Abstract
Activation of T cells induces the production of T cell growth and survival factor interleukin (IL) 2. Regulatory T cells intrinsically fail to induce IL-2 expression upon activation and can suppress IL-2 production in conventional T cells. Thus, the control of IL-2 expression is critically important to T cell immune responses, yet the mechanisms remain incompletely understood. Nuclear factor (NF) 90 is a zinc-finger DNA- and double-stranded RNA-binding protein subunit that binds specifically to the antigen receptor response element (ARRE)/NF of activated T cells target sequence in the IL-2 proximal promoter. Inducible binding of NF90 to the IL-2 promoter in vivo is shown by chromatin immunoprecipitation. NF90 gene-targeted mice exhibit perinatal lethality. Compared with newborn NF90+/+ mice, newborn NF90−/− mice demonstrate severe impairment of IL-2 expression. Compared with wild-type cells, T cells deficient in NF90 are impaired in ARRE and IL-2 transcriptional activation and IL-2 mRNA stabilization. Fetal liver cells from NF90 gene-targeted mice were transplanted into irradiated adult recombination activating gene (RAG)–2−/− and IL-2Rγ−/− mice deficient in T cells, B cells, and natural killer cells. NF90+/+- and NF90−/−-RAG chimeric mice showed grossly normal repopulation of the thymus and spleen, but only NF90−/− T cells were severely impaired in IL-2 gene expression. Compared with littermates, NF90−/− RAG chimeric mice exhibited profound T cell lymphocytopenia in the peripheral circulation. Thus, NF90 regulates inducible IL-2 transcription, mRNA stability, and gene expression in T cells and represents a novel therapeutic target for the modulation of T cell immune responses.
Nuclear factor (NF) 90 and NF45 are each zinc-finger DNA- and RNA-binding proteins purified and cloned from activated T cells based on their specific binding to the A-T–rich purine-box/antigen receptor response element (ARRE)/NFAT site in the IL-2 promoter (1, 2). NF90 also contains two double-stranded RNA-binding motifs that bind IL-2 and other RNAs (3–5). Antisera to NF90 and NF45 specifically inhibited ARRE/NFAT DNA binding and in vitro transcription (2). NF90 and NF45 copurify with two systemic lupus erythematosis autoantigens, Ku80 and Ku70, and the catalytic subunit of DNA-dependent protein kinase (6). Monoclonal antibodies against Ku80 and Ku70 specifically inhibited ARRE DNA binding (7, 8).
Proteins with double-stranded RNA-binding motifs—such as NF90 (also referred to as IL enhancer binding factor 3), RNA helicase A, adenosine-to-inosine editase (RNA-specific adenosine deaminase [ADA]), staufen, protein kinase R, and Dicer—can bind structured nucleic acids and regulate gene expression at the levels of transcription, RNA processing and transport, translation, and RNA interference (9). NF90 regulates transcription (10–12), posttranscriptional RNA stabilization (3, 5), nuclear export (3), and translation (4, 13). NF90 has been implicated in host antiviral responses and as a cellular cofactor involved in viral replication and translation (9, 13, 14).
The ARRE site serves a unique role in regulating IL-2 transcription: a specific transcriptional repressor preexisting in the nucleus of resting T cells is converted during T cell activation into a potent transcriptional activator (15). DNA-footprinting experiments demonstrated that proteins bound to the distal ARRE-2 site in the IL-2 proximal promoter in resting Jurkat and EL-4 T cells and T cell activation expanded the footprint of this purine-box regulator complex (16, 17). The ARRE site was proposed as key for regulating chromatin melting and providing concerted access of all other transcription factors to the IL-2 promoter (18). This model predicts that targeted disruption of the genes that encode critical regulators operating at the purine-box/NFAT site in the IL-2 promoter may confer phenotypes of defective IL-2 transcription and T cell activation and may exhibit T cell immunodeficiency in vivo.
To elucidate the roles of NF90 in development and immune regulation, we generated mice with a targeted disruption of NF90. NF90−/− mice die within 6 h of birth because of diaphragmatic respiratory failure related to insufficient expression of the myogenic regulators MyoD, myogenin, and p21Cip1/Waf1 (5). To circumvent the perinatal lethality of the NF90-deficient mice, we transplanted NF90 fetal liver cells into irradiated recombination activating gene (RAG)–2−/−/IL-2Rγ−/− mice that lack T cells, B cells, and NK cells and characterized immune reconstitution and function in the NF90-RAGγ chimeric mice. In this report, we demonstrate that NF90 inducibly binds to the IL-2 promoter and regulates ARRE/NFAT and IL-2 transcriptional activation, IL-2 mRNA stability, IL-2 gene expression, and peripheral T lymphocyte survival in vivo.
RESULTS AND DISCUSSION
NF90 binds specifically to the IL-2 promoter in vivo in activated T cells
We used chromatin immunoprecipitation (ChIP) to characterize in vivo binding of NF90 to the IL-2 proximal promoter, to IL-2 intron 3 (2.5 kB removed), and to the ADA origin of DNA replication to which Ku80 and Ku70 specifically bind in vivo (Fig. 1) (19). Primary mouse spleen cells were nonstimulated or stimulated for 4 h with PMA + ionomycin (PMA/Iono) and treated with 1% formaldehyde to cross-link transcription factors to their chromatin targets in vivo. Genomic PCR of sheared and restricted input chromatin demonstrated equal amplification of all targets from nonstimulated and stimulated cells (Fig. 1 A). We performed NF90 ChIP using a monoclonal antibody and showed specific binding to the IL-2 proximal promoter in vivo that increased upon stimulation (Fig. 1, B, lane 2 vs. lane 1), and no binding to IL-2 intron 3 (Fig. 1 B, lanes 3 and 4). NF90 bound equally to the ADA origin of DNA replication in nonstimulated and stimulated spleen cells (Fig. 1 B, lanes 5 and 6). Control antibody against hemagglutinin (HA) epitope did not precipitate the IL-2 promoter, intron 3, or ADA chromatin targets in our experimental conditions (Fig. 1 C). This ChIP result validates our original purification using EMSA that identified NF90 and NF45 as subunits of an inducible ARRE/NFAT DNA-binding complex in the nucleus of Jurkat T cells (1).
Figure 1.

NF90 binds specifically to the IL-2 promoter in vivo in activated T cells. Spleen cells were nonstimulated (lanes 1, 3, and 5; N) or stimulated for 4 h with PMA/Iono (lanes 2, 4, and 6; S), and nuclear proteins were cross-linked to chromatin in vivo with 1% formaldehyde. (A) Sheared and restricted chromatin was used as a template for PCR amplifications of IL-2 proximal promoter (lanes 1 and 2), IL-2 intron 3 (lanes 3 and 4), and ADA origin of replication sequences (lanes 5 and 6). (B) NF90 ChIP. (C) HA epitope ChIP.
NF90 genotype determines phenotype of maximal IL-2 gene expression
Adult NF90 heterozygous mice are indistinguishable from wild-type NF90 littermates in terms of size, activity, and longevity. We examined whether adult NF90 heterozygous mice might express less IL-2 than wild-type littermates. Compared with NF90+/+ mice, thymocytes and splenocytes from NF90+/− mice stimulated with PMA/Iono for 6, 12, and 18 h produced ∼50% less IL-2 (Fig. 2 A). We also characterized maximal IL-2 gene expression by newborn NF90+/+, NF90+/−, and NF90−/− littermates (Fig. 2 B). Compared with NF90+/+ mice, lymphocytes from NF90+/− and NF90−/− mice stimulated with PMA/Iono for 6 h mice demonstrated moderate and severe impairment of IL-2 expression. The substantial reduction in IL-2 expression in NF90 gene-targeted mice as early as 6 h suggests that expression levels of NF90 regulate transcriptional activation of the IL-2 gene.
Figure 2.
Targeted disruption of NF90 impairs T cell IL-2 gene expression and T cell homeostasis. (A) Thymocytes and splenocytes were isolated from adult NF90+/+ and NF90+/− littermate mice and stimulated with for 6–18 h with PMA/Iono, and secreted IL-2 was measured by ELISA. The data are representative of two independent pairs of littermates. NF90+/− IL-2 reduction was significant (P < 0.05). (B) Thymocytes and splenocytes were isolated from two newborn NF90+/+, two NF90+/−, and one NF90−/− littermate mice and stimulated for 6 h with PMA/Iono, and IL-2 was measured. IL-2 reduction was significant (P < 0.001). Nonstimulated cells produced no detectable IL-2. (C) Immune reconstitution analysis of NF90-RAGγ mice. Representative flow cytometry of CD4+ and CD8+ lymphocytes in the thymus and spleen of NF90−/−- and NF90+/+-RAG−/−/IL-2Rγ−/− chimeric mice. NF90−/−-RAGγ chimera yielded 107 thymocytes, 4.2 × 108 splenocytes, and 1,666 peripheral lymphocytes per mm3, and NF90+/+-RAGγ chimera yielded 107 thymocytes, 4.5 × 108 splenocytes, and 3,455 peripheral lymphocytes per mm3. Recipient mice received 900 rads conditioning radiation and were killed at 14 wk after transplantation with NF90 E14 fetal liver cells. Peripheral blood lymphocyte subset analyses of the same mice at 9 wk after transplantation demonstrated a nearly complete absence of circulating CD4+ and CD8+ T cells associated with the NF90−/− genotype (row 3). Spleen cells were gated on CD3 and analyzed for expression of CD4 and CD25 in nonstimulated conditions (row 4) and after 24 h of stimulation with anti-CD3 (row 5). The percentage of cells in each quadrant is shown. (D) Impaired IL-2 secretion in NF90−/−-RAGγ compared with NF90+/−- or NF90+/+-RAGγ chimeric mice. Single-cell suspensions of thymocytes, splenocytes, and CD4+ T cells from individually numbered chimeras were stimulated with PMA/Iono or anti-CD3 + CD28. After 16 h, secreted IL-2 was measured. IL-2 reduction in the NF90−/−-RAGγ chimeric T cells was significant (P < 0.02). Data in A, B, and D represent the mean ± SD.
NF90 fetal liver RAG-2−/−/IL-2Rγ−/− chimeric mice characterization
We transplanted NF90 fetal liver cells, enriched in hematopoietic stem cells, into irradiated adult RAG-2−/−/IL-2Rγ−/− mice that lack T cells, B cells, and NK cells. The resultant chimeric mice developed T and B lymphocytes, allowing us to determine the contributions of NF90 to T cell homeostasis, activation, and IL-2 gene expression.
We performed four independent NF90 fetal liver transplantation experiments that generated 21 NF90-RAGγ chimeric mice. A total of 10 NF90−/−-RAGγ animals were compared with 10 NF90+/+- or NF90+/−-RAGγ chimeric littermates. Complete blood counts demonstrated no substantial defects in the reconstitution of erythroid, myeloid, or megakaryocytic lineages. The most consistent defect we observed in NF90−/−-RAGγ chimeras was lymphocytopenia (Table I).
Table I.
Complete blood counts of NF90 donors, RAG-2−/−/IL-2Rγ−/− recipients, and NF90-RAG chimeric mice at 14 wk after transplantation of fetal liver stem cells
| WBC K/μl |
Neutrophils absolute |
Lymphocytes absolute |
Hemoglobin gm/dL |
Platelets K/μl |
|
|---|---|---|---|---|---|
| Donor NF90+/+ | 8 | 400 | 7,600 | 16.1 | 732 |
| Donor NF90+/− | 8.4 | 1,590 | 6,529 | 15.0 | 1,233 |
| RAG recipient | 0.4 L | 180 | 180 L | 12.9 L | 876 |
| (−/−) BMT | 3.8 L | 2,172 | 1,295 L | 13.1 L | 1,429 |
| (−/−) BMT | 1.2 L | 234 | 913 L | 13.5 L | 1,277 |
| (−/−) BMT | 2.5 L | 446 | 2,009 L | 13.4 L | 1,177 |
| (−/+) BMT | 7.8 | 853 | 6,510 | 13.3 L | 943 |
| (+/+) BMT | 7.3 | 584 | 6,570 | 13.7 | 608 |
| (+/+) BMT | 7.1 | 1,343 | 5,090 | 12.1 L | 1,430 |
| (−/−) BMT2 | 2.4 L | 643 | 1,666 L | 12.8 L | 883 |
| (+/+) BMT2 | 6.9 | 2,971 | 3,455 | 13.5 L | 1,723 |
Conditioning radiation for BMT was 450 cGy and for BMT2 was 900 cGy. Abnormal values are identified by L (low) and shown in bold.
Flow cytometry analyses of T cell development
NF90-RAGγ chimeric mice were killed 14 wk after transplantation. The thymi and spleens in all NF90-RAGγ chimeric mice were substantially larger than in adult RAGγ-null mice and were approximately two thirds the size of wild-type donor mice. We analyzed surface expression of CD4, CD8, CD3, and CD45R-B220 on thymocytes and splenocytes (Fig. 2 C). The thymi of all NF90-RAGγ chimeric mice contained a major population of CD4+ and CD8+ (double-positive) thymocytes (Fig. 2 C, row 1). The spleens of all NF90-RAGγ chimeric mice showed maturation of T cells to CD4+ or CD8+ (single-positive) T cells. B cells represented 70–80% of splenic lymphocytes (Fig. 2 C, row 2). We consistently observed a modest decrease in splenic CD4+ T cells and a moderate decrease in CD8+ T cells between the NF90−/−-RAGγ and NF90+/−- or NF90+/+-RAGγ chimeras (Fig. 2 C, row 2). The reconstitution of T cells and B cells in the NF90−/−-RAGγ chimeras demonstrated that NF90 is not absolutely required for T or B cell development.
Analyses of lymphocyte subsets in peripheral blood at 4 and 9 wk after transplantation revealed no reconstitution of T cells in NF90−/−-RAGγ chimeras compared with NF90+/+- and NF90+/−-RAGγ chimeras. We observed severe deficiencies of CD4+ and CD8+ T cells only in NF90−/−-RAGγ chimeric animals (Fig. 2 C, row 3). This T cell lymphocytopenia was present in five out of six NF90−/−-RAGγ chimeras compared with zero out of seven NF90+/−- or NF90+/+-RAGγ chimeras. The residual lymphocytes in the peripheral circulation of NF90−/−-RAGγ chimeras were predominantly B cells. From this result, we infer that NF90 is necessary for T cell survival in the blood but not in the spleen.
Spleen cells were analyzed for the proportion of CD4+CD25+ regulatory T cells (Fig. 2 C, rows 4 and 5). Compared with NF90+/+-RAG chimeras, NF90−/−-RAG chimeras showed an increased proportion of CD4+CD25+ regulatory T cells in nonstimulated spleen cells and a decreased proportion of regulatory T cells after anti-CD3 stimulation. The results shown are representative of multiple NF90-RAG chimeric mice and support our hypothesis that NF90 modulates T cell immune responses.
NF90−/−-RAG chimeric T cells demonstrate impaired secretion of IL-2
We characterized IL-2 production by NF90-RAGγ chimeric thymocytes, splenocytes, and purified splenic CD4+ T cells in response to primary stimulations for 16 h with PMA/Iono and anti-CD3 + CD28 (Fig. 2 D). Stimulated NF90−/−-RAGγ chimeric T cells (eight mice) consistently demonstrated severely reduced IL-2 expression compared with NF90+/+ or NF90+/−-RAGγ T cells (seven littermate mice).
NF90−/− thymocytes are impaired in ARRE/NFAT transcriptional activation
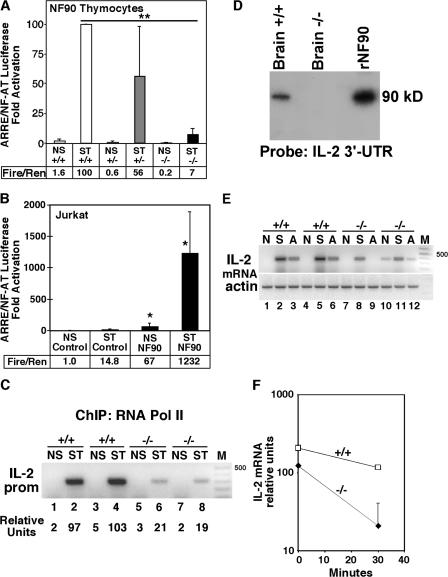
To address the mechanisms of impaired IL-2 expression in T cells lacking NF90, we characterized transcriptional activation of an ARRE/NFAT luciferase reporter gene transfected into NF90+/+, NF90+/−, and NF90−/− thymocytes (Fig. 3 A). Compared with NF90+/+ and NF90+/− cells, NF90−/− thymocytes demonstrated severe impairment of ARRE/NFAT transcriptional activation upon stimulation (Fig. 3 A).
Figure 3.
NF90 regulates ARRE/NFAT and IL-2 transcriptional activation and binds to and regulates IL-2 mRNA stability. (A) Primary cultured thymocytes from NF90+/+, NF90+/−, and NF90−/− littermates were nucleofected with 3× ARRE/NFAT firefly luciferase and EF-1α Renilla luciferase reporter plasmids. Lysates were prepared from nonstimulated (NS) and 6-h PMA/Iono-stimulated (ST) cells and analyzed for the ratio of firefly/Renilla luciferase activities. Data represent the mean ± SD. (B) Jurkat T cells stably expressing NF90 cDNA were established. Control and NF90 T cells were transiently cotransfected with 3× ARRE/NFAT firefly luciferase and EF-1α Renilla luciferase. Lysates prepared from NS and ST cells were analyzed for luciferase activities. *, P < 0.05 for NF90 versus control (n = 4). (C) IL-2 promoter ChIP using mAB 8WG16 to RNA polymerase II. Thymocytes from newborn NF90+/+ or NF90−/− mice (two littermate animals for each genotype) were nonstimulated (NS) or stimulated (ST) for 6 h with anti-CD3/CD28, and RNA pol II ChIP was performed. (D) Northwestern analysis. Mouse brain extracts and recombinant NF90 protein were fractionated by SDS-PAGE, transferred to nitrocellulose, and hybridized with 32P-labeled mouse IL-2 3′ UTR RNA probe (558–939 nt). (E) RT-PCR analysis of IL-2 mRNA induction and stability in NF90+/+ and NF90−/− splenocytes nonstimulated (N) or stimulated (S) for 6 h with anti-CD3/CD28. At 6 h, actinomycin D (A) was added, and the remaining IL-2 mRNA after 30 min was determined. (F) Semilog plot of IL-2 mRNA present at 0 and 30 min after addition of actinomycin D. The data shown are the mean and SD of two replicates.
NF90 transcriptionally activates an ARRE/NFAT luciferase reporter gene in Jurkat T cells
We recently reported that transgenic overexpression of NF45 in Jurkat T cells conferred 120-fold enhancement of IL-2 promoter– and NFAT-luciferase reporter gene activation (20). In this paper we demonstrate that stable transgenic expression of NF90 in Jurkat T cells is associated with 70–80-fold specific enhancement of ARRE/NFAT luciferase reporter gene activation in nonstimulated and PMA/Iono-stimulated T cells (Fig. 3 B). These results indicate that NF90 and NF45 specifically transactivate the ARRE sequence in activated T cells.
NF90−/− thymocytes are impaired in IL-2 gene transcription
We performed ChIP to examine recruitment and binding of the large subunit of RNA polymerase II to the IL-2 proximal promoter in nonstimulated and stimulated NF90+/+ and NF90−/− thymocytes. In nonstimulated T cells, we observed no binding of RNA pol II to the chromatin at the IL-2 proximal promoter, consistent with a closed chromatin conformation and no transcription. Wild-type thymocytes stimulated with anti-CD3/CD28 showed prominent induction of RNA pol II binding to the IL-2 promoter, consistent with transcriptional activation (Fig. 3 C, lanes 2 and 4 vs. lanes 1 and 3). In contrast, NF90-deficient thymocytes showed substantially reduced binding of RNA pol II to the IL-2 promoter upon stimulation (Fig. 3 C, lanes 6 and 8 vs. lanes 2 and 4). This result supports our conclusion that NF90 regulates inducible IL-2 transcription.
NF90 binds to and stabilizes IL-2 RNA
We used Northwestern blotting to demonstrate that NF90 is a principal IL-2 3′ untranslated region (UTR) RNA-binding protein (Fig. 3 D) (3). Brain extracts are suitable for these studies because NF90 is highly expressed in the brain (5), and IL-2 is expressed in rodent brains (21). A 90-kD protein with the same electrophoretic mobility and IL-2 RNA-binding properties as recombinant NF90 is present in NF90+/+ extracts and is absent in extracts prepared from NF90−/− mice. We observed similar results with NF90−/− neonatal thymocytes and a shorter IL-2 RNA probe that contains the four proximal ARREs (unpublished data). We examined IL-2 mRNA stability in splenocytes from newborn NF90+/+ and NF90−/− mice. Upon stimulation with anti-CD3/CD28, NF90+/+ splenocytes prominently induced IL-2 mRNA (Fig. 3 E, lanes 2 and 5 vs. lanes 1 and 4), whereas NF90−/− splenocytes show reduced IL-2 induction (Fig. 3 E, lanes 8 and 11 vs. lanes 2 and 5). Transcription was blocked after 6 h of stimulation by the addition of actinomycin D (22). Compared with NF90+/+ splenocytes, NF90−/− splenocytes showed faster disappearance of IL-2 mRNA after 30 min (Fig. 3 F). Assuming first order exponential decay kinetics, the IL-2 mRNA half-life in NF90+/+ mice is 37 min, whereas the IL-2 mRNA half-life in NF90−/− mice is 12 min. Thus, NF90 binds to and stabilizes newly transcribed IL-2 RNA, thereby posttranscriptionally enhancing IL-2 gene expression.
We propose that NF90 regulates and facilitates rapid expression of IL-2 and other important, tightly controlled genes (including MyoD and p21Cip1) (5). T cell activation triggers IL-2 chromatin remodeling that increases the binding of NF90 and RNA polymerase II to the IL-2 proximal promoter in vivo, as revealed by ChIP. NF90 regulates transcriptional activation through the ARRE sequence in the IL-2 proximal promoter in activated T cells. NF90-deficient thymocytes exhibit specific impairment of ARRE/NFAT-regulated transcriptional activation and binding of RNA pol II to the IL-2 promoter upon stimulation. Binding of newly transcribed IL-2 RNA to NF90 stabilizes the transcript against degradation (3). Operating as an RNA chaperone, NF90 regulates nuclear export of IL-2 RNA and can associate with ribosomes and regulate protein translation (4, 13).
Purine-box regulator ARRE DNA-binding subunits NF90, NF45, Ku80, and Ku70 (1, 2, 7, 8) are distinct from the ∼120-kD NFAT proteins isolated from T cell cytoplasm (23). Both NF90 and NFAT proteins can specifically bind to the same ARRE sequence in vitro. Our ChIP results demonstrate inducible binding of NF90 to the IL-2 proximal promoter in vivo. Targeted disruptions of individual NFAT proteins failed to impair IL-2 gene expression, which prompted the characterization of NFATc1 and NFATc2 double-knockout (DKO) fetal liver–RAG chimeric mice (24). These DKO mice showed normal reconstitution of T cells and B cells, but in vitro cultured DKO T cells showed deficient IL-2 production when restimulated with anti-CD3. It will be important to elucidate the relative contributions of NF90 and NFATs to regulating the individual steps of IL-2 gene expression.
Our study identifies NF90 as an important regulator of T cell IL-2 transcription, mRNA stability, and gene expression and, therefore, a novel target for therapeutic modulation of T cell immune responses. The T cell lymphocytopenia associated with homozygous deficiency of NF90 may be a consequence of attenuated proliferation or survival of circulating T cells caused by impaired expression of IL-2 or other genes. Future studies should also address the roles of NF90 as a host cellular factor that may be subverted during viral pathogenesis (9, 14, 25).
MATERIALS AND METHODS
Animal studies.
Animal studies were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
ChIP.
Mouse spleen cells (C57BL/6) were nonstimulated or stimulated for 4 h with 20 ng/ml PMA (Sigma-Aldrich) + 2 μM Iono (Calbiochem). 106 thymocytes per condition from newborn NF90+/+ or NF90−/− mice were nonstimulated or stimulated for 6 h with anti-CD3 + anti-CD28 (1 μg/ml each). Formaldehyde cross-linking and ChIP were performed as previously described (19). Antibodies were against NF90 (mAb DRBP76; Becton Dickinson), HA (sc-805; Santa Cruz Biotechnology, Inc.), and the large subunit of RNA polymerease II (8WG16; Covance). PCR primers were as follows: IL-2 proximal promoter (299 bp), (forward) 5′-CTGCCACCTAAGTGTGGGCTAACCCGACC-3′ and (reverse) 5′-GCATGCTGTACATGCCTGCAGGACTTGAGG-3′; IL-2 intron 3 (424 bp), (forward) 5′-CCACAATGTGGGTGGGTCACTGCAATTGAAC-3′ and (reverse) 5′-GTTAGGCCACTCTAGTGAGCTCTTCTGGC-3′; and ADA origin of DNA replication (268 bp), (forward) 5′-CTGAGACTATCCTCCAGGTCTTCTAATGGGG-3′ and (reverse) 5′-GGATGACCCTTTCATGGCTGCCTATGACCAACAG-3′. PCR amplifications used a three-step protocol with annealing temperatures of 58°C or 59°C and 35 cycles.
NF90 fetal liver–RAGγ transplantations.
NF90 gene-targeted donor mice and RAG-2−/−/IL-2Rγ−/− recipient mice were maintained on a C57BL/6 background. NF90+/− mice were intercrossed and killed at E14–15, and fetal livers were dissociated into single-cell suspensions. Genomic DNA from embryonic skin was used for genotyping by PCR (5). Adult recipient RAG-2−/−/IL-2Rγ−/− mice received conditioning radiation from a cesium source (450 or 900 cGy), followed within 24 h by tail vein injection of 4 × 106 NF90+/+, NF90+/−, or NF90−/− fetal liver cells.
Flow cytometry.
Engraftment and lymphocyte subsets in peripheral blood were monitored in NF90-RAG chimeric mice at 4 and 9 wk after transplantation. Peripheral blood mononuclear cells were labeled with anti-CD4, -CD8, -CD19, and -DX5 antibodies (Becton Dickinson) and analyzed by flow cytometry (LSR; Becton Dickinson) using FloJo software (TreeStar Inc.). NF90-RAGγ chimeras were killed at 15 wk after transplantation. Dissociated thymic and spleen cells were labeled with anti-CD4, -CD8, -CD25, -CD11b, -CD11c, and -B220 antibodies (Becton Dickinson) and analyzed by flow cytometry.
IL-2 analysis.
Thymocytes, splenocytes, and CD4+ T cells (enriched by magnetic bead negative selection; Miltenyi Biotec) were stimulated (106 cells/ml) for 6–18 h with 20 ng/ml anti-CD3 (Becton Dickinson) + 20 ng/ml anti-CD28 (Becton Dickinson) or 20 ng/ml PMA + 2 μM Iono, and supernatants were analyzed by IL-2 ELISA (Becton Dickinson) in triplicate.
ARRE/NFAT luciferase reporter gene assays.
Primary thymocytes were isolated from newborn NF90+/+, NF90+/−, and NF90−/− littermate mice and cultured in vitro with 20 ng/ml anti-CD3 and 100 U/ml recombinant IL-2 (Becton Dickinson). After 3–7 d, 0.8 × 106 cells of each genotype were nucleofected (Amaxa) with 3× ARRE/NFAT firefly luciferase (3 μg) and EF-1α Renilla luciferase (1 μg) reporter genes. Jurkat T cells were stably transfected with expression plasmid pEF-1α NF90 ires neo and selected in G418. Control or NF90 T cells were transiently transfected with ARRE/NFAT firefly and EF Renilla luciferase. After 18 h in culture, thymocytes or Jurkat T cells were divided into nonstimulated and 6-h PMA/Iono-stimulated pools, and cell lysates were analyzed for the ratios of firefly/Renilla luciferase activities (Dual Luciferase Reporter; Promega).
Northwestern blotting.
The radiolabeled IL-2 3′ UTR RNA probe was transcribed in vitro (nt 558–939 available from GenBank/EMBL/DDBJ under accession no. NM_008366). 80 μg of brain extracts or 10 μg of recombinant NF90 protein were fractionated by SDS-PAGE, transferred to nitrocellulose, and probed as previously described (5).
IL-2 mRNA stability.
0.7 × 106 splenocytes per condition from newborn NF90+/+ or NF90−/− mice (two littermate animals for each genotype) were nonstimulated, stimulated for 6 h with anti-CD3/CD28 (1 μg/ml each), or stimulated for 6 h followed by addition of 10 μg/ml actinomycin D for 30 min. Total RNA was extracted (TRIzol; Invitrogen) and reverse transcribed using oligo dT primer, and IL-2 and β-actin were amplified by PCR (35 cycles of 95°C for 20 s, 58°C for 40 s, and 72°C for 40 s) (5). The primer sequences used are IL-2 (forward) 5′-GCTCCTGAGCAGGATGGAGAATTACAG-3′ and (reverse) 5′-GAGAGCCTTATGTGTTGTAAGCAGGAGG-3′. The intensities of the ethidium bromide–stained bands were quantified using ImageJ software (National Institutes of Health). The means of the relative intensities at 0 and 30 min of actinomycin D were averaged and used to calculate the IL-2 mRNA half-life, assuming first order exponential decay.
Data analysis.
Data are presented as the mean ± SD. Statistical significance was determined by analysis of variance or the Student's paired t test (Excel; Microsoft), and P < 0.05 was considered significant.
Acknowledgments
We thank Mark Krasnow and Arthur Karlin for encouragement, and Blaise Corthesy, Garry Fathman, Jane Parnes, Irv Weissman, Tushar Desai, Glenn Rosen, and the anonymous reviewers for constructive suggestions.
This work was supported by National Institutes of Health grants R01AI39624 and R01HL62588 and a gift from the Donald E. and Delia B. Baxter Foundation to P.N. Kao.
The authors have no conflicting financial interests.
References
- 1.Kao, P.N., L. Chen, G. Brock, J. Ng, J. Kenny, A.J. Smith, and B. Corthesy. 1994. Cloning and expression of cyclosporin A- and FK506-sensitive nuclear factor of activated T-cells: NF45 and NF90. J. Biol. Chem. 269:20691–20699. [PubMed] [Google Scholar]
- 2.Corthesy, B., and P.N. Kao. 1994. Purification by DNA affinity chromatography of two polypeptides that contact the NF-AT DNA binding site in the interleukin 2 promoter. J. Biol. Chem. 269:20682–20690. [PubMed] [Google Scholar]
- 3.Shim, J., H. Lim, J.R. Yates, and M. Karin. 2002. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol. Cell. 10:1331–1344. [DOI] [PubMed] [Google Scholar]
- 4.Xu, Y.H., and G.A. Grabowski. 1999. Molecular cloning and characterization of a translational inhibitory protein that binds to coding sequences of human acid beta-glucosidase and other mRNAs. Mol. Genet. Metab. 68:441–454. [DOI] [PubMed] [Google Scholar]
- 5.Shi, L., G. Zhao, D. Qiu, W.R. Godfrey, H. Vogel, T.A. Rando, H. Hu, and P.N. Kao. 2005. NF90 regulates cell cycle exit and terminal myogenic differentiation by direct binding to the 3′-untranslated region of MyoD and p21WAF1/CIP1 mRNAs. J. Biol. Chem. 280:18981–18989. [DOI] [PubMed] [Google Scholar]
- 6.Ting, N.S., P.N. Kao, D.W. Chan, L.G. Lintott, and S.P. Lees-Miller. 1998. DNA-dependent protein kinase interacts with antigen receptor response element binding proteins NF90 and NF45. J. Biol. Chem. 273:2136–2145. [DOI] [PubMed] [Google Scholar]
- 7.Aoki, Y., G. Zhao, D. Qiu, L. Shi, and P.N. Kao. 1998. CsA-sensitive purine-box transcriptional regulator in bronchial epithelial cells contains NF45, NF90, and Ku. Am. J. Physiol. 275:L1164–L1172. [DOI] [PubMed] [Google Scholar]
- 8.Shi, L., D. Qiu, G. Zhao, B. Corthesy, S. Lees-Miller, W.H. Reeves, and P.N. Kao. 2007. Dynamic binding of Ku80, Ku70 and NF90 to the IL-2 promoter in vivo in activated T-cells. Nucleic Acids Res. 10.1093/nar/gkm117. [DOI] [PMC free article] [PubMed]
- 9.Tian, B., P.C. Bevilacqua, A. Diegelman-Parente, and M.B. Mathews. 2004. The double-stranded-RNA-binding motif: interference and much more. Nat. Rev. Mol. Cell Biol. 5:1013–1023. [DOI] [PubMed] [Google Scholar]
- 10.Saunders, L.R., D.J. Perkins, S. Balachandran, R. Michaels, R. Ford, A. Mayeda, and G.N. Barber. 2001. Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 276:32300–32312. [DOI] [PubMed] [Google Scholar]
- 11.Reichman, T.W., L.C. Muniz, and M.B. Mathews. 2002. The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells. Mol. Cell. Biol. 22:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie, Y., L. Ding, P.N. Kao, R. Braun, and J.H. Yang. 2005. ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing. Mol. Cell. Biol. 25:6956–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langland, J.O., P.N. Kao, and B.L. Jacobs. 1999. Nuclear factor-90 of activated T-cells: A double-stranded RNA-binding protein and substrate for the double-stranded RNA-dependent protein kinase, PKR. Biochemistry. 38:6361–6368. [DOI] [PubMed] [Google Scholar]
- 14.Isken, O., C.W. Grassmann, R.T. Sarisky, M. Kann, S. Zhang, F. Grosse, P.N. Kao, and S.E. Behrens. 2003. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 22:5655–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouzaki, A., R. Weil, L. Muster, and D. Rungger. 1991. Silencing and trans-activation of the mouse IL-2 gene in Xenopus oocytes by proteins from resting and mitogen-induced primary T-lymphocytes. EMBO J. 10:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw, J.P., P.J. Utz, D.B. Durand, J.J. Toole, E.A. Emmel, and G.R. Crabtree. 1988. Identification of a putative regulator of early T cell activation genes. Science. 241:202–205. [DOI] [PubMed] [Google Scholar]
- 17.Randak, C., T. Brabletz, M. Hergenrother, I. Sobotta, and E. Serfling. 1990. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 9:2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrity, P.A., D. Chen, E.V. Rothenberg, and B.J. Wold. 1994. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol. Cell. Biol. 14:2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novac, O., D. Matheos, F.D. Araujo, G.B. Price, and M. Zannis-Hadjopoulos. 2001. In vivo association of Ku with mammalian origins of DNA replication. Mol. Biol. Cell. 12:3386–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, G., L. Shi, D. Qiu, H. Hu, and P.N. Kao. 2005. NF45/ILF2 tissue expression, promoter analysis, and interleukin-2 transactivating function. Exp. Cell Res. 305:312–323. [DOI] [PubMed] [Google Scholar]
- 21.Hanisch, U.K., and R. Quirion. 1995. Interleukin-2 as a neuroregulatory cytokine. Brain Res. Brain Res. Rev. 21:246–284. [DOI] [PubMed] [Google Scholar]
- 22.Ogilvie, R.L., M. Abelson, H.H. Hau, I. Vlasova, P.J. Blackshear, and P.R. Bohjanen. 2005. Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay. J. Immunol. 174:953–961. [DOI] [PubMed] [Google Scholar]
- 23.McCaffrey, P.G., B.A. Perrino, T.R. Soderling, and A. Rao. 1993. NF-ATp, a T lymphocyte DNA-binding protein that is a target for calcineurin and immunosuppressive drugs. J. Biol. Chem. 268:3747–3752. [PubMed] [Google Scholar]
- 24.Peng, S.L., A.J. Gerth, A.M. Ranger, and L.H. Glimcher. 2001. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 14:13–20. [DOI] [PubMed] [Google Scholar]
- 25.Langland, J.O., P. Kao, and B.L. Jacobs. 2003. Regulation of IL-2 gene expression and nuclear factor-90 translocation in vaccinia virus-infected cells. J. Interferon Cytokine Res. 23:489–500. [DOI] [PubMed] [Google Scholar]