Abstract
The salivary glands represent a major site of cytomegalovirus replication and transmission to other hosts. Despite control of viral infection by strong T cell responses in visceral organs cytomegalovirus replication continues in the salivary glands of mice, suggesting that the virus exploits the mucosal microenvironment. Here, we show that T cell immunity in the salivary glands is limited by the induction of CD4 T cells expressing the regulatory cytokine interleukin (IL)-10. Blockade of IL-10 receptor (IL-10R) with an antagonist antibody dramatically reduced viral load in the salivary glands, but not in the spleen. The mucosa-specific protection afforded by IL-10R blockade was associated with an increased accumulation of CD4 T cells expressing interferon γ, suggesting that IL-10R signaling limits effector T cell differentiation. Consistent with this, an agonist antibody targeting the tumor necrosis factor receptor superfamily member OX40 (TNFRSF4) enhanced effector T cell differentiation and increased the number of interferon γ–producing T cells, thus limiting virus replication in the salivary glands. Collectively, the results indicate that modulating effector T cell differentiation can counteract pathogen exploitation of the mucosa, thus limiting persistent virus replication and transmission.
Mucosal tissues serve as major sites of entry, replication, and exit for many pathogens. Indeed, herpesviruses often persist in and are shed from mucosal tissues for long periods of time despite strong adaptive immune responses in their host. Understanding how pathogens persist in mucosal tissue may assist the design of effective vaccination and immunotherapeutic strategies.
Human CMV (HCMV) is a β-herpesvirus that infects the majority of the world's population. Although this persistent/latent infection is asymptomatic in healthy individuals, HCMV causes multiorgan disease in the immunologically immature (e.g., congenital infection) and the immune-compromised population (e.g., AIDS patients and organ transplant recipients). Asymptomatic shedding in saliva is an important source of virus in natural transmission of HCMV (1–3), implying that virus replication in the salivary glands is pivotal for horizontal transmission. After systemic infection, mouse CMV (MCMV) initially replicates in visceral organs such as the spleen and liver, but virus production is limited by NK cell and adaptive cellular immunity within a week after infection. Despite strong cellular immunity, infectious virus is produced in the acinar glandular epithelial cells within the submaxillary salivary glands (4) and can be detected for several months after primary infection (4–6). Like all herpesvirus, MCMV establishes latency (5, 7), yet very little is known about how CMV persists within the salivary glands. CD4 T cells producing IFN-γ have been described to afford protection at this site (5, 8), but it is not clear if or how these cells are regulated. CMV can inhibit CD4 T cell activation by interfering with IFN-γ–induced MHC class II expression (9). Viral genes that interfere with antigen presentation to CD8 T cells influence virus replication in the salivary glands, suggesting mucosal CD8 T responses are also targeted (10). In BALB/c mice, NK cells and γδ T cells have also been detected within the salivary glands after MCMV infection (11), but their role in clearance is not known. As a target of persistent infection, the salivary glands are exposed to substantial antigenic burden without obvious pathology. Thus, it is likely that inflammatory immune cells at this site may be regulated differently than in other tissues. Potential mechanisms could include induction of T cell anergy or apoptosis (12). Immune responses may also be actively suppressed by regulatory T cells, such as naturally occurring CD4+CD25+ T regulatory (T reg) or T reg cells induced by antigen, which are defined by the expression of suppressive cytokines, including IL-10 or TGF-β (13, 14).
We now report that cellular immunity in the salivary glands to a persistent virus is regulated through an IL-10– dependent mechanism. We observed that persistent MCMV replication in the salivary glands was accompanied by the appearance of IL-10–expressing CD4 T cells specifically within this organ, but not elsewhere. Blockade of IL-10R signaling during infection dramatically decreased virus replication that correlated with increased accumulation of CD4 T cells expressing antiviral IFN-γ. Further, targeting CD4 T cell differentiation through activation of the TNFR family member OX40 (15) increased the ratio of CD4 T cells expressing IFN-γ compared with IL-10 in the salivary glands and reduced persistent replication of MCMV. Collectively, these data show that MCMV exploits the salivary gland environment favoring IL-10 expression by virus-induced CD4 T cells, thus enabling persistent virus replication and horizontal transmission to susceptible hosts.
RESULTS
IL-10–expressing CD4 T cells accumulate in the salivary glands as MCMV replicates
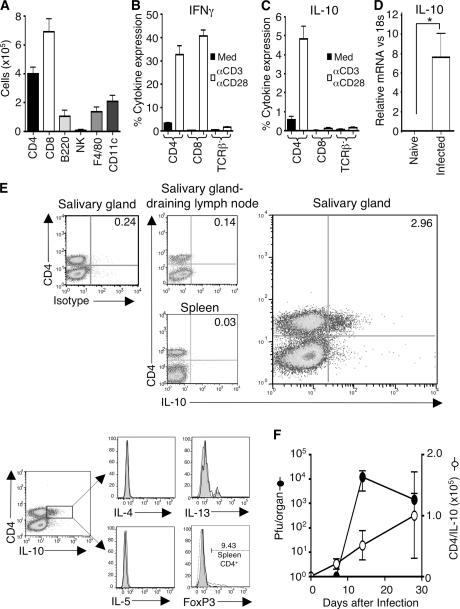
MCMV replication can be detected for 1–2 mo in the submaxillary salivary glands, but not in the spleen, after a primary infection in C57BL/6 (B6) mice (6). Persistent MCMV replication in the salivary glands induced the accumulation of CD4 and CD8 T cells, B cells, NK cells, and antigen-presenting cells (Fig. 1 A). Cytokine expression analysis of salivary gland–derived leukocytes revealed that T cells (primarily CD4 T cells) were the predominant source of low spontaneous expression of antiviral cytokine IFN-γ, which was amplified by ex vivo polyclonal stimulation (Fig. 1 B).
Figure 1.
IL-10–expressing CD4 T cells accumulate in the salivary glands during persistent MCMV infection. (A) 14 d after MCMV infection of mice, accumulation of CD4+CD3+, CD8+CD3+, B220+, NK+CD3−, F4/80+, and CD11c+ cells (A) were measured. (B and C) After 5 h with (white bars) or without (black bars) stimulation with anti-CD3/anti-CD28, IFN-γ (B), and IL-10 (C) expression by CD4+TCRβ+ and CD8+TCRβ+ cells and TCR-β− cells derived from the salivary glands was measured. (D) IL-10 expression in salivary gland extracts from naive and MCMV-infected mice 14 d after infection was measured by quantitative PCR and was normalized to 18S RNA. *, P < 0.05, with student's t test. All results are expressed as mean ± SD of three to four mice per group. (E) IL-10 expression by TCR-β+ cells in the salivary glands, draining lymph nodes, and spleen was analyzed by intracellular stain 5 h after stimulation with anti-CD3/anti-CD28 (top). Data are representative plots (n = 4) staining for CD4 versus IL-10 or isotype control on gated live lymphocytes. (bottom) Expression of IL-4, IL-5, IL-13, and FoxP3 (unshaded lines) compared with isotype control (shaded lines) by gated CD4+IL-10+ cells visualized 5 h after in vitro stimulation of salivary gland leukocytes 14 d after infection. Positive control for FoxP3 staining (dashed line) is CD4 T cells derived from spleens of naive mice. (F) On 0, 7, 14, and 30 d after infection, the number of CD4+IL-10+ cells (◯) and virus titers (•) in the salivary glands were measured. Results are expressed as mean ± SD of four mice per group, and all results are representative of three experiments.
Interestingly, we observed a considerable accumulation in the salivary glands of CD4 T cells expressing the immunosuppressive cytokine IL-10 during persistent MCMV replication, although these cells were not detected in visceral sites such as the spleen or in the lymph nodes draining the salivary glands (Fig. 1, C and E). We found a low percentage of CD4 T cells making detectable IL-10 (0.5–1%) without ex vivo restimulation (Fig. 1 C), suggesting that the results derived from stimulation with CD3/CD28 represented an amplification of cytokine production by these cells. Further reinforcing the notion that this IL-10 production reflected a physiological event in vivo, we found strong expression of IL-10 in salivary gland extracts from infected but not naive mice (Fig. 1 D). Importantly, CD8 T cells and non–T cells did not express notable levels of IL-10, suggesting CD4 T cells were the predominant source of this cytokine (Fig. 1 C). IL-10–producing CD4 T cells did not co-express IL-4, IL-5, IL-13, or FoxP3, implying these cells were not conventional T helper (Th)2 cells or naturally occurring T reg cells (Fig. 1 E). Moreover, the accumulation of IL-10–expressing CD4 T cells accompanied MCMV replication in the salivary glands (Fig. 1 F), demonstrating these T cells were actively induced by the virus infection.
OX40 induces IFN-γ expression and limits persistent MCMV replication
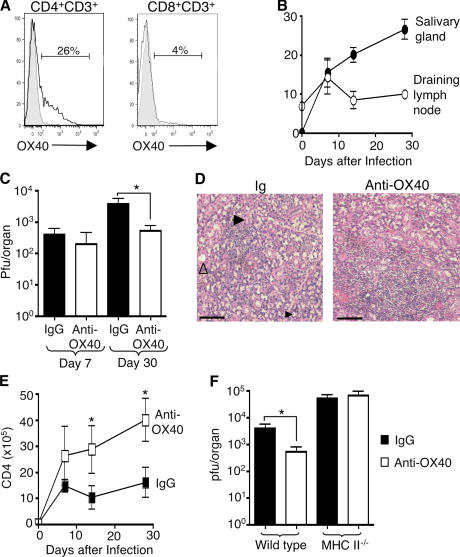
IFN-γ–expressing CD4 T cells inhibit MCMV replication in the salivary glands (8). Although CD4+IFN-γ+ T cells were present (Fig. 1 B), the results suggested that IL-10– secreting cells might limit either antiviral activity of IFN-γ+ T cells or the IFN-γ itself. We hypothesized that shifting the ratio of CD4 T cells to favor IFN-γ production might limit virus replication. To accomplish this, we targeted the TNFR OX40 with an agonist antibody based on the ability of this receptor to enhance IFN-γ secretion (16, 17) and suppress IL-10 production by T cells (18, 19). OX40 was detected on CD4 but not CD8 T cells infiltrating the infected salivary glands (Fig. 2, A and B) and in draining lymph nodes (Fig. 2 B), indicating that ectopic targeting of OX40 with an agonist antibody was feasible. Mice treated with an agonist anti-OX40 antibody at the time of infection had no discernable effect on early (day 7 after infection) viral load in the salivary glands (Fig. 2 C). However, anti-OX40–treated mice had substantially reduced titers of replicating virus at 30 d after infection (Fig. 2 C). This was associated with increased infiltration of mononuclear cells compared with control mice (Fig. 2 D). Further analysis revealed that this cellular infiltrate consisted primarily of CD4 T cells (Fig. 2 E), and we found that the elevated CD4 response was critical for OX40-mediated inhibition of MCMV-persistent replication, as the protective effect was abolished in MHC class II–deficient mice (Fig. 2 F). In contrast, OX40 stimulation did not alter the accumulation of total or MCMV-specific CD8 T cells, or granzyme B–expressing CD8 T cells in the salivary glands (Fig. S1, A–C, available at http://www.jem.org/cgi/content/full/jem.20062424/DC1).
Figure 2.
OX40 activation inhibits virus replication and enhances cellular infiltration in the salivary glands during MCMV infection. (A) OX40 expression by salivary gland–derived CD4+CD3+ (left) and CD8+CD3+ (right) cells 14 d after MCMV infection (unshaded line) compared with isotype-stained control (shaded line). (B) The percentage of OX40-expressing CD4+CD3+ T cells isolated from the salivary glands (•) and draining lymph nodes (○) 0, 7, 14, and 30 d after infection. (C) MCMV-infected mice were treated with IgG (black bars) or anti-OX40 (white bars), and after 7 and 30 d salivary glands were assayed for infectious virus (C). (D) Hematoxylin and eosin–stained salivary gland sections 14 d after infection from control Ig (left) and anti-OX40– (right) treated mice. Acinus (large black arrowhead), duct (white arrowhead), and glandular epithelial cells with an intranuclear inclusion indicative of virus cytopathic effect (small black arrowhead) are shown in the section from Ig-treated mice. Bars, 200 μm. (E) Numbers of CD4 T cells in the salivary glands of IgG (▪) and anti-OX40– (□) treated mice 0, 7, 14, and 30 d after infection. (F) Wild-type C57BL/6 and MHCII−/− mice were infected with MCMV and treated with IgG (black bars) or anti-OX40 (white bars), and 30 d later salivary glands were assayed for infectious virus. Data presented in B, C, E, and F represent mean ± SD of four mice per group, and all data is representative of three to five independent experiments. *, P < 0.05, with student's t test.
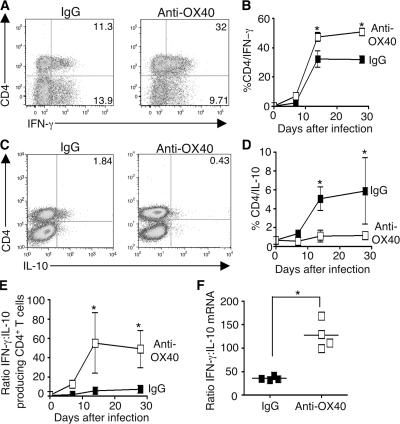
OX40 activation increased the percentage (Fig. 3, A and B) and total numbers (unpublished data) of CD4 T cells, but not CD8 T cells (Fig. S1 D), expressing IFN-γ. Moreover, anti-OX40 treatment also reduced the percentage of IL-10–expressing CD4 T cells (Fig. 3, C and D), altering the ratio of T cells expressing IFN-γ to IL-10 in the salivary glands (Fig. 3 E). This effect of OX40 activation was also reflected in a shift in the expression of IFN-γ and IL-10 in situ (Fig. 3 F). These data show that virus replication in the salivary glands is reduced when OX40 is activated, which is associated with an increased ratio of IFN-γ to IL-10–expressing CD4 T cells.
Figure 3.
OX40 activation alters the ratio of IFN-γ to IL-10–expressing cells in the salivary glands during MCMV infection. B6 mice were infected with MCMV and treated with rat IgG or anti-OX40 at the time of infection. IFN-γ and IL-10 expression was measured in salivary gland–derived CD4 T cells from IgG and anti-OX40–treated mice at various times after MCMV infection, after ex vivo stimulation with anti-CD3 and anti-CD28. (A and C) Representative plots of IFN-γ (A) and IL-10 (C) expression by CD4 T cells from Ig (left) or anti-OX40– (right) treated mice 14 d after infection. (B and D) The percentage of CD4+ T cells expressing IFN-γ (B) or IL-10 (D) in the salivary glands of Ig and anti-OX40–treated mice over time. (E) Ratio of IFN-γ to IL-10–producing CD4 cells, calculated as the ratio of the percentages of IFN-γ–expressing CD4 cells to IL-10–expressing CD4 cells. All results are expressed as mean ± SD of four to seven mice per group over a 30-d time-course infection. (F) IFN-γ and IL-10 expression in salivary gland extracts measured by quantitative PCR and normalized to 18S RNA. Data are presented as a ratio of IFN-γ to IL-10 expression per mouse. All results represent two to three experiments. *, P < 0.05, with student's t test.
Blockade of IL-10R limits MCMV replication in the salivary glands
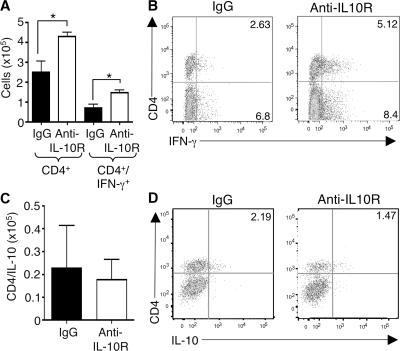
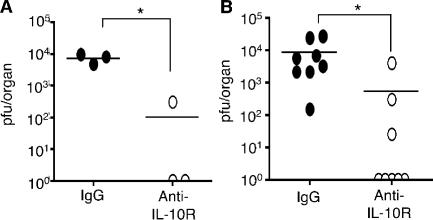
To determine whether the restricted virus replication and the increased ratio of IFN-γ to IL-10–secreting CD4 T cells involved IL-10, we used an antagonist IL-10R antibody (anti–IL-10R) to block signaling. Administration of this antibody enhanced the accumulation of total and IFN-γ–expressing CD4 T cells in the salivary glands (Fig. 4, A and B), but had no effect on the accumulation of CD8+ T cells or NK+CD3− cells expressing IFN-γ (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20062424/DC1). The accumulation of CD4+IL-10+ T cells was not a result of IL-10R signaling in this organ (Fig. 4, C and D). Importantly, virus replication was dramatically inhibited by blocking IL-10R, with no virus detectable in two treated mice (n = 3) after 30 d of infection (Fig. 5 A). Moreover, when anti–IL-10R treatment was started at 7 d after infection to coincide with the appearance of IL-10+ cells (Fig. 1 E), virus levels in salivary glands remained undetectable in five out of eight mice (Fig. 5 B). Collectively, these data highlight the salivary glands as sites of strong IL-10–dependent immune regulation and demonstrate that IL-10–producing CD4 T cells suppress antiviral effector CD4 T cells. The results demonstrate that modulation of the OX40 and IL-10 pathways can block virus replication in this important excretory organ.
Figure 4.
IL-10R blockade augments accumulation of IFN-γ– producing CD4 T cells in the salivary glands. C57BL/6 mice were infected with MCMV and treated with rat IgG or anti–IL-10R on days 0 and 7 after infection. Total CD4 T cells (A) and IFN-γ (A) and IL-10– (C) expressing CD4 T cells in the salivary glands of IgG (black bars) and anti–IL-10R– (white bars) treated mice were quantified 14 d after infection. Results are expressed as mean ± SD from three to four mice per group. *, P < 0.05, with student's t test. (B and D) Representative FACS plots of CD4 versus IFN-γ (B) or IL-10 (D). Quadrants were set using isotype control antibodies, and results represent four mice per group. All results are representative of three similar experiments.
Figure 5.
Blockade of IL-10R signaling inhibits MCMV persistence. MCMV-infected mice were treated with IgG (•) or anti–IL-10R on days 0 and 7 (A) or 7 and 14 (B) after infection. After 30 d, salivary glands were assayed for infectious virus. Individual mice plus mean are shown. Results are representative of three similar experiments. Undetectable titers are expressed as an arbitrary value of 1 for display on a logarithmic scale.
DISCUSSION
The salivary glands are a site of IL-10–dependent immune regulation. We demonstrated that IL-10–producing CD4 T cells suppressed effector CD4 T cells in a model of persistent virus replication at this mucosal site. The results establish that the IL-10 immunoregulatory pathway enables MCMV to persistently replicate in this mucosal organ. Counteracting this viral strategy by modulation of either the costimulatory OX40 pathway with an agonist antibody or targeting the immunoregulatory IL-10 pathway with an antagonist antibody, limited persistent virus replication in the salivary glands, attenuating shedding of virus, thus potentially reducing transmission to susceptible hosts.
Sustained activation of the TNFR OX40 with an agonist antibody favored the differentiation and accumulation of CD4 T cells that produced IFN-γ in the salivary glands to counteract the inhibitory effects of the IL-10–dominated immune response. OX40 was expressed on antigen-activated CD4 T cells, which were readily detected in the spleen, salivary glands, and draining lymph nodes from MCMV-infected mice. T cells express OX40 for short periods of time after antigen activation; thus, virus reactive T cells were the mostly likely targets of the antibody, and therefore, treatment subsequently altered virus-specific cytokine expression. Activation of OX40 can favor generation of effector Th1 or Th2 cells (16, 17, 20, 21), which are distinguished by their expression of IFN-γ or IL-4, IL-5, and IL-13, respectively. OX40 stimulation during MCMV infection enhanced the accumulation of CD4 T cells coexpressing IFN-γ and the activation-associated isoform of CD43 (unpublished data), suggesting that OX40 signaling directly impacted the differentiation of antiviral Th1 primary effector cells.
CD4 T cells in the salivary glands were identified as the predominant source of IL-10, representing ∼80–90% of all IL-10–expressing cells. In contrast to peripheral chronic arenavirus infection (22, 23), MCMV replication in the salivary glands did not induce expression of IL-10 by CD11c+ dendritic cells (unpublished data). IL-10–expressing CD4 T cells did not co-express the Th2-associated cytokines IL-4, IL-5, or IL-13, nor did they express the transcription factor FoxP3, which is a marker of naturally occurring CD4+CD25+ T reg cells. Collectively, the data indicated these T cells were IL-10– producing T reg cells, which have been described to mediate peripheral tolerance (24, 25) and suppresses mucosal inflammation in the gut (26). Interestingly, recent studies have reported that conventional Th1 cells can express IL-10 during chronic parasitic infections (27, 28). Whether MCMV-induced IL-10–expressing CD4 T cells represent T regulatory 1 or Th1 cells, or a combination of both, is currently unknown. Regardless of the exact lineage of these cells, however, this current study is the first description of a role for IL-10– expressing CD4 T cells in the salivary glands.
IL-10 suppresses inflammatory immune responses by inhibiting the expression of proinflammatory cytokines and chemokines, and decreasing expression of MHC class II and costimulatory molecules by APC, which subsequently limits differentiation or expansion of CD4 T cell effector responses (29). The dramatic reduction in persistent virus replication after IL-10R blockade was associated with increased IFN-γ–producing Th1 cells in the salivary glands, implying that IL-10 might act directly on effector T cells in the local microenvironment. Alternatively, IL-10 may suppress T cells indirectly by down-regulating one or more functions associated with APC. A previous study showed that MCMV- induced IL-10 in APC suppressed MHC class II expression by macrophages in vitro (30). However, we observed no change in MHC class II expression by cells infiltrating the salivary glands after IL-10R blockade (Fig. S2, B and C), suggesting this explanation cannot account for the action of IL-10 in vivo. The strong effect of anti-OX40 treatment in promoting IFN-γ–secreting CD4 T cell accumulation in the salivary glands suggests that IL-10 may predominantly act to suppress expression of costimulatory ligands such as OX40L and/or production of antiviral cytokines by APC, either in the salivary glands, or in the case of dendritic cells, after migration to draining lymph nodes.
In this mouse model of persistent virus replication, we have identified two distinct approaches to counteracting immune regulation. Regardless of the mechanism of IL-10–mediated suppression, the efficacy of IL-10R blockade in preventing viral replication was remarkable. Furthermore, delaying treatment until a week after infection, after CD4 and CD8 primary responses were established, dramatically altered virus replication in the salivary glands, showing the importance of IL-10 during this late stage of the antiviral response.
Targeting OX40 was also efficacious perhaps because of the expression of OX40 on antigen-reactive T cells, altering cytokine expression. This conclusion is guarded because MCMV peptides presented by class II MHC have not yet been defined to directly visualize antigen-specific T cells. The blockade of persistent viral replication by OX40 activation was associated with the accumulation of IFN-γ–secreting CD4 T cells previously described to afford protection from MCMV replication in the salivary glands (5, 8). Furthermore, the protective effect of OX40 stimulation was accompanied by a strong alteration in the ratio of IFN-γ to IL-10–expressing CD4 T cells. In a tissue culture model, the presence of OX40L on dendritic cells inhibited IL-10 expression in human CD4 T cells differentiating under both Th1 and Th2 priming conditions (19), suggesting that OX40 may directly antagonize IL-10 expression. However, the transcription factor GATA-3, which is implicated in the transcriptional control of IL-10 expression (31), can also be induced by OX40 stimulation in an IL-4R–dependent manner (21). This latter result suggests that, in certain conditions, OX40 could enhance IL-10 expression. Indeed, although we found an altered ratio of IFN-γ to IL-10–expressing T cells after OX40 stimulation, the actual numbers of IL-10–producing CD4 T cells did not change significantly. Furthermore, the level of IL-10 expression by CD4+IL-10+ T cells, as indicated by mean fluorescent intensity, was also unaltered by OX40 stimulation (unpublished data). Thus, the action of OX40 most likely reflected a preferential increase of Th1 effector cell differentiation and/or accumulation of Th1 cells in the salivary glands independent of IL-10. This conclusion is consistent with data where OX40 was shown to promote Th1 cell accumulation at the site of infection with influenza (32, 33) and Cryptococcus neoformans (20). Moreover, OX40 signaling can directly induce IFN-γ secretion and promote Th1 differentiation in tissue culture (16, 17). Anti-OX40 treatment reduced the viral load in the salivary glands by 1–2 log10, whereas anti–IL-10R reduced titers by 3 log10. The difference between the two pathways may reflect the finding that the IL-10–expressing CD4 T cells were not eliminated by treatment with anti-OX40. The finding that neither treatment altered the numbers of CD4+IL-10+ T cells indicates the difference in efficacy in reducing virus replication represented a fundamental distinction between the OX40 and IL-10R signaling pathways.
The ratio of T cells expressing IFN-γ to IL-10 provides a useful marker of immune regulation in the salivary glands and perhaps at other mucosal sites. A shift to IFN-γ expression pattern is critical for effective clearance of chronic parasitic infections (34), and correspondingly IFN-γ is necessary for the control of chronic MCMV infection (35). Elevated IFN-γ expression in the salivary glands may reduce viral persistence by directly inhibiting virus replication (35–37) or activation of additional effector mechanisms, such as NK, γδ T cells, and macrophages in the salivary glands (11, 38–40). IFN-γ also promotes CD8 T cell responses; however, in the context of the salivary glands, CD8 T cells do not limit virus replication (5), and neither anti-OX40 nor anti–IL-10R treatment had any effect on virus-specific CD8 T cell numbers, IFN-γ expression, or granzyme B expression, suggesting a minimal role for CD8 T cells in the protective effect of either treatment.
Induction of CD4+IL-10+ T cells after MCMV infection may represent a mucosal tolerance mechanism used by the salivary glands that is exploited by MCMV. It is likely that MCMV takes an active role in the exploitation of the mucosal tissue. Interestingly, MCMV alters the microarchitecture of the spleen by selective depression of CCL21 chemokine mRNA expression, which alters T cell trafficking within the spleen (41). Mutations in the viral genes M155 (42), M35 (43), or M133 (44, 45) abrogate replication in the salivary glands, although the role of these genes in promoting IL-10–expressing T cells is not understood. Of significance is the presence of viral IL-10 orthologs in primate CMV (46, 47), Epstein-Barr virus (48), and equine herpesvirus 2 (49). Although MCMV does not encode an obvious IL-10 ortholog, the observation that MCMV utilizes an IL-10–dependent mechanism to persist in salivary glands suggests a strong selective pressure is driving the conservation of targeting the IL-10 pathway in herpesviridae. The results raise the intriguing possibility that the primary function of cmvIL-10 in vivo may be to mimic IL-10–expressing T reg cells to block the action of the effector T cells secreting IFN-γ, allowing persistent virus replication specifically at mucosal sites. Thus, viral IL-10 orthologs may represent important targets to limit persistent virus replication in mucosal tissues such as the salivary glands, which in turn may block transmission of virus to new hosts.
MATERIALS AND METHODS
Mice.
Wild-type female C57BL/6 (B6) mice were purchased from The Jackson Laboratory, and MHC class II–deficient mice (50) were purchased from Taconic. All experiments were approved by institutional Animal Care and Use Committee.
Virus infections and antibody treatment
MCMV Smith (American Type Culture Collection) was prepared and titered as described previously (51). Mice were infected with 5 × 104 pfu MCMV by intraperitoneal route and, in some experiments, injected i.p. at the time of infection with 100 μg of either rat IgG (Chemicon) or anti-OX40 (clone OX86 [52]) purified by protein A affinity chromatography. In other experiments, mice were injected i.p. with 250 μg rat IgG1κ (clone R3-34; BD Biosciences) or anti-CD210 (anti–IL-10Rα clone 1B1.3a; BD Biosciences [53]).
Leukocyte isolation, intracellular cytokine staining, and flow cytometry
Salivary glands were surgically excised and tissue leukocytes were isolated as previously described (11). In brief, salivary glands, free of associated lymph nodes, were cut into small pieces and incubated in RPMI 1640 supplemented with 5 mM CaCl2, 5% fetal bovine serum, 1 mg/ml collagenase D (Roche Diagnostics), and 10 μg/ml DNase I (USB) at 37°C with mild agitation. After two consecutive incubations, dissociated cells and salivary gland tissue was passed through a 40-μm nylon cell strainer, and extruded cells were washed with PBS before analysis. The draining lymph nodes of the salivary glands were also excised, and cells were isolated by passage through a 40-μm nylon cell strainer.
Isolated leucocytes were then incubated at 5 × 106 cells/ml for 5 h at 37°C in the presence of 2 μg/ml brefeldin A (Sigma-Aldrich) and stimulated with or without 1 μg/ml anti-CD3 (clone 145–2C11) and 1 μg/ml anti-CD28 (clone 37.51; BD Biosciences), or 2 μg/ml of MCMV-derived peptides (Genemed Synthesis Inc.). The peptides used were the H-2Db–restricted M45-derived peptide HGIRNASFI and the H-2Kb–restricted peptides derived from the M38 (SSPPMFRVP), M57 (SCLEFWQRV), and M139 (TVYGFCLL) open reading frames, as previously described (54). Cells were then washed and incubated with Fc receptor blocking reagent and then stained with anti–CD8-PE-Cy7, anti–TCRβ-PE-cy5, and anti-CD4 antibody conjugated to FITC or APC-cy7 (BD Biosciences). Cells were then fixed with 4% formalin and permeabilized with saponin buffer (PBS, 2% FBS, 0.05% sodium azide, and 0.5% saponin) and stained with anti–IFN-γ-FITC or PE and, in some experiments, anti–IL-10-APC and PE-labeled anti–IL-5, anti–IL4, anti-FoxP3, or anti–IL-13 biotin (R&D Systems) followed by streptavidin-PE (BD Biosciences). In some experiments, unstimulated cells treated with brefeldin A were stained with anti-CD8 and anti–human granzyme B–PE (Catag/Invitrogen Life Technologies) or anti–NK1.1-APC and anti–IFN-γ. In other experiments, splenocytes were immediately stained with anti–MHC class II, anti-B220, anti-CD11c, anti–CD8-PE-cy7, anti–CD4-APC-cy7 (BD Biosciences), anti-F4/80 (eBioscience), and/or OX40-PE (Serotec). All cells were acquired on an LSR II flow cytometer (BD Bioscience).
Histology
Salivary glands were fixed in 10% formalin, paraffin-embedded, and 5-μm sections were stained with hematoxylin and eosin. Three sections from each gland were examined from four mice per group.
Gene expression
IFN-γ and IL-10 mRNA were assayed by quantitative RT-PCR using Stratagene Mx 4000 and SYBR green reagent (Bio-Rad Laboratories). Salivary glands from mock or MCMV-infected mice were snap frozen in liquid nitrogen before homogenization in TRIzol reagent (Invitrogen Life Technologies). Total cellular RNA was then isolated and quantified before performing DNase digestion and reverse transcription as described (55). The primer sequences for detection of mouse IFN-γ are GCCGTGGCAGTAACAGCC and AACGCTACACACTGCATCTGGG, and for IL-10 are CACTGCTATGCTGCCTTCTCT and TGTCTTCCCTGCTGTACTGT.
Online supplemental material
Fig. S1 shows time-course analysis of numbers of CD8 T cells (a), granzyme B expression by CD8 T cells (b), and accumulation of IFN-γ–expressing CD8 T cells (d), in the salivary glands of anti-OX40 or control IgG-treated mice. Panel c shows MCMV-specific CD8 T cell accumulation in the salivary glands at day 7 after infection in anti-OX40–treated or control IgG-treated mice.
Fig. S2 shows the numbers of CD8+/IFN-γ+ and NK+/CD3−/IFN-γ+ cells (a) and the percentage (b) and the mean fluorescent intensity (c) of MHC class II expression by leukocytes in the salivary glands of anti–IL-10R or control IgG-treated mice 14 d after infection.
Supplemental Material
Acknowledgments
The authors wish to thank Dr. Kirsten Schneider and Professor Brigitte Askonas for valuable discussions; Xiaohong Tang, Yan Fei Adams, and Antje Rhodes for excellent technical support; Dr. Ann Hill for MCMV peptide sequences recognized by CD8 T cells; and Dr. Tim Cheung for assistance with figure preparation.
This work was supported in part by National Institutes of Health (NIH) grants CA91837 and AI67341 (to M. Croft); NIH Predoctoral Fellowship T32 AI060536 (to A. Kinkade); and AI48073, AI057840, and R37AI33068 (to C.F. Ware). This is publication #793 from the La Jolla Institute for Allergy and Immunology.
The authors have no conflicting financial interests.
Abbreviations used: HCMV, human CMV; MCMV, mouse CMV; T reg, T regulatory.
References
- 1.Britt, W.J., and C.A. Alford. 1996. Cytomegalovirus. In Virology. B.N. Fields and D.M. Knipe, editors. Lippincott-Raven Publishers, Philadelphia, PA. 2493–2523.
- 2.Zanghellini, F., S.B. Boppana, V.C. Emery, P.D. Griffiths, and R.F. Pass. 1999. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J. Infect. Dis. 180:702–707. [DOI] [PubMed] [Google Scholar]
- 3.Rowe, W.P., J.W. Hartley, Cramblett, and F.M. Mastrota. 1958. Detection of human salivary gland virus in the mouth and urine of children. Am. J. Hyg. 67:57–65. [DOI] [PubMed] [Google Scholar]
- 4.Henson, D., and A.J. Strano. 1972. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am. J. Pathol. 68:183–202. [PMC free article] [PubMed] [Google Scholar]
- 5.Jonjic, S., W. Mutter, F. Weiland, M.J. Reddehase, and U.H. Koszinowski. 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 169:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukowski, J.F., B.A. Woda, and R.M. Welsh. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddehase, M.J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U.H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U.H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise, M.T., M. Connick, and H.W. Virgin IV. 1998. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II–associated genes. J. Exp. Med. 187:1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu, X., A.K. Pinto, A.M. Kelly, K.S. Cho, and A.B. Hill. 2006. Murine cytomegalovirus interference with antigen presentation contributes to the inability of CD8 T cells to control virus in the salivary gland. J. Virol. 80:4200–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanaugh, V.J., Y. Deng, M.P. Birkenbach, J.S. Slater, and A.E. Campbell. 2003. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J. Virol. 77:1703–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer, L., and L. Shao. 2004. Therapeutic potential of oral tolerance. Nat. Rev. Immunol. 4:407–419. [DOI] [PubMed] [Google Scholar]
- 13.O'Garra, A., and P. Vieira. 2004. Regulatory T cells and mechanisms of immune system control. Nat. Med. 10:801–805. [DOI] [PubMed] [Google Scholar]
- 14.Roncarolo, M.G., R. Bacchetta, C. Bordignon, S. Narula, and M.K. Levings. 2001. Type 1 T regulatory cells. Immunol. Rev. 182:68–79. [DOI] [PubMed] [Google Scholar]
- 15.Croft, M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609–620. [DOI] [PubMed] [Google Scholar]
- 16.Gramaglia, I., A. Jember, S.D. Pippig, A.D. Weinberg, N. Killeen, and M. Croft. 2000. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 165:3043–3050. [DOI] [PubMed] [Google Scholar]
- 17.Rogers, P.R., and M. Croft. 2000. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J. Immunol. 164:2955–2963. [DOI] [PubMed] [Google Scholar]
- 18.Ito, T., Y.H. Wang, O. Duramad, S. Hanabuchi, O.A. Perng, M. Gilliet, F.X. Qin, and Y.J. Liu. 2006. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc. Natl. Acad. Sci. USA. 103:13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, T., Y.H. Wang, O. Duramad, T. Hori, G.J. Delespesse, N. Watanabe, F.X. Qin, Z. Yao, W. Cao, and Y.J. Liu. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys, I.R., L. Edwards, G. Walzl, A.J. Rae, G. Dougan, S. Hill, and T. Hussell. 2003. OX40 ligation on activated T cells enhances the control of Cryptococcus neoformans and reduces pulmonary eosinophilia. J. Immunol. 170:6125–6132. [DOI] [PubMed] [Google Scholar]
- 21.So, T., J. Song, K. Sugie, A. Altman, and M. Croft. 2006. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc. Natl. Acad. Sci. USA. 103:3740–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks, D.G., M.J. Trifilo, K.H. Edelmann, L. Teyton, D.B. McGavern, and M.B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejrnaes, M., C.M. Filippi, M.M. Martinic, E.M. Ling, L.M. Togher, S. Crotty, and M.G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundstedt, A., I. Hoiden, A. Rosendahl, T. Kalland, N. van Rooijen, and M. Dohlsten. 1997. Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J. Immunol. 158:180–186. [PubMed] [Google Scholar]
- 25.Sundstedt, A., E.J. O'Neill, K.S. Nicolson, and D.C. Wraith. 2003. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170:1240–1248. [DOI] [PubMed] [Google Scholar]
- 26.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 27.Anderson, C.F., M. Oukka, V.J. Kuchroo, and D. Sacks. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankovic, D., M.C. Kullberg, C.G. Feng, R.S. Goldszmid, C.M. Collazo, M. Wilson, T.A. Wynn, M. Kamanaka, R.A. Flavell, and A. Sher. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, K.W., R.B. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 30.Redpath, S., A. Angulo, N.R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701–6707. [PubMed] [Google Scholar]
- 31.Shoemaker, J., M. Saraiva, and A. O'Garra. 2006. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J. Immunol. 176:3470–3479. [DOI] [PubMed] [Google Scholar]
- 32.Kopf, M., C. Ruedl, N. Schmitz, A. Gallimore, K. Lefrang, B. Ecabert, B. Odermatt, and M.F. Bachmann. 1999. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL responses after virus infection. Immunity. 11:699–708. [DOI] [PubMed] [Google Scholar]
- 33.Humphreys, I.R., G. Walzl, L. Edwards, A. Rae, S. Hill, and T. Hussell. 2003. A critical role for OX40 in T cell–mediated immunopathology during lung viral infection. J. Exp. Med. 198:1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sher, A., and R.L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385–409. [DOI] [PubMed] [Google Scholar]
- 35.Presti, R.M., J.L. Pollack, A.J. Dal Canto, A.K. O'Guin, and H.W Virgin, 4th. 1998. Interferon gamma regulates acute and lethal murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucin, P., S. Jonjic, M. Messerle, B. Polic, H. Hengel, and U.H. Koszinowski. 1994. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J. Gen. Virol. 75:101–110. [DOI] [PubMed] [Google Scholar]
- 37.Gribaudo, G., S. Ravaglia, A. Caliendo, R. Cavallo, M. Gariglio, M.G. Martinotti, and S. Landolfo. 1993. Interferons inhibit onset of murine cytomegalovirus immediate-early gene transcription. Virology. 197:303–311. [DOI] [PubMed] [Google Scholar]
- 38.Weigent, D.A., G.J. Stanton, and H.M. Johnson. 1983. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect. Immun. 41:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heise, M.T., and H.W.t. Virgin. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J.Virol. 69:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noda, S., K. Tanaka, S. Sawamura, M. Sasaki, T. Matsumoto, K. Mikami, Y. Aiba, H. Hasegawa, N. Kawabe, and Y. Koga. 2001. Role of nitric oxide synthase type 2 in acute infection with murine cytomegalovirus. J. Immunol. 166:3533–3541. [DOI] [PubMed] [Google Scholar]
- 41.Benedict, C.A., C. De Trez, K. Schneider, S. Ha, G. Patterson, and C.F. Ware. 2006. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boname, J.M., and J.K. Chantler. 1992. Characterization of a strain of murine cytomegalovirus which fails to grow in the salivary glands of mice. J. Gen. Virol. 73:2021–2029. [DOI] [PubMed] [Google Scholar]
- 43.Tam, A., J. Zhu, R. Hai, E. Haghjoo, T. Tong, X. Zhan, S. Lu, and F. Liu. 2003. Murine cytomegalovirus with a transposon insertional mutation at open reading frame M35 is defective in growth in vivo. J. Virol. 77:7746–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning, W.C., C.A. Stoddart, L.A. Lagenaur, G.B. Abenes, and E.S. Mocarski. 1992. Cytomegalovirus determinant of replication in salivary glands. J. Virol. 66:3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagenaur, L.A., W.C. Manning, J. Vieira, C.L. Martens, and E.S. Mocarski. 1994. Structure and function of the murine cytomegalovirus sgg1 gene: a determinant of viral growth in salivary gland acinar cells. J. Virol. 68:7717–7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotenko, S.V., S. Saccani, L.S. Izotova, O.V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA. 97:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockridge, K.M., S.S. Zhou, R.H. Kravitz, J.L. Johnson, E.T. Sawai, E.L. Blewett, and P.A. Barry. 2000. Primate cytomegaloviruses encode and express an IL-10-like protein. Virology. 268:272–280. [DOI] [PubMed] [Google Scholar]
- 48.Hsu, D.H., R. de Waal Malefyt, D.F. Fiorentino, M.N. Dang, P. Vieira, J. de Vries, H. Spits, T.R. Mosmann, and K.W. Moore. 1990. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 250:830–832. [DOI] [PubMed] [Google Scholar]
- 49.Rode, H.J., W. Janssen, A. Rosen-Wolff, J.J. Bugert, P. Thein, Y. Becker, and G. Darai. 1993. The genome of equine herpesvirus type 2 harbors an interleukin 10 (IL10)-like gene. Virus Genes. 7:111–116. [DOI] [PubMed] [Google Scholar]
- 50.Grusby, M.J., R.S. Johnson, V.E. Papaioannou, and L.H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 253:1417–1420. [DOI] [PubMed] [Google Scholar]
- 51.Reddehase, M.J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U.H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al Shamkhani, A., M.L. Birkeland, M. Puklavec, M.H. Brown, W. James, and A.N. Barclay. 1996. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur. J. Immunol. 26:1695–1699. [DOI] [PubMed] [Google Scholar]
- 53.O'Farrell, A.M., Y. Liu, K.W. Moore, and A.L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17:1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munks, M.W., M.C. Gold, A.L. Zajac, C.M. Doom, C.S. Morello, D.H. Spector, and A.B. Hill. 2006. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 176:3760–3766. [DOI] [PubMed] [Google Scholar]
- 55.Benedict, C.A., T.A. Banks, L. Senderowicz, M. Ko, W.J. Britt, A. Angulo, P. Ghazal, and C.F. Ware. 2001. Lymphotoxins and cytomegalovirus cooperatively induce interferon-β, establishing host-virus détente. Immunity. 15:617–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.