Abstract
Interleukin-1 receptor–associated kinase 4 (IRAK-4) was reported to be essential for the Toll-like receptor (TLR)– and T cell receptor (TCR)–mediated signaling leading to the activation of nuclear factor κB (NF-κB). However, the importance of kinase activity of IRAK family members is unclear. In this study, we investigated the functional role of IRAK-4 activity in vivo by generating mice carrying a knockin mutation (KK213AA) that abrogates its kinase activity. IRAK-4 KN/KN mice were highly resistant to TLR-induced shock response. The cytokine production in response to TLR ligands was severely impaired in IRAK-4 KN/KN as well as IRAK-4 −/− macrophages. The IRAK-4 activity was essential for the activation of signaling pathways leading to mitogen-activated protein kinases. TLR-induced IRAK-4/IRAK-1–dependent and –independent pathways were involved in early induction of NF-κB–regulated genes in response to TLR ligands such as tumor necrosis factor α and IκBζ. In contrast to a previous paper (Suzuki, N., S. Suzuki, D.G. Millar, M. Unno, H. Hara, T. Calzascia, S. Yamasaki, T. Yokosuka, N.J. Chen, A.R. Elford, et al. 2006. Science. 311:1927–1932), the TCR signaling was not impaired in IRAK-4 −/− and IRAK-4 KN/KN mice. Thus, the kinase activity of IRAK-4 is essential for the regulation of TLR-mediated innate immune responses.
The innate immune system senses pathogen-specific molecular patterns via pattern recognition receptors, such as Toll-like receptors (TLRs; references 1–3). 12 TLR family members have been identified in mammals, and the pathogen-specific molecular patterns recognized by these TLRs have been mostly identified. The cytoplasmic portion of TLRs, called TIR (Toll/IL-1 receptor [IL-1R]) domain, resembles that of IL-1R family members, and these two receptor families in part share intracellular signaling machineries. Stimulation with TLR ligands or IL-1 family cytokines recruits a TIR domain–containing adaptor, MyD88, to the receptors. IL-1R–associated kinases (IRAKs) are recruited to MyD88 through a homophilic interaction of the death domains and associate with TNF receptor–associated factor 6 (TRAF6), which acts as an ubiquitin protein ligase. TRAF6 catalyzes the formation of a K63-linked polyubiquitin chain on TRAF6 itself and on IκB kinase γ (IKK-γ)/NF-κB essential modulator. TGF-β–activated kinase 1 is also recruited to TRAF6 and then phosphorylates IKK-β and mitogen-activated protein (MAP) kinase kinase 6. Phosphorylation of IκB by the IKK complex leads to its degradation, and freed NF-κB translocates into the nucleus, resulting in induction of genes involved in inflammatory responses as well as increase in the surface expression of costimulatory molecules on innate immune cells. The activation of MAP kinase cascade is responsible for AP-1–induced gene expression. In addition to the MyD88-dependent signaling pathway, the TLR4 signaling also activates a MyD88-independent signaling cascade via another TIR domain–containing adaptor protein inducing IFN-β, TRIF (4). It triggers the signaling cascade leading to the production of type I IFNs via IKK-related kinases, TANK-binding kinase 1 (TBK1) and IKK-i. The TLR3 signaling also entirely relies on TRIF to activate NF-κB and IFN-regulatory factors.
The IRAK family is comprised of four members and is characterized by the presence of an N-terminal death domain and a serine/threonine kinase domain (5). IRAK-1 was initially identified as a kinase that is coprecipitated with IL-1R in response to IL-1 stimulation (6). IRAK-1 associates with MyD88 through a homophilic interaction of the death domains (7). Whereas IRAK-1 has a nonredundant role in the production of type I IFNs in response to TLR9 ligands in plasmacytoid DCs (pDCs; reference 8), IRAK1-deficient (IRAK-1 −/−) macrophages show modest impairment in IL-1R– and TLR-mediated proinflammatory cytokine production (9, 10). IRAK-2 is suggested to be involved in the signaling via TIRAP/Mal, an adaptor protein responsible for TLR2 and TLR4 responses (11). In contrast, IRAK-M was identified as the negative regulator of the TLR/IL-1R signaling (12). The fourth member of IRAK family members, IRAK-4, has been discovered by a database search (13). Generation of IRAK-4 −/− mice revealed its essential role in IL-1R/TLR–mediated responses (14, 15). Furthermore, the poor defenses against bacterial infection were observed in patients having autosomal recessive amorphic mutations in IRAK-4 (16, 17). It was suggested that IRAK-4 can directly phosphorylate IRAK-1 for the signaling. Recently, IRAK-4 has been reported to be a requisite for TCR-induced NF-κB activation by associating with ZAP-70 (18).
Although IRAK family members are involved in TLR/IL-1R signaling, the role of their kinase activity is still controversial. Among IRAK family members, IRAK-1 and -4 were shown to possess intrinsic kinase activity (13, 19). Nevertheless, it has been shown that IRAK-1 kinase activity is dispensable for its ability to activate NF-κB (20). IRAK-1 could act as a scaffold protein recruiting MyD88 and TRAF6 for the signaling (13). Second, a critical aspartate residue in the catalytic domain has changed to an asparagine or a serine in IRAK-2 or -M, and their kinase domains have been shown to be inactive (21). Regarding the requirement of IRAK-4 activity for the IL-1R signaling, two controversial observations have been reported to date (22, 23). One paper showed that the reconstitution with the kinase-inactive mutant IRAK-4 fully restored IL-1 responsiveness (22), whereas the other showed that the same reconstitution was capable of restoring only a partial cytokine response to IL-1β (23). Therefore, the requirement of kinase activity in IRAK family members has not been well understood.
In the present study, we generated mice carrying a knockin mutation that abrogated IRAK-4 activity. For the assessment of the roles of kinase activity, we also generated IRAK-4 −/− mice. The analysis of these mice revealed that the kinase activity of IRAK-4 is essential for the physiological function of IRAK-4, and the TLR-mediated proinflammatory responses are severely impaired in these mice. Nevertheless, we did not observe any defects in the T cell responses in either IRAK-4 −/− or IRAK-4 KN/KN mice. This study demonstrates that IRAK-4 functions as an actual kinase for relaying the TLR signaling.
RESULTS
Generation of IRAK-4 KN/KN and IRAK-4 −/− mice
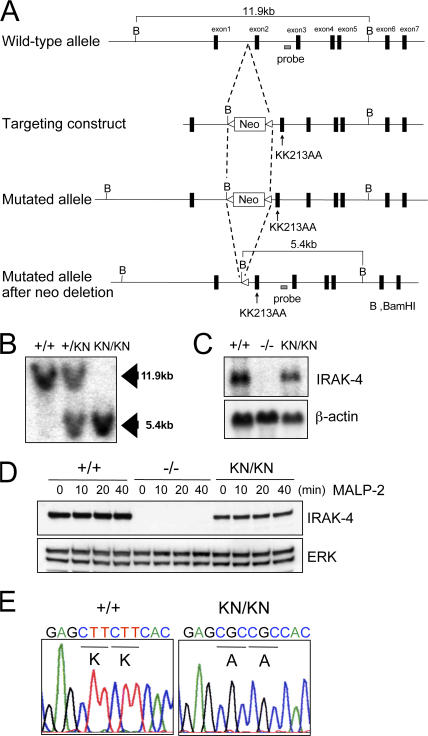
It has been shown that a mutation in ATP binding pocket (K239S) of IRAK-1 abrogated its kinase activity. Nevertheless, overexpression of this mutant IRAK-1 still efficiently induced NF-κB activation. Corresponding mutations in IRAK-4 (KK213AA) was capable of inducing activation of NF-κB in response to IL-1β stimulation. These results suggested that IRAK family members function as adaptor molecules for the signaling, and the IRAK kinase activity was dispensable for their function. To identify the role of IRAK-4 activity in TLR signaling, we inserted a mutation (KK213AA) of IRAK-4. To replace serines 213 and 214 of IRAK-4 with alanines, a loxP-flanked Neo cassette was inserted. Serine to alanine substitutions were introduced by site-directed mutagenesis (Fig. 1 A). A targeting vector containing these mutations were electroporated into embryonic stem (ES) cells, clones with homologous recombination at the IRAK-4 locus were obtained, and IRAK-4–mutated mice were generated. The mice were crossed with CAG-Cre transgenic mice to excise the neo resistant gene. Homologous recombination of IRAK-4 locus was confirmed by Southern blotting, and the sequencing analysis revealed that the mutations were correctly introduced (Fig. 1, B and E). The Northern blot and immunoblot analysis showed that IRAK-4 messenger RNA (mRNA) and protein were expressed in wild-type and IRAK-4 KN/KN macrophages, although the expression of IRAK-4 protein was slightly reduced in IRAK-4 KN/KN macrophages (Fig. 1, C and D).
Figure 1.
Generation of IRAK-4KN/KN mice. (A) Schematic representation of IRAK-4 (KK213AA) knockin allele. The targeting vector contains point mutations in exon 2 that change lysines at position 213 and 214 to alanine (KK213AA). (B) Southern blot analysis of offspring from the heterozygote intercrosses. Genomic DNA was extracted from mouse tails, digested with BamHI, separated by electrophoresis, and hybridized with the radiolabeled probe indicated in A. (C) Nothern blot analysis of the expression of IRAK-4 mRNA. Total RNA from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN peritoneal macrophages were extracted and subjected to the Northern blot analysis for the expression of IRAK-4 mRNA. The same membrane was rehybridized with a β-actin probe. (D) Immunoblot analysis for the expression of IRAK-4 protein expression in wild-type (+/+), IRAK-4 −/− (−/−), and IRAK-4 KN/KN (KN/KN) macrophages. Cell lysates from peritoneal macrophages stimulated with 10 ng/ml MALP-2 for the indicated times were immunoblotted with Ab to IRAK-4. Probing for ERK1/2 was used to ensure equal loading. (E) PCR amplification products from wild-type (+/+) and IRAK-4 KN/KN (KN/KN) macrophages were subcloned and sequenced to demonstrate the presence of the KK213AA mutation; representative traces are shown.
We also generated IRAK-4 −/− mice by homologous recombination to compare the importance of kinase activity of IRAK-4 (Fig. S1, A and B, available at http://www.jem.org/cgi/content/full/jem.20061523/DC1). For generation of IRAK-4 −/− mice, we targeted exon 2 of mouse IRAK-4 gene with the neo cassette in ES cells and established IRAK-4 −/− mice. Absence of IRAK-4 protein in IRAK-4 −/− macrophages was confirmed by Northern blotting and immunoblotting (Fig. 1, C and D).
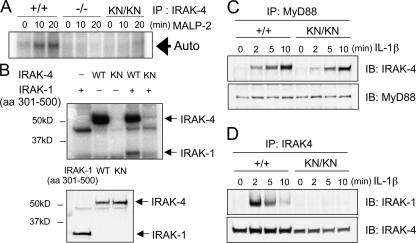
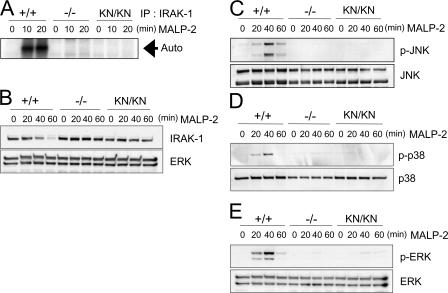
In vitro kinase assay revealed that IRAK-4 autophosphorylation was induced in wild-type macrophages in response to TLR2 stimulation (Fig. 2 A). In contrast, the autophosphorylation of IRAK-4 was not observed either in IRAK-4 KN/KN or IRAK-4 −/− cells. To further investigate whether the mutant IRAK-4 can phosphorylate IRAK-1, we isolated IRAK-4 cDNA from wild-type and IRAK-4 KN/KN cells. The IRAK-4 proteins were expressed using the rabbit reticulocyte lysate system, and in vitro kinase assay was performed with mouse IRAK-1 protein (aa 301–500), which contains an activation loop, as a substrate. As shown in Fig. 2 B, the wild-type IRAK-4, but not IRAK-4 (KK213AA), phosphorylated IRAK-1. Immunoblot analysis revealed that the amounts of wild-type and IRAK-4 (KK213AA) expressed were not altered (Fig. 2 B).
Figure 2.
The kinase activity in IRAK-4KN/KN mice. (A) Peritoneal macrophages were stimulated with 10 ng/ml MALP-2 for 0, 10, and 20 min. The cell lysates were prepared and immunoprecipitated with anti–IRAK-4 Ab. The kinase activity of IRAK-4 was measured by in vitro kinase assay. The data shown are representative of three independent experiments. Auto, autophosphorylation. (B) IRAK-4 cDNA was obtained from total RNA of wild-type and IRAK-4 KN/KN macrophages. Wild-type and KK213AA IRAK-4 proteins were expressed in the rabbit reticulocyte lysates, and in vitro kinase assay was performed in the presence of IRAK-1 (aa 301–500; top panel). The expression of wild-type and KK213AA IRAK-4 and IRAK-1 (aa 301–500) was determined by immunoblot analysis (bottom panel). (C) Interaction of MyD88 and IRAK-4. MEFs were stimulated with 10 ng/ml IL-1β for the indicated periods. The cell lysates were immunoprecipitaed with anti-MyD88, followed by immunoblot with anti–IRAK-4 and anti-MyD88. (D) IL-1β–induced coprecipitation of IRAK-1 and -4. The cell lysates prepared in C were immunoprecipitated with anti–IRAK-4, followed by immunoblot with anti–IRAK-1 and anti–IRAK-4.
We next examined whether IRAK-4 activity was required for the recruitment of IRAK-4 in the complex of MyD88 and IRAK-1 in response to IL-1R stimulation. When mouse embryonic fibroblasts (MEFs) were stimulated with IL-1β, IRAK-4 was coprecipitaited with MyD88 in both wild-type and IRAK-4 KN/KN cells (Fig. 2 C). In contrast, interaction between IRAK-4 and -1 was not induced in IRAK-4 KN/KN macrophages (Fig. 2 D). These results indicate that IRAK-4 activity is dispensable for the recruitment of IRAK-4 to MyD88, although the kinase activity is essential for the recruitment of IRAK-1 to -4.
The role of IRAK-4 activity in TLR-induced responses
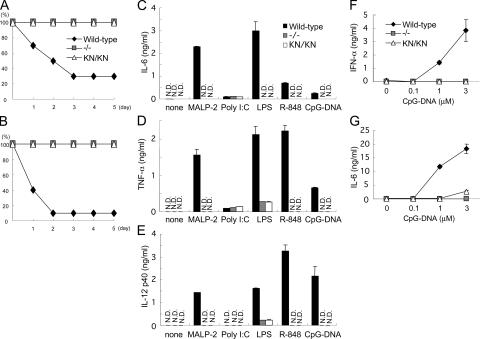
We first examined the role of IRAK-4 activity in response to TLR ligand stimulation in vivo. After challenge with LPS or CpG-DNA together with d-galactosamine, wild-type mice succumbed to shock and died, whereas all IRAK-4 −/− and IRAK-4 KN/KN mice survived, indicating that the kinase activity of IRAK-4 is critical for the TLR-induced shock in vivo (Fig. 3, A and B). We examined cytokine production of macrophages possessing mutated IRAK-4 against TLR ligands, including macrophage-activating lipopeptide-2 (MALP-2; TLR6/TLR2), poly I:C (TLR3), LPS (TLR4), R-848 (TLR7), and CpG-DNA (TLR9). Thioglycollate-elicited peritoneal macrophages were stimulated with each TLR ligand and the production of proinflammatory cytokines was measured by ELISA. In accordance with a previous paper, production of IL-6, TNF-α, and IL-12p40 in response to these TLR ligands except poly I:C was severely impaired in IRAK-4 −/− macrophages compared with wild-type cells (14). The production of IL-6, TNF-α, and IL-12p40 was also profoundly impaired in IRAK-4 KN/KN cells, and the extent of reduction was similar to that of IRAK-4 −/− cells (Fig. 3, C–E). In contrast, IL-6 and TNF-α production in response to poly I:C was not altered between wild-type, IRAK-4 −/−, and IRAK-4 KN/KN macrophages. DCs from IRAK-4 KN/KN mice also showed defective cytokine production in response to these TLR ligands (unpublished data). Thus, the IRAK-4 activity is important for evoking cytokine production in response to various TLR ligands, except for TLR3 ligand.
Figure 3.
Essential role of IRAK-4 activity in TLR-mediated cytokine responses. (A and B) Age-matched wild-type (n = 10), IRAK-4 −/− (n = 5), and IRAK-4 KN/KN (n = 5) mice were challenged with 2 mg LPS (A) and 20 nmol CpG-DNA together with 20 mg d-galactosamine (B). The survival of mice was monitored for 5 d. (C–E) Thioglycollate-elicited peritoneal macrophages from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with MALP-2, poly I:C, LPS, R-848, and CpG-DNA for 24 h. For measuring IL-12 p40 concentration, macrophages were stimulated with the indicated TLR ligands in the presence of 30 ng/ml IFN-γ. Concentrations of IL-6 (C), TNF-α (D), and IL-12 p40 (E) in the culture supernatants were measured by ELISA. Data are shown as mean ± SD of triplicates. Similar results were obtained in three independent experiments. (F and G) Flt3L-DCs from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with the indicated concentrations of A/D type CpG-DNA. Production of IFN-α (D) and IL-6 (E) was measured by ELISA. Data are representative of three independent experiments. Indicated values are mean ± SD of triplicates.
Not only proinflammatory cytokines but also type I IFNs are strongly induced in response to TLR7 and TLR9 stimulation in pDCs (8, 17, 24). It has been shown that IRAK-4 and -1 are essential for TLR9-induced type I IFN production in pDCs. To examine the role of IRAK-4 activity in type I IFN response, we generated pDCs by cultivating bone-marrow cells in the presence of Flt3 ligand. Whereas wild-type Flt3L-DCs produced IFN-α and IL-6 in response to A/D-type CpG-DNA, cells from neither IRAK-4 −/− nor IRAK-4 KN/KN mice produced both IFN-α and IL-6 (Fig. 3, F and G).
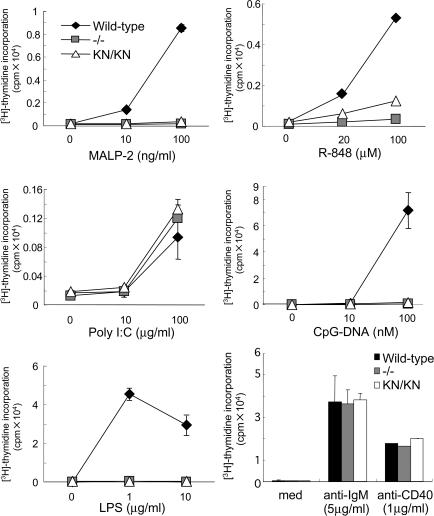
Next we investigated the proliferation of B cells in response to these TLR ligands. When we stimulated purified B cells with MALP-2, poly I:C, LPS, R-848, and CpG-DNA, wild-type B cells proliferated in a dose-dependent manner (Fig. 4). In contrast, IRAK-4 −/− as well as IRAK-4 KN/KN B cells failed to proliferate in response to MALP-2, LPS, R-848, and CpG-DNA. Stimulation with poly I:C induced proliferation of B cells even in IRAK-4 −/− and IRAK-4KN/KN mice, suggesting that TLR3 signals independent of IRAK-4 in B cells. The proliferative responses against anti-IgM, anti-CD40 antibodies (Abs) were not altered in wild-type, IRAK-4 −/−, and IRAK-4 KN/KN splenocytes, indicating that the MyD88-dependent responses were specifically impaired in IRAK-4 −/− or IRAK-4 KN/KN cells. Collectively, the kinase activity of IRAK-4 is essential for the pleiotrophic effects in response to TLR stimulation.
Figure 4.
IRAK-4 activity is critical for TLR-mediated proliferation of splenocytes. Splenocytes were cultured with the indicated concentrations of MALP-2, poly I:C, LPS, R-848, CpG-DNA, anti-IgM, or anti-CD40 for 48 h. Samples were pulsed with 1 μCi [3H]thymidine for the last 16 h. [3H]thymidine incorporation was measured by a scintillation counter. Data are representative of three independent experiments. Indicated values are mean ± SD of triplicates.
Expression of TLR-mediated gene expression in IRAK-4 mutated mice
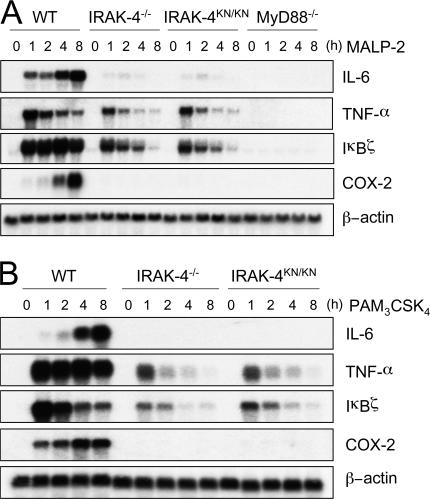
We examined whether the defects in cytokine response to TLR stimulation in IRAK-4 mutation were regulated in a gene expression level by Northern blot analysis. We chose TLR2 ligands as the stimulant because TLR2 signals only via the MyD88-dependent pathway. In response to MALP-2 stimulation, wild-type macrophages induced expression of IL-6, TNF-α, IκBζ, and cyclooxygenase-2 (COX-2) genes (Fig. 5 A). In contrast, IRAK-4 −/− and IRAK-4 KN/KN macrophages failed to express IL-6 and COX-2 in response to MALP-2 stimulation. However, TNF-α and IκBζ were expressed even in the absence of IRAK-4, albeit the expression was weaker than wild-type cells. Thus, the kinase activity of IRAK-4 is critical for regulating IRAK-4–mediated controlling of gene expression. Indeed, TNF bioassay revealed that a subtle amount of TNF activity was induced 1 and 2 h after MALP-2 stimulation in IRAK-4 −/− and IRAK-4 KN/KN macrophages, although the amount was much smaller than in wild-type cells (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061523/DC1). Interestingly, MyD88−/− macrophages failed to induce any detectable amount of these genes in response to MALP-2 stimulation (Fig. 5 A). In addition, PAM3CSK4, a synthetic lipopeptide known to be recognized by TLR1/TLR2 heterodimer, also induces expression of TNF-α and IκBζ even in the absence of IRAK-4 (Fig. 5 B; reference 25). These results indicate that the early expression of TNF-α and IκBζ genes in response to TLR2 ligands is regulated in part in a IRAK-4–independent fashion, although IRAK-4 plays a major role in the expression of TLR2-inducible genes.
Figure 5.
IRAK-4–independent induction of gene expression in TLR signaling. (A and B) Peritoneal macrophages were stimulated with 10 ng/ml MALP-2 (A) and 10 ng/ml PAM3CSK4 (B) for the indicated periods. Total RNA was extracted and subjected to Northern blot analysis for expression of IL-6, TNF-α, Iκ Bζ, and COX-2. The same membrane was rehybridized with a β-actin probe.
The role of IRAK-4 activity in the TLR-mediated signaling pathway
These observations prompted us to examine intracellular signaling pathways in response to TLR stimulation. It has been shown that IRAK-4 is recruited to IL-1R in response to ligand stimulation, where it phosphorylates and activates IRAK-1. It was also shown that IRAK-4 is essential for the initiation of IL-1R–mediated signaling pathways leading to the activation of NF-κB and MAP kinases in MEFs (14).
TLR2-mediated autophosphorylation of IRAK-1 was completely abrogated in IRAK-4 −/− and IRAK-4 KN/KN macrophages (Fig. 6 A). TLR/IL-1R stimulation induces not only phosphorylation but also degradation of IRAK-1. MALP-2 stimulation decreased the IRAK-1 expression in wild-type macrophages (Fig. 6 B). However, TLR2-mediated degradation of IRAK-1 was not observed in IRAK-4 −/− and IRAK-4 KN/KN macrophages. Thus, IRAK-4 activity is essential for IRAK-1 activation in response to TLR2 activation.
Figure 6.
IRAK-4 is critical for TLR-mediated activation of IRAK-1 and MAP kinases. (A) In vitro kinase assay for IRAK-1 activation. Peritoneal macrophages were stimulated with 10 ng/ml MALP-2 for the indicated periods. The cell lysates were prepared and immunoprecipitated with anti-IRAK-1 Ab, and the kinase activity of IRAK-1 was measured by in vitro kinase assay. The data shown are representative of three independent experiments. Auto, autophosphorylation. (B) Immunoblot analysis for the change in IRAK-1 expression in response to MALP-2 stimulation. Peritoneal macrophages from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with MALP-2 for the indicated periods. Whole cell lysates were subject to immunoblot analysis using anti–IRAK-1. ERK1/2 levels are shown as loading control. (C–E) Macrophages from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with MALP-2 for the indicated periods, and whole cell lysates were subject to immunoblot analysis using anti–phospho-JNK (C), anti–phospho-P38 (D), and anti–phospho-ERK (E). The blots of JNK, p38, and ERK are shown as loading controls. Similar results were obtained in three independent experiments.
We examined the role of IRAK-4 activity in the activation of MAP kinases and NF-κB in macrophages. First, activation of c-Jun N-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK) induced by MALP-2 was profoundly impaired in IRAK-4 −/− as well as IRAK-4 KN/KN macrophages (Fig. 6, C–E). These results indicate that IRAK-4 activity is critical for the activation of MAP kinases in the TLR signaling.
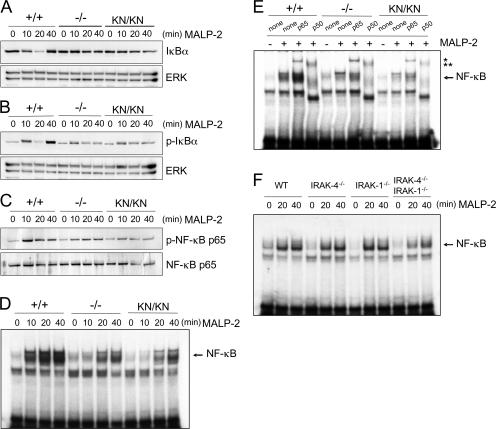
We analyzed activation of NF-κB. Phosphorylation and degradation of IκBα were also severely impaired in IRAK-4 −/− and IRAK-4 KN/KN macrophages (Fig. 7, A and B). MALP-2–induced phosphorylation of NF-κB p65 was not observed in IRAK-4 −/− and IRAK-4 KN/KN macrophages (Fig. 7 C). Nevertheless, an electrophoretic mobility shift assay (EMSA) revealed that the NF-κB–DNA binding activity was clearly induced even in the absence of IRAK-4, although the activation was ∼10 min delayed compared with wild-type cells (Fig. 7 D). Consistent with our previous study, MyD88 −/− macrophages failed to induce NF-κB–DNA binding activity in response to MALP-2 (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20061523/DC1; reference 26). These results indicate that TLR2 activates a MyD88-dependent and IRAK-4–independent signaling pathway leading to the activation of NF-κB. To assess the subunits of the NF-κB complexes observed in response to MALP-2 stimulation, we performed supershift assays using anti-p65 or anti-p50 Ab and nuclear extracts from macrophages stimulated with MALP-2 for 40 min (Fig. 7 E). In wild-type and IRAK-4–mutated cells, the bands were supershifted with anti-p65 and anti-p50 Ab, suggesting that the NF-κB complex is mainly composed of p65/p50 heterodimers in both wild-type and IRAK-4–mutated cells. These findings indicate that TLR2 activates a MyD88-dependent and IRAK-4–independent signaling pathway leading to the activation of NF-κB. Stimulation with R-848 and CpG-DNA also induced NF-κB activation in an IRAK-4–independent manner without degrading IκBα (Fig. S4). When IRAK-4–mutated cells were stimulated with LPS, degradation of IκBα as well as NF-κB–DNA binding was delayed as observed in MyD88 −/− macrophages (Fig. S4). These data indicate that the IRAK- 4–independent pathway is activated downstream of various TLRs.
Figure 7.
IRAK-4–independent activation of NF-κB in TLR2 signaling. (A–C) Macrophages from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with MALP-2 for the indicated periods. Whole cell lysates were subject to immunoblot analysis using anti-IκBα (A), anti–phospho-IκBα (B), and anti–phospho-NF-κB p65 Abs (C). The blots of ERK1/2 and NF-κB p65 are shown as loading controls. The data are representative of three independent experiments. (D) Wild-type, IRAK-4 −/−, and IRAK-4 KN/KN macrophages were stimulated with 10 ng/ml MALP-2 for the indicated periods. Nuclear extracts were then prepared, and NF-κB–DNA binding activity was determined by EMSA using an NF-κB–specific probe. (E) Nuclear extracts from macrophages stimulated with MALP-2 for 40 min were incubated with Abs specific to NF-κB p65 and p50 before addition of NF-κB probe. The single and double asterisks indicate the supershifts induced by Ab to p65 and p50, respectively. (F) IRAK-1/IRAK-4–independent activation of NF-κB in response to TLR stimulation. Wild-type, IRAK-4 −/−, IRAK-1 −/−, and IRAK-4 −/− IRAK-1 −/− doubly deficient macrophages were stimulated with 10 ng/ml MALP-2 for the indicated periods. NF-κB–DNA binding activity was determined by EMSA.
Activation of NF-κB in the IRAK-1–independent and IRAK-4–dependent signaling pathway
It was revealed that the death domain of MyD88 is responsible for triggering downstream signaling cascades. Given that only IRAK-4 and -1 have intrinsic kinase activity, it was hypothesized that IRAK-4 and -1 function redundantly in activating NF-κB. Therefore, we generated IRAK-1/IRAK-4 doubly deficient mice and examined the response to MALP-2. As shown in Fig. 7 F, the activation of NF-κB–DNA binding activity was induced even in the absence of both IRAK-1 and -4. Furthermore, TLR2-induced TNF-α gene induction was still observed in IRAK-1 −/− IRAK-4 −/− macrophages (unpublished data). Thus, IRAK-1– and IRAK-4–independent mechanisms are responsible for the signaling pathway leading to the activation of NF-κB.
Normal TCR responses in IRAK-4 −/− and IRAK-4 KN/KN T cells
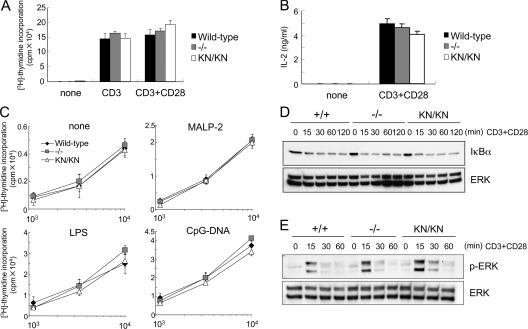
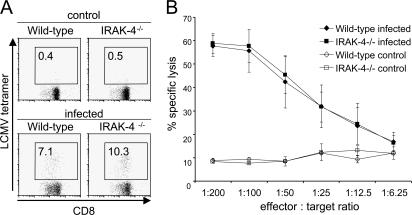
A recent study has shown that deficiency in IRAK- 4 results in the impaired responses to TCR stimulation (18). IRAK-4 interacts with ZAP-70 in the cells and regulates TCR- mediated activation of NF-κB. We then analyzed responses of IRAK-4 KN/KN mice to TCR stimulation. Surprisingly, proliferation of purified T cells in response to either immobilized or soluble anti-CD3 was not impaired in either IRAK-4 −/− or IRAK-4 KN/KN mice compared with wild-type mice (Fig. 8 A and Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20061523/DC1). In addition, production of IL-2 in response to TCR stimulation was not impaired in T cells from these mice (Fig. 8 B). Furthermore, wild-type, IRAK-4 −/−, and IRAK-4 KN/KN T cells have equivalent ability to proliferate in response to allogenic DCs either untreated or treated with various TLR ligands, including MALP-2, LPS, and CpG-DNA (Fig. 8 C). Moreover, TCR-mediated activation of NF-κB as well as MAP kinases was also not altered between wild-type, IRAK-4 −/−, and IRAK-4 KN/KN T cells (Fig. 8, D and E). We investigated whether IRAK-4 was involved in adaptive T cell responses in vivo. Wild-type and IRAK-4 −/− mice were infected with lymphocytic choriomeningitis virus (LCMV). Splenocytes were prepared 8 d after infection, and induction of LCMV-specific CD8+ T cells was analyzed by tetramer staining. As shown in Fig. 9 A, LCMV-specific CD8+ T cells were induced both in wild-type and IRAK-4 −/− mice in a similar manner after infection. Similarly, wild-type and IRAK-4 −/− mice induced comparable ex vivo CTL responses as determined in a 51Cr release assay (Fig. 9 B). These results indicate that IRAK-4 is not involved in the TCR signaling leading to the activation of NF-κB as well as T cell responses in vivo.
Figure 8.
IRAK-4 is dispensable for TCR signaling. (A) Splenic T cells from wild-type, IRAK-4 −/−, and IRAK-4 KN/KN mice were stimulated with immobilized (plate-bound) anti-CD3 (5 μg/ml) alone or anti-CD3 (5 μg/ml) plus anti-CD28 (2 μg/ml) for 48 h. The cells were pulsed with 1 μCi [3H]thymidine for the last 16 h. [3H]thymidine incorporation was measured by a β-scintillation counter. (B) Splenic T cells were stimulated with 5 μg/ml anti-CD3 plus 2 μg/ml anti-CD28 for 72 h. IL-2 concentrations in the culture supernatant were measured by ELISA. (C) Allogenic activity of wild-type, IRAK-4 −/−, and IRAK-4 KN/KN T cells. T cells from indicated mice were mixed with bone-marrow DCs from BALB/c mice stimulated with MALP-2, LPS, and CpG-DNA. (D) Normal activation of NF-κB in response to TCR in IRAK-4 −/− and IRAK-4 KN/KN mice. The cell lysates from cells stimulated with plate-bound anti-CD3 plus anti-CD28 were immunoblotted with anti-IκBα. ERK1/2 levels are shown as loading control. (E) Normal activation of ERK in IRAK-4 −/− and IRAK-4 KN/KN mice. Wild-type, IRAK-4 −/−, and IRAK-4 KN/KN T cells were stimulated with plate-bound anti-CD3 plus anti-CD28, and the cell lysates were immunoblotted with anti–phospho-ERK. ERK1/2 levels are shown as loading control.
Figure 9.
Responses of IRAK-4−/− T cells to LCMV infection. Wild-type and IRAK-4 −/− mice were intravenously infected with 5 × 105 PFU of LCMV, and splenocytes were harvested at day 8 after infection. (A) The cells were stained with LCMV MHC class I tetramer and CD8a and were analyzed by flow cytometry. The data shown are representative of six different mice tested. (B) Ex vivo CTL activity in splenocytes was determined using 5-h 51Cr release assay and GP33-loaded EL-4 cells as targets. Indicated values are mean ± SD of three mice. The data are representative of two separate experiments.
DISCUSSION
In the present study, we analyzed the role of IRAK-4 activity in vivo by generating mice with knockin mutation KK213AA and with null mutation. In agreement with previous papers, IRAK-4 −/− macrophages showed severe defects in TLR-mediated cytokine responses (14, 15). Although the expression of IRAK-4 protein was slightly lower than wild-type cells, IRAK-4 KN/KN macrophages also showed profound defects in the responses to various TLR ligands to the same extent as IRAK-4 −/− cells. These results clearly indicate that the kinase activity of IRAK-4 is essential for the function of IRAK-4 in vivo. Previous in vitro studies implicated that the IRAK family members could activate NF-κB and inflammatory responses even in the absence of their kinase activity (19, 20). In the case of IRAK-4, one group has shown that the mutant IRAK-4 (KK213AA) restored IL-1β responsiveness (22), and the other group reported that the same mutation could restore the response only partially (23). It has been shown that expression of kinase-inactive IRAK-1 could also restore IL-1β–induced NF-κB activation. It may be possible that overexpressed IRAK-4 behaved differently compared with the physiological expression. In the physiological level of expression, the kinase activity of IRAK-4 is critical for its function. So far IRAK-4 substrates responsible for the signaling have not been well understood. Although it was shown that IRAK-4 phosphorylated IRAK-1 for activating TRAF6, TLR-mediated production of proinflammatory cytokines in IRAK-1 −/− cells was not impaired in peritoneal macrophages. Further studies are required for identifying substrates other than IRAK-1 that are responsible for the TLR signaling pathway. Nevertheless, this is the first paper showing that the kinase activity of IRAK family members plays a critical role in their function in vivo.
The association between MyD88 and IRAK-4 was induced in response to IL-1β stimulation in both wild-type and IRAK-4 KN/KN cells. A previous study showed that MyD88 interacted with kinase-negative, but not with wild-type, IRAK-4 when they were overexpressed in human embryonic kidney 293 cells (13). In contrast, it was reported that IL-1 stimulation induced an interaction between endogenous MyD88 and wild-type IRAK-4 (16). In that study, the kinase-truncated mutant of IRAK-4 was shown to constitutively interact with MyD88 even before IL-1 stimulation. Given that overexpression of wild-type IRAK-4 immediately activates NF-κB without further stimulation, the localization of overexpressed IRAK-4 may be different from endogenous protein. Based on our observation and that study (16), the endogenous IRAK-4 is probably recruited to MyD88 in response to stimulation, and IRAK-4 with KK213AA point mutation behaves similarly to wild-type IRAK-4 regarding association with MyD88.
Although IRAK-4 deficiency profoundly affected TLR2-mediated cytokine production, TNF-α gene induction was impaired, but not abrogated, as observed in MyD88 deficiency. TLR2-mediated expression of TNF-α and IκBζ genes was induced even in the absence of IRAK-4, though the expression in IRAK-4 −/− and IRAK-4 KN/KN cells was reduced and transient compared with wild-type cells. Furthermore, induction of NF-κB–DNA binding activity was also induced in IRAK-4 −/− and IRAK-4 KN/KN macrophages, although the activation was ∼10 min delayed compared with wild-type cells. This finding is in contrast to the complete abrogation of TLR2 signaling in MyD88−/− macrophages and indicates the existence of an IRAK-4–independent signaling pathway. Stimulation with R-848 and CpG-DNA also induced NF-κB activation in an IRAK-4–independent manner without degrading IκBα, suggesting that the IRAK-4–independent pathway is not TLR2 specific. Given that the death domain of MyD88 is responsible for downstream signaling, other IRAK family members that contain an N-terminal death domain are candidates for mediating IRAK-4–independent signaling. Nevertheless, the activation of NF-κB in response to a TLR2 ligand was observed even in the absence of both IRAK-1 and -4. Because it was shown that IRAK-2 also positively regulated the IL-1β–signaling pathway, IRAK-2 may be responsible for the signaling pathway. Future studies will clarify if IRAK family members redundantly function in IL-1R/TLR responses in vivo. Although we clearly detected NF-κB–DNA binding activity, which is supershifted by anti-p50 and p65 Ab, we failed to detect activation of IKKs and phosphorylation of IκBα in the absence of IRAK-4 or its kinase activity in response to TLR2 stimulation. Induction of NF-κB activation without degradation of IκBα is quite unique, although the mechanism of activation is enigmatic. It has been reported that TLR2 stimulation leads to the recruitment of active Rac1 and phosphatidylinositol-3 kinase to the TLR2 cytosolic domain (27). Therefore, it is possible that the signaling is mediated through the small G protein–phosphatidylinositol-3 kinase pathway.
The TLR/IL-1R and antigen-receptor signaling share signaling molecules for activating NF-κB. In addition to IKK complex, TRAF6 was also reported to be involved in TCR-mediated NF-κB activation (28). TRAF6 can associate with MALT1, which forms a complex with BCL10 and CARMA1/CARD11. TRAF6 is oligomerized by the complex and activates IKKs by inducing polyubiquitination of IKK-γ/NF-κB essential modulator and activation of TGF-β–activated kinase 1. A recent paper showed that IRAK-4 was also involved in TCR responses via suppressing NF-κB activation by associating with ZAP-70 (18). However, the newly generated IRAK-4 −/− mice did not show any defects in the T cell response as well as the TCR signaling pathway. Furthermore, IRAK-4 was not required for LCMV-induced CTL responses. IRAK-4 KN/KN T cells also showed normal responses against TCR stimulation. We do not have a clear explanation for the discrepancy, and it may be due to the difference in the genetic background of the strains. However, it is unlikely that the critical TCR signaling components are different between mouse strains, suggesting that IRAK-4 is not critically involved in TCR signaling.
In summary, this study demonstrates that IRAK-4 activity plays a critical role in the physiological function of IRAK-4. Macrophages and DCs from IRAK-4 KN/KN mice as well as IRAK-4 −/− mice were profoundly defective in TLR-mediated proinflammatory cytokine production. In addition, IRAK-4 KN/KN mice were highly resistant to LPS-induced shock response. The exploration of small compounds targeting kinase activity of IRAKs has been challenged by the fact that expression of even kinase-inactive IRAK-4 mutant results in the activation of the intracellular signaling pathway. However, this study clearly indicates that the kinase activity of IRAK-4 is essential for the physiological functions, and the kinase activity of IRAK-4 is a good therapeutic target for inflammatory diseases and septic shock, without affecting acquired immune responses.
MATERIALS AND METHODS
Generation of IRAK-4 KN/KN mice.
The IRAK-4 gene was isolated from genomic DNA extracted from ES cells (GSI) by PCR. A genomic fragment containing exon 2 of IRAK-4 was cloned into a pT7blue vector (Nugen), and point mutations resulting in the KK213AA conversion in the kinase domain were introduced by a site-directed mutagenesis. A targeting vector has a neomycin-resistance gene cassette (neo) flanked with two loxP sites, and a HSV thymidine kinase driven by PGK promoter was inserted into the genomic fragment for negative selection. The targeting vector was linearized and electroporated into ES cells (GSI). G418 and gancyclovir doubly resistant clones were selected and screened by PCR and further confirmed by Southern blotting. Three clones with homologous recombination were injected into blastocysts from C57BL/6 females, the obtained chimeric males were crossed with C57BL/6 females, and the obtained F1 generations with mutated IRAK-4 mice were crossed with CAG-Cre transgenic mice to excise the neo cassette. CAG-Cre transgene was removed from IRAK-4 KN/+ mice without a neo cassette by crossing the mice with C57BL/6 mice. IRAK-4 KN/+ mice were further intercrossed to obtain IRAK-4 KN/KN mice. The IRAK-4 KN/KN mice used were under 129Sv × C57BL/6 background. Mice were maintained in our animal facility and treated in accordance with the guidelines of Osaka University.
Generation of IRAK-4 −/− mice.
The IRAK-4 gene was isolated from genomic DNA extracted from ES cells (GSI) by PCR. The targeting vector was constructed by replacing a 4.3-kb fragment encoding the IRAK-4 ORF with a neo cassette, and a HSV thymidine kinase driven by PGK promoter was inserted into the genomic fragment for negative selection. After the targeting vector was transfected into ES cells, G418 and gancyclovir doubly resistant colonies were selected and screened by PCR and further confirmed by Southern blotting. Homologous recombinants were microinjected into blastocysts from C57BL/6 female mice, and heterozygous F1 progenies were intercrossed to obtain IRAK-4 −/− mice. The IRAK-4 −/− mice used were under 129Sv × C57BL/6 background.
Cells.
Peritoneal exudate cells were isolated from the peritoneal cavity of mice 3 d after injection with 2 ml of 4.0% thioglycollate medium (Sigma-Aldrich) by washing with ice cold Hanks' balanced salt solution (Invitrogen). Bone-marrow DCs were prepared by cultivating either in the presence of 100 ng/ml human Flt3 ligand (PeproTech) or 10 ng/ml mouse GM-CSF (PeproTech) as described previously (29). Splenic T cells were isolated using MACS (Miltenyi Biotec).
Reagents.
MALP-2 and PAM3CSK4 were synthesized as described previously (25, 26). LPS from Salmonella minesota Re-595 was purchased from Sigma-Aldrich. Poly I:C was purchased from GE Healthcare. R-848 was provided by the Pharmaceuticals and Biotechnology Laboratory of the Japan Energy Corporation. CpG oligonucleotide was synthesized as described previously (30). Polyclonal Ab to phosphorylated JNK (anti– phospho-JNK), anti–phospho-p38, anti–phospho-ERK, anti–phospho- IκBα (Ser32), and anti–phospho-NF-κB p65 (Ser536) were purchased from Cell Signaling. Polyclonal anti-JNK, anti-p38, anti-ERK, and anti–IκB-α were obtained from Santa Cruz Biotechnology, Inc. Abs to NF-κB p50 and p65 were purchased from Santa Cruz Biotechnology, Inc. Anti-MyD88 Ab was purchased from ProSci, and anti–IRAK-1 Ab was made as described previously (25). Rabbit anti–IRAK-4 polyclonal Ab was raised against a peptide corresponding to aa 436 to 459 of mouse IRAK-4. Specificity of this Ab was tested on overexpressed IRAK-4 (unpublished data) and on IRAK-4 −/− cells (Fig. 1 D).
Measurement of cytokine production.
Concentrations of cytokines in the culture supernatants were measured by ELISA. ELISA kits for mouse TNF-α, IL-6, IL-12 p40, and IL-2 were purchased from R&D Systems, and the kit for mouse IFN-α was purchased from PBL Biomedical Laboratories.
[3H]thymidine uptake.
Splenocytes were cultured with the indicated concentrations of MALP-2, poly I:C, LPS, CpG-DNA, anti-IgM (Jackson ImmunoResearch Laboratories), or anti-CD40 (BD Biosciences) for 48 h. For examining T cell responses, splenic T cells were activated with 10 μg/ml of plate-bound anti-CD3 (BD Biosciences) and 2 μg/ml of plate-bound anti-CD28 (BD Biosciences) for 48 h. Cells were pulsed with 1 μCi [3H]thymidine for the last 16 h. [3H]thymidine incorporation was measured by a scintillation counter (Packard Instrument Co.).
Synthesis of IRAK proteins and in vitro kinase assay.
IRAK-4 cDNA was obtained by RT-PCR from mRNAs prepared from wild-type and IRAK-4 KN/KN macrophages. The cDNAs were cloned into a pcDNA3 vector, which contains a T7 promoter and a Myc tag sequence. Recombinant Myc-tagged IRAK-4 proteins were expressed in the rabbit reticulocyte lysates using TNT T7 Quick coupled transcription/translation systems (Promega). A part of mouse IRAK-1 protein (aa 301–500), which contains the IRAK-1 activation loop, was also prepared the by same system. 10 μl of reticulocyte lysates, which contained recombinant kinase or exogenous substrate, was diluted with cell lysis buffer and combined as indicated. Kinase and substrate were immunoprecipitated with anti-Myc Ab (Cell Signaling), and then in vitro kinase assay was performed as described previously (31). For assessing autophosphorylation of endogenous IRAK proteins, peritoneal macrophages stimulated with 10 ng/ml MALP-2 were lysed and immunoprecipitated with anti–IRAK-1 and anti–IRAK-4 Ab. The kinase activity was then measured by in vitro kinase assay.
Northern blot analysis.
Peritoneal macrophages were treated with 10 ng/ml MALP-2 for 0, 1, 2, 4, and 8 h, and total RNA was extracted using TRIzol reagent (Invitrogen). RNA was electrophoresed, transferred to nylon membranes, and hybridized with the indicated cDNA probes. To detect the expression of IRAK4 mRNA, a 394-bp fragment (707–1,101) was used as a probe. The same membrane was rehybridized with a β-actin probe.
Western blot analysis.
Peritoneal macrophages were treated with 10 ng/ml MALP-2 for the indicated times. Cells were then lysed in a lysis buffer containing 1.0% NP-40, 150 mM NaCl, 20 mM Tris-Cl, pH 7.5, 1 mM EDTA, and protease inhibitor cocktail (Roche). Cell lysates were dissolved by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membrane was blotted with the specific Ab to indicated proteins and visualized with an enhanced chemiluminescence system (NEN Life Science Products). For immunoprecipitation, 107 MEFs were treated with 10 ng/ml IL-1β for the indicated periods, and cell lysates were immunoprecipitated with anti-MyD88 or anti–IRAK-4 Ab, followed by immunoblot with the indicated Abs.
EMSA.
The nuclear extracts were prepared from peritoneal macrophages (5 × 106) stimulated with MALP-2 as described previously (4). Nuclear extracts were incubated with or without Abs against NF-κB p65 or p50, and then further incubated with a specific probe for NF-κB DNA binding sites, electrophoresed, and visualized by autoradiography.
Allogenic T cell response assay.
The allogenic T cell responses were analyzed as described previously (32). In brief, bone marrow–derived DCs stimulated with 10 ng/ml MALP-2, 1 μg/ml LPS, or 100 nM CpG-DNA for 48 h from BALB/c mice were harvested at day 8, irradiated at a dose of 30 Gy, and plated at threefold serial dilutions in 96-well round-bottom plates. These DCs were incubated for 3 d with 5 × 104/well of splenic CD4+ T cells from wild-type, IRAK4 −/−, and IRAK-4 KN/KN mice isolated using MACS with CD4 microbeads (Miltenyi Biotec). [3H]thymidine was added for the last 16 h. [3H]thymidine incorporation was measured by a scintillation counter.
LCMV infection and analysis of T cell responses.
LCMV-WE strain was obtained from T. Otheki (Akita University, Akita, Japan). Wild-type and IRAK4 −/− mice were intravenously infected with 5 × 105 PFU of LCMV-WE and splenocytes were harvested at day 8 after infection. To investigate the induction of LCMV-specific T lymphocytes, splenocytes were incubated with T-select H-2Db LCMV tetramer-KAVYNFATC-PE (MBL International Corporation) and CD8a-APC Ab. Samples were acquired on a FACS Calibur (BD Biosciences) and analyzed with FlowJo software (TreeStar).
For assessment of cytotoxicity of LCMV-specific T cells, splenocytes prepared from LCMV-infected mice were incubated for 5 h with EL-4 target cells that had been loaded with a peptide (GP33; KAVYNFATM; Peptide Institute) and labeled with 51Cr. The percentage of specific lysis was calculated as [(experimental release − spontaneous release)/(maximal release − spontaneous release)] × 100%.
TNF bioassay.
TNF activity was measured in macrophage culture supernatant after stimulation with MALP-2 for 1 and 2 h by cytotoxicity on L929 fibroblasts. L929 cells were plated on 96-well plates in RPMI 1640 medium supplemented with 2% FCS. Serial twofold dilutions of supernatants in 8 mg/ml actinomycin D were added to each well and incubated for 20 h. Viability of cells was determined using CellTiter-Glo (Promega) according to the manufacturer's instructions. Mouse recombinant TNF-α (R&D systems) was used to derive a standard curve, and the concentration of TNF-α was determined based on the standard curve.
Online supplemental material.
Fig. S1 shows the generation of IRAK-4 −/− mice. Fig. S2 shows the induction of TNF activity in response to MALP-2 stimulation. Fig. S3 shows that activation of NF-κB in response to MALP-2 was dependent on MyD88. Fig. S4 shows the activation of NF-κB in IRAK-4 −/− and IRAK-4 KN/KN macrophages in response to LPS, R-848, and CpG-DNA. Fig. S5 shows the proliferative responses of IRAK-4 −/− and IRAK-4 KN/KN T cells to soluble anti-CD3 plus anti-Ig Ab. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061523/DC1.
Supplemental Material
Acknowledgments
We thank all colleagues in our laboratory, M. Hashimoto for secretarial assistance, and Y. Fujiwara, M. Shiokawa, A. Shibano, and N. Kitagaki for technical assistance.
This work was in part supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, from the 21st Century Center of Excellence Program of Japan, and from the National Institutes of Health (grant AI070167).
The authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; COX-2, cyclooxygenase-2; EMSA, electrophoretic mobility shift assay; ERK, extracellular signal-regulated kinase; ES, embryonic stem; IKK-γ, IκB kinase γ; IL-1R, IL-1 receptor; IRAK, IL-1R–associated kinase; JNK, c-Jun N-terminal kinase; LCMV, lymphocytic choriomeningitis virus; MALP-2, macrophage-activating lipopeptide-2; MAP, mitogen-activated protein; MEF, mouse embryonic fibroblast; mRNA, messenger RNA; pDC, plasmacytoid DC; TIR, Toll/IL-1R; TLR, Toll-like receptor; TRAF6, TNF receptor–associated factor 6.
References
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801. [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B. 2004. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 430:257–263. [DOI] [PubMed] [Google Scholar]
- 3.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 5.Janssens, S., and R. Beyaert. 2003. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell. 11:293–302. [DOI] [PubMed] [Google Scholar]
- 6.Croston, G.E., Z. Cao, and D.V. Goeddel. 1995. NF-kappa B activation by interleukin-1 (IL-1) requires an IL-1 receptor-associated protein kinase activity. J. Biol. Chem. 270:16514–16517. [DOI] [PubMed] [Google Scholar]
- 7.Wesche, H., W.J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 7:837–847. [DOI] [PubMed] [Google Scholar]
- 8.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K.J. Ishii, T. Kawai, et al. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J. Exp. Med. 201:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakaraj, P., P.H. Schafer, D.E. Cavender, Y. Wu, K. Ngo, P.F. Grealish, S.A. Wadsworth, P.A. Peterson, J.J. Siekierka, C.A. Harris, and W.P. Fung-Leung. 1998. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J. Exp. Med. 187:2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas, J.A., J.L. Allen, M. Tsen, T. Dubnicoff, J. Danao, X.C. Liao, Z. Cao, and S.A. Wasserman. 1999. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol. 163:978–984. [PubMed] [Google Scholar]
- 11.Fitzgerald, K.A., E.M. Palsson-McDermott, A.G. Bowie, C.A. Jefferies, A.S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M.T. Harte, et al. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 413:78–83. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi, K., L.D. Hernandez, J.E. Galan, C.A. Janeway Jr., R. Medzhitov, and R.A. Flavell. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 110:191–202. [DOI] [PubMed] [Google Scholar]
- 13.Li, S., A. Strelow, E.J. Fontana, and H. Wesche. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA. 99:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki, N., S. Suzuki, G.S. Duncan, D.G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, et al. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 416:750–756. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, N., S. Suzuki, U. Eriksson, H. Hara, C. Mirtosis, N.J. Chen, T. Wada, D. Bouchard, I. Hwang, K. Takeda, et al. 2003. IL-1R-associated kinase 4 is required for lipopolysaccharide-induced activation of APC. J. Immunol. 171:6065–6071. [DOI] [PubMed] [Google Scholar]
- 16.Medvedev, A.E., K. Thomas, A. Awomoyi, D.B. Kuhns, J.I. Gallin, X. Li, and S.N. Vogel. 2005. Cutting edge: expression of IL-1 receptor-associated kinase-4 (IRAK-4) proteins with mutations identified in a patient with recurrent bacterial infections alters normal IRAK-4 interaction with components of the IL-1 receptor complex. J. Immunol. 174:6587–6591. [DOI] [PubMed] [Google Scholar]
- 17.Yang, K., A. Puel, S. Zhang, C. Eidenschenk, C.L. Ku, A. Casrouge, C. Picard, H. von Bernuth, B. Senechal, S. Plancoulaine, et al. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 23:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki, N., S. Suzuki, D.G. Millar, M. Unno, H. Hara, T. Calzascia, S. Yamasaki, T. Yokosuka, N.J. Chen, A.R. Elford, et al. 2006. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 311:1927–1932. [DOI] [PubMed] [Google Scholar]
- 19.Knop, J., and M.U. Martin. 1999. Effects of IL-1 receptor-associated kinase (IRAK) expression on IL-1 signaling are independent of its kinase activity. FEBS Lett. 448:81–85. [DOI] [PubMed] [Google Scholar]
- 20.Maschera, B., K. Ray, K. Burns, and F. Volpe. 1999. Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-kappaB. Biochem. J. 339:227–231. [PMC free article] [PubMed] [Google Scholar]
- 21.Wesche, H., X. Gao, X. Li, C.J. Kirschning, G.R. Stark, and Z. Cao. 1999. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 274:19403–19410. [DOI] [PubMed] [Google Scholar]
- 22.Qin, J., Z. Jiang, Y. Qian, J.L. Casanova, and X. Li. 2004. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J. Biol. Chem. 279:26748–26753. [DOI] [PubMed] [Google Scholar]
- 23.Lye, E., C. Mirtsos, N. Suzuki, S. Suzuki, and W.C. Yeh. 2004. The role of interleukin 1 receptor-associated kinase-4 (IRAK-4) kinase activity in IRAK-4-mediated signaling. J. Biol. Chem. 279:40653–40658. [DOI] [PubMed] [Google Scholar]
- 24.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W.C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA. 101:15416–15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato, S., O. Takeuchi, T. Fujita, H. Tomizawa, K. Takeda, and S. Akira. 2002. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14:783–791. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P.F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554–557. [DOI] [PubMed] [Google Scholar]
- 27.Arbibe, L., J.P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P.J. Godowski, R.J. Ulevitch, and U.G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533–540. [DOI] [PubMed] [Google Scholar]
- 28.Sun, L., L. Deng, C.K. Ea, Z.P. Xia, and Z.J. Chen. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 14:289–301. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi, H., T. Kaisho, K. Takeda, and S. Akira. 2003. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J. Immunol. 170:3059–3064. [DOI] [PubMed] [Google Scholar]
- 30.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 31.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 32.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688–5694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.