells provides a constitutive procoagulant stimulus in the placental vascular bed (15, 19). The protein C anticoagulant pathway sustained by expression of Thbd and Procr on trophoblast cells appears to be necessary to suppress this stimulus. Localized disruption of this pathway at the fetomaternal interface—either caused by the complete lack of Thbd or Procr from trophoblast cells or a combination of fetal and maternal risk factors—permits amplification of the coagulation reaction and formation of activated clotting factors, including thrombin. We find that platelets and Par4 are critical mediators of placental failure. The impairment of placental growth and morphogenesis is therefore caused by a process similar to the initial tissue factor– and thrombin-dependent stages of arterial thrombosis, likely involving platelet activation, but does not require thrombotic occlusion. Possible explanations for the lack of occlusive thrombi in the setting of an active coagulation cascade include inhibition of ADP-driven platelet aggregation caused by abundant expression of ecto-ATPDase/CD39 on the trophoblast cell surface (19).
The nature of the mechanism by which activation of maternal platelets causes pregnancy failure is unknown, but the possibility that platelet-released factors negatively influence placental development or function is an obvious candidate. Peptide mediators with (anti)angiogenic activity have been suggested to alter trophoblast function and contribute to the etiology of pregnancy disorders such as preeclampsia (28–30). Of note, platelets have been shown to adhere to endovascular trophoblasts within the lumen of spiral arteries in normal pregnancy (30), indicating that the recruitment of platelets to the fetomaternal interface is a physiological process that links platelet function to the regulation of placental development.
It is noteworthy that fetal loss in FvL animals occurs in the absence of overt thrombosis in the mother's systemic vasculature and is caused by a highly localized defect precipitated by the fetal genotype, which affects individual placental beds. Local dysregulation of the protein C anticoagulant pathway at the fetomaternal interface alone (Thbd−/− embryos) is also sufficient to cause fetal loss in a mother with near-normal hemostatic function (Thbd+/− mothers) (15). Complete disruption of the protein C pathway (≤3% of protein C), on the other hand, is accompanied by active thrombotic disease, consumptive coagulopathy, and a severe proinflammatory phenotype (31). These animals suffer fetal genotype-independent early pregnancy loss (E6.5) with a markedly different disease etiology caused by the pronounced secondary effects of acute protein C deficiency in the mother. Increasing maternal protein C to 18% of wild-type levels overcomes systemic thrombosis and inflammation and restores normal pregnancies (31).
In summary, we have presented animal models of placental malfunction and fetal loss secondary to inherited defects in the protein C anticoagulant pathway, including a clinically relevant gene defect (FvL) in the mother. The loss of ThbdProPro embryos in FvL mothers correlates with the early fetal loss observed in women with the Leiden mutation. We also observed a partial loss of embryos with a heterozygous defect in Thbd function (ThbdPro+) later in pregnancy (around E12.5, correlating with Leiden-associated late fetal loss in women) that we did not characterize further in this study. The early fetal loss in FvL mice is preceded by disrupted placental growth and morphogenesis during developmental events that lead to the formation of a functional labyrinth and is mediated through a platelet and Par4 receptor–driven mechanism but does not involve formation of occlusive thrombi in the placenta. The described animal model of thrombophilia-associated fetal loss provides an opportunity to test the efficacy of antithrombotic treatments and, possibly, of antiplatelet treatments targeting the recruitment and activation of platelets in the placental vascular bed.
MATERIALS AND METHODS
Mice.
Animal experiments were conducted according to standards and procedures approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Fv Leiden (provided by D. Ginsburg, University of Michigan, Ann Arbor, MI), Thbd Pro, Procr∂/∂ (provided by F. Castellino, University of Notre Dame, Notre Dame, IN), and TFPI+/− (provided by G. Broze, Washington University School of Medicine, Saint Louis, MO) mice have been previously described (13, 17, 20, 32). These mice were maintained on C57BL/6 genetic background.
Histology.
Embryonic development was assessed from dpc, assuming midday of plug as 0.5 dpc, and as previously described (15). Dissections were performed, and pictures of embryos were taken under a microscope (SMZ-U; Nikon) equipped with a color camera (SPOT Insight; Diagnostic Instruments) and SPOT software (version 3.2.4; Diagnostic Instruments). 7-μm serial sections of formalin-fixed (Sigma-Aldrich), paraffin-embedded tissues were stained with hematoxylin and eosin for general morphology, identification of thrombi, and morphometric analysis. Thrombi were validated by immunostaining with antibodies that recognize fibrinogen (DakoCytomation) and p-selectin (Santa Cruz Biotechnology, Inc.). Immunostaining with antibodies against cytokeratin (DakoCytomation) was done after antigen retrieval with 40 μg/ml proteinase K in 10 mM Tris, pH 7.5, at 37°C for 30 min. ImageJ software (version 1.36b; National Institutes of Health) was used for morphometric analysis. Sizes of the decidua and placenta were measured on every 30th section, and the totals were used to arrive at decidua/placenta ratio.
Perfusion studies and TAT and D-dimer measurements.
Placental perfusion was examined by injecting pregnant females with 100 μl of 25 mg/ml FITC-labeled dextran (mol wt 200 × 104; Sigma-Aldrich) via the tail vein at 9.5 dpc. After 15 min, the uteroplacental units were dissected under a microscope (SteREO Discovery.V12 PentaFluar S; Carl Zeiss MicroImaging, Inc.) equipped with a GFP filter cube (excitation, BP470/40; emission, BP525/50) and a camera (Axiocam MRc5; Carl Zeiss MicroImaging, Inc.). The fetal aspect of the placenta was photographed using Axiovision software (version 4.5; Carl Zeiss MicroImaging, Inc.). The uteroplacental units were embedded in tissue-freezing medium (Triangle Biomedical Sciences) and flash frozen in a dry ice–ethanol bath. Serial sections of the frozen units were examined and photographed under a microscope (Eclipse TE200; Nikon) equipped with a camera (Photometrics CoolSNAP ES; Roper Scientific, Inc.) using Metamorph software (version 6.2r6; Universal Imaging Corp.). D-dimer and TAT levels were measured as previously described (33).
Platelet depletions.
For platelet depletion, a mixture of anti-GPIbα rat monoclonal antibodies (34) or polyclonal nonimmune IgG (control; R300 or C301, Emfret Analytics) was injected via the tail vein at 4 μg/g body weight. Anti-GPIbα antibodies depleted 95% of the platelets within 1 h, as counted manually and with an automated cell counter (ABX Diagnostics). Injections were repeated on the fourth day.
Statistical analysis.
The significance of survival differences between groups was determined using the χ2 analysis. The Student's t test (two-tailed with unequal variance) was used for all other statistical analysis. P < 0.05 was considered significant.
Acknowledgments
R. Sood conceptually designed, executed, and interpreted experiments and wrote the manuscript; M. Zogg maintained the animal colonies; R.J. Westrick contributed to the analysis of TFPI-deficient mice; Y.-h. Guo contributed to embryo analysis from FvQQThbdPro+ intercrosses; E.J. Kerschen genotyped Procrδ animals; G. Girardi, J.E. Salmon, and S.R. Coughlin provided critical reagents and assisted in writing the manuscript; and H. Weiler conceptually designed and performed experiments and wrote the manuscript.
We thank Barbara Fleming for preparing histological sections, Dr. Francis Castellino for making endothelial Procr–deficient mice available, Dr. George Broze for TFPI-deficient mice, Dr. Lynette Sholl for critiquing the manuscript, and Dr. David Ginsburg for providing FvL mice and for critiquing the manuscript.
This work was supported by grants HL60655 (to H. Weiler); HL44907 and HL65590 (to S.R. Coughlin); and AI055007 (to J.E. Salmon and G. Girardi), as well as an American Heart Association predoctoral fellowship (to R.J. Westrick).
The authors have no conflicting financial interests.
Abbreviations used: dpc, days post coitum; FvL, factor V Leiden; Par4, protease-activated receptor 4; Procr, endothelial protein C receptor; TAT, thrombin–antithrombin; Tfpi, tissue factor pathway inhibitor; Thbd, thrombomodulin.
References
- 1.Dudding, T.E., and J. Attia. 2004. The association between adverse pregnancy outcomes and maternal factor V Leiden genotype: a meta-analysis. Thromb. Haemost. 91:700–711. [DOI] [PubMed] [Google Scholar]
- 2.Rey, E., S.R. Kahn, M. David, and I. Shrier. 2003. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 361:901–908. [DOI] [PubMed] [Google Scholar]
- 3.Kovalevsky, G., C.R. Gracia, J.A. Berlin, M.D. Sammel, and K.T. Barnhart. 2004. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: a meta-analysis. Arch. Intern. Med. 164:558–563. [DOI] [PubMed] [Google Scholar]
- 4.Mousa, H.A., and Z. Alfirevic. 2000. Do placental lesions reflect thrombophilia state in women with adverse pregnancy outcome? Hum. Reprod. 15:1830–1833. [DOI] [PubMed] [Google Scholar]
- 5.Sikkema, J.M., A. Franx, H.W. Bruinse, N.G. van der Wijk, H.W. de Valk, and P.G. Nikkels. 2002. Placental pathology in early onset pre-eclampsia and intra-uterine growth restriction in women with and without thrombophilia. Placenta. 23:337–342. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, B., R. Hoffman, H. Carp, M. Dulitsky, and J. Younis. 2005. Efficacy and safety of two doses of enoxaparin in women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study. J. Thromb. Haemost. 3:227–229. [DOI] [PubMed] [Google Scholar]
- 7.Gris, J.C., E. Mercier, I. Quere, G. Lavigne-Lissalde, E. Cochery-Nouvellon, M. Hoffet, S. Ripart-Neveu, M.L. Tailland, M. Dauzat, and P. Mares. 2004. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood. 103:3695–3699. [DOI] [PubMed] [Google Scholar]
- 8.Carp, H., M. Dolitzky, and A. Inbal. 2003. Thromboprophylaxis improves the live birth rate in women with consecutive recurrent miscarriages and hereditary thrombophilia. J. Thromb. Haemost. 1:433–438. [DOI] [PubMed] [Google Scholar]
- 9.Sanson, B.J., A.W. Lensing, M.H. Prins, J.S. Ginsberg, Z.S. Barkagan, E. Lavenne-Pardonge, B. Brenner, M. Dulitzky, J.D. Nielsen, Z. Boda, et al. 1999. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb. Haemost. 81:668–672. [PubMed] [Google Scholar]
- 10.Walker, I.D., J.L. Kujovich, I.A. Greer, E. Rey, M. David, J.E. Salmon, B.J. Hunt, R.B. Zotz, A. Gerhardt, R.E. Scharf, et al. 2005. The use of LMWH in pregnancies at risk: new evidence or perception? J. Thromb. Haemost. 3:778–793. [DOI] [PubMed] [Google Scholar]
- 11.Gris, J.C., and P. Mares. 2005. The long and winding road…towards LMWH for pregnancy loss. J. Thromb. Haemost. 3:224–226. [DOI] [PubMed] [Google Scholar]
- 12.Lindqvist, P.G., and J. Merlo. 2005. Low molecular weight heparin for repeated pregnancy loss: is it based on solid evidence? J. Thromb. Haemost. 3:221–223. [DOI] [PubMed] [Google Scholar]
- 13.Cui, J., D.T. Eitzman, R.J. Westrick, P.D. Christie, Z.J. Xu, A.Y. Yang, A.A. Purkayastha, T.L. Yang, A.L. Metz, K.P. Gallagher, et al. 2000. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 96:4222–4226. [PubMed] [Google Scholar]
- 14.Isermann, B., S.B. Hendrickson, K. Hutley, M. Wing, and H. Weiler. 2001. Tissue-restricted expression of thrombomodulin in the placenta rescues thrombomodulin-deficient mice from early lethality and reveals a secondary developmental block. Development. 128:827–838. [DOI] [PubMed] [Google Scholar]
- 15.Isermann, B., R. Sood, R. Pawlinski, M. Zogg, S. Kalloway, J.L. Degen, N. Mackman, and H. Weiler. 2003. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat. Med. 9:331–337. [DOI] [PubMed] [Google Scholar]
- 16.Li, W., X. Zheng, J.M. Gu, G.L. Ferrell, M. Brady, N.L. Esmon, and C.T. Esmon. 2005. Extra-embryonic expression of EPCR is essential for embryonic viability. Blood. 106:2716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiler-Guettler, H., P.D. Christie, D.L. Beeler, A.M. Healy, W.W. Hancock, H. Rayburn, J.M. Edelberg, and R.D. Rosenberg. 1998. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J. Clin. Invest. 101:1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eitzman, D.T., R.J. Westrick, X. Bi, S.L. Manning, J.E. Wilkinson, G.J. Broze, and D. Ginsburg. 2002. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor V Leiden. Circulation. 105:2139–2142. [DOI] [PubMed] [Google Scholar]
- 19.Sood, R., S. Kalloway, A.E. Mast, C.J. Hillard, and H. Weiler. 2006. Fetomaternal cross talk in the placental vascular bed: control of coagulation by trophoblast cells. Blood. 107:3173–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellino, F.J., Z. Liang, S.P. Volkir, E. Haalboom, J.A. Martin, M.J. Sandoval-Cooper, and E.D. Rosen. 2002. Mice with a severe deficiency of the endothelial protein C receptor gene develop, survive, and reproduce normally, and do not present with enhanced arterial thrombosis after challenge. Thromb. Haemost. 88:462–472. [PubMed] [Google Scholar]
- 21.Ohlin, A.K., L. Norlund, and R.A. Marlar. 1997. Thrombomodulin gene variations and thromboembolic disease. Thromb. Haemost. 78:396–400. [PubMed] [Google Scholar]
- 22.Le Flem, L., V. Picard, J. Emmerich, S. Gandrille, J.N. Fiessinger, M. Aiach, and M. Alhenc-Gelas. 1999. Mutations in promoter region of thrombomodulin and venous thromboembolic disease. Arterioscler. Thromb. Vasc. Biol. 19:1098–1104. [DOI] [PubMed] [Google Scholar]
- 23.Biguzzi, E., G. Merati, P.C. Liaw, P. Bucciarelli, N. Oganesyan, D. Qu, J.M. Gu, R. Fetiveau, C.T. Esmon, P.M. Mannucci, and E.M. Faioni. 2001. A 23bp insertion in the endothelial protein C receptor (EPCR) gene impairs EPCR function. Thromb. Haemost. 86:945–948. [PubMed] [Google Scholar]
- 24.von Depka, M., A. Czwalinna, R. Eisert, C. Wermes, I. Scharrer, A. Ganser, and S. Ehrenforth. 2001. Prevalence of a 23bp insertion in exon 3 of the endothelial cell protein C receptor gene in venous thrombophilia. Thromb. Haemost. 86:1360–1362. [PubMed] [Google Scholar]
- 25.Kunz, G., A.K. Ohlin, A. Adami, B. Zoller, P. Svensson, and D.A. Lane. 2002. Naturally occurring mutations in the thrombomodulin gene leading to impaired expression and function. Blood. 99:3646–3653. [DOI] [PubMed] [Google Scholar]
- 26.Lissalde-Lavigne, G., P. Fabbro-Peray, E. Cochery-Nouvellon, E. Mercier, S. Ripart-Neveu, J.P. Balducchi, J.P. Daures, T. Perneger, I. Quere, M. Dauzat, et al. 2005. Factor V Leiden and prothrombin G20210A polymorphisms as risk factors for miscarriage during a first intended pregnancy: the matched case-control ‘NOHA first’ study. J. Thromb. Haemost. 3:2178–2184. [DOI] [PubMed] [Google Scholar]
- 27.Girardi, G., J. Berman, P. Redecha, L. Spruce, J.M. Thurman, D. Kraus, T.J. Hollmann, P. Casali, M.C. Caroll, R.A. Wetsel, et al. 2003. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112:1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, Y., M. McMaster, K. Woo, M. Janatpour, J. Perry, T. Karpanen, K. Alitalo, C. Damsky, and S.J. Fisher. 2002. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 160:1405–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, L., M.D. Hollenberg, and J.L. Wallace. 2001. Thrombin-induced platelet endostatin release is blocked by a proteinase activated receptor-4 (PAR4) antagonist. Br. J. Pharmacol. 134:701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato, Y., H. Fujiwara, B.X. Zeng, T. Higuchi, S. Yoshioka, and S. Fujii. 2005. Platelet-derived soluble factors induce human extravillous trophoblast migration and differentiation: platelets are a possible regulator of trophoblast infiltration into maternal spiral arteries. Blood. 106:428–435. [DOI] [PubMed] [Google Scholar]
- 31.Lay, A.J., Z. Liang, E.D. Rosen, and F.J. Castellino. 2005. Mice with a severe deficiency in protein C display prothrombotic and proinflammatory phenotypes and compromised maternal reproductive capabilities. J. Clin. Invest. 115:1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, Z.F., D. Higuchi, N. Lasky, and G.J. Broze Jr. 1997. Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood. 90:944–951. [PubMed] [Google Scholar]
- 33.Isermann, B., S.B. Hendrickson, M. Zogg, M. Wing, M. Cummiskey, Y.Y. Kisanuki, M. Yanagisawa, and H. Weiler. 2001. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J. Clin. Invest. 108:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergmeier, W., K. Rackebrandt, W. Schroder, H. Zirngibl, and B. Nieswandt. 2000. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 95:886–893. [PubMed] [Google Scholar]