Abstract
Newborns and infants are highly susceptible to viral and bacterial infections, but the underlying mechanism remains poorly understood. We show that neonatal B cells effectively control the production of proinflammatory cytokines by both neonatal plasmacytoid and conventional dendritic cells, in an interleukin (IL) 10–dependent manner, after Toll-like receptor (TLR) 9 triggering. This antiinflammatory property of neonatal B cells may extend to other TLR agonists (Pam3CSK4, lipopolysaccharide, and R848) and viruses. In the absence of B cells or of CD5+ B cell subsets, neonatal mice developed stronger inflammatory responses and became lethally susceptible to CpG challenge after galactosamine sensitization, whereas wild-type (WT) mice were resistant. Paradoxically, interferon (IFN)-α/β enhanced the inflammatory response to CpG challenge in adult mice, whereas they helped to control neonatal acute inflammation by stimulating the secretion of IL-10 by neonatal B cells. Finally, WT neonatal B cells rescued IL-10−/− neonates from a lethal CpG challenge, whereas IFN-α/β receptor–deficient B cells did not. Our results show that type I IFNs support a negative regulatory role of neonatal B cells on TLR-mediated inflammation, with important implications for neonatal inflammation and infection.
Neonatal immune responses are generally characterized by low antibody, poor cytotoxic, and Th2 cell–skewed T cell responses to intracellular pathogens and vaccines (1). The mechanisms underlying the weakness of immune responses in newborns remain poorly understood. In neonates, the maturation of the immune system is not totally achieved, which leads to quantitative and qualitative differences in immune cell subsets between neonates and adults (2). Defects in neonatal adaptive immunity have been thoroughly investigated, but little is known about neonatal innate immunity. Neonatal T cells may develop adult-like responses under appropriate stimulation (2). The key issue is therefore the absence of such appropriate stimulatory conditions after infection or vaccination early in life.
Innate immune cells, including macrophages, DCs, and natural killer cells (3), operate upstream and are critical for early host defense and the establishment of acquired immunity. The innate immune system recognizes microorganisms via a limited number of germline-encoded pattern-recognition receptors, among which 11 Toll-like receptors (TLRs) have been identified. The patterns of TLR expression on innate cells display differential regulation, as shown by the expression of TLR7 and TLR9 on plasmacytoid DCs (pDCs), whereas a wider range of TLRs are expressed by conventional DCs (cDCs) (4). The stimulation of cells with TLR ligands (TLR-Ls) activates myeloid differentiation factor 88 (MyD88)–dependent or –independent pathways, resulting in production of the cytokines and chemokines required to establish the inflammatory environment necessary for antimicrobial activity, the activation and recruitment of immune effector cells, and the subsequent development of adaptive immune responses (5).
Cord blood IL-12 and TNF-α responses to microbial and TLR-L stimulation are much weaker than adult peripheral blood responses (6–8). Human DCs derived from cord blood or neonatal monocytes have been shown to have a defective IL-12 response to microbial stimulation in some studies (9, 10), whereas others reported no difference between these and adult cells (8, 11). In mice, we and others have shown that purified neonatal DCs are fully competent for IL-12 and IFN-α production in response to TLR-L, demonstrating the maturity of the DC compartment for innate responses (12, 13). We have shown that innate activation of IL-10–producing neonatal CD5+ B cells influences DC functions for T cell activation in vivo, highlighting the critical influence of the neonatal environment (14). The TNF-α response of cord blood monocytes to TLR-L has been shown to be impaired by plasma factors from cord blood (15). These findings indicate that both humans and mice have regulatory mechanisms controlling innate responses that are specific to the neonatal period. A more detailed understanding of these mechanisms is essential for the improvement of vaccines and the treatment of infectious diseases early in life.
Mouse B cells can be divided into B1 cells (including CD5+ and CD5− B cells), conventional B2 cells, and marginal zone B cells based on their different tissue distribution, function, and phenotype. CD5+ B cell development has been found to occur primarily in the fetal and perinatal periods, and this subset has self-renewal capacity (16). In contrast, the B2 cell compartment is poorly developed at the neonatal stage, and marginal zone B cells are only found by 3 wk of life. The existence of a progenitor specific for B1 cells has been a long-running debate. Recently, a pregenitor with the Lin−B220lo–negCD19+ phenotype has been identified that specifically develops into B1 cells (17). The total number and activity of this pregenitor peak at the fetal and perinatal stages and gradually decrease with age. Several cytokines, including IL-7, thymic stromal lymphopoietin, and Flt-3 ligand are involved in the generation and expansion of CD5+ B cells (18, 19). CD5+ B cells are an important source of natural IgM, which act as the first line of defense to prevent bacterial and viral infections in several mouse models (20–22). However, one striking feature of these CD5+ B cells is their ability to produce IL-10 after TLR triggering (14, 23).
In this paper, we show that neonatal CD5+ B cells play an active role in the in vivo control of neonatal inflammation. This protection of neonates against acute inflammation is mediated by the production of IL-10 in response to a large array of TLR-Ls and viruses. IFN-α/β were found to be critical for this function, as they cooperated with regulatory neonatal CD5+ B cells to dampen the inflammatory response induced by TLR-L, thereby potentially contributing to neonatal susceptibility to infection.
RESULTS
Neonatal B cells inhibit proinflammatory cytokine production by neonatal pDCs after CpG stimulation
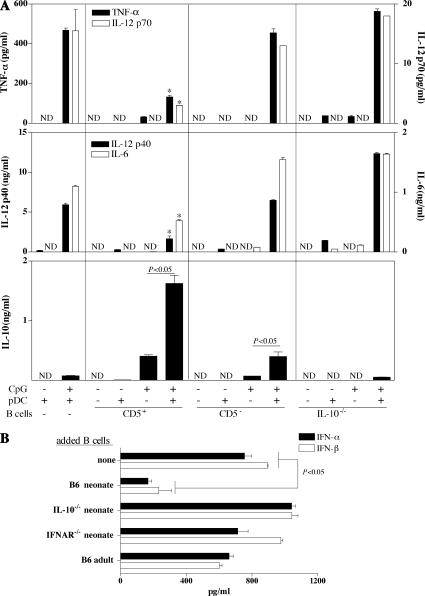
We initially investigated the potential role of neonatal B cells in regulating pDC function. Neonatal splenic CD5+ and CD5− B cells were co-cultured with neonatal splenic pDCs (B220+CD11c+mPDCA-1+NK1.1−) in the presence of CpG. Under these conditions, neonatal pDCs produced smaller amounts of proinflammatory cytokines (TNF-α, IL-12p70/p40, and IL-6) in the presence of CD5+ but not CD5− B cells in an IL-10–dependent manner (Fig. 1 A). The IFN-α/β response of neonatal pDCs to CpG was also strongly decreased in the presence of WT neonatal B cells, but not in the presence of IL-10−/− neonatal B cells or WT adult B cells (Fig. 1 B). Thus, in response to TLR9 signaling, neonatal CD5+ B cells inhibit neonatal pDC function in an IL-10–dependent manner. These results are consistent with the ability of IL-10 to impair pDC responses (24). Similar results were obtained with neonatal splenic cDCs (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062013/DC1) and macrophages (not depicted).
Figure 1.
Neonatal CD5+ B cells inhibit the innate pDC response to CpG. (A) 4 × 104 neonatal splenic pDCs were cultured alone or in the presence of CD5+ or CD5−CD19+ B cells from B6 neonates, or with CD19+ B cells from IL-10−/− neonates, and stimulated with 1 μg/ml CpG for 48 h. The ratio of CD5+ or CD5− B cells to pDCs was 3:1, and the ratio of IL-10−/− B cells to pDCs was 10:1. TNF-α, IL-10, IL-6, and IL-12p70 were detected in supernatants with Luminex, and IL-12p40 was detected by ELISA. *, P < 0.05 versus pDCs alone, pDCs plus CD5− B cells, or pDCs plus IL-10−/− B cells. (B) 2 × 104 neonatal splenic pDCs were cultured alone or with 2 × 105 splenic B cells from WT, IL-10−/−, or IFNAR−/− neonatal mice or from WT adult mice in the presence of 1 μg/ml CpG for 48 h. The supernatants were tested for IFN-α and IFN-β content by ELISA. pDCs, B cells, or pDCs with B cells cultured in medium alone produced no detectable IFN-α/β (not depicted). Data are presented as mean values ± SD. ND, not detectable.
Neonatal CD5+ B cells produced much larger amounts of IL-10 than CD5− B cells after CpG stimulation (Fig. 1 A), and significantly larger amounts of IL-10 were produced in the presence of pDCs (Fig. 1 A). The capacity of neonatal pDCs to support IL-10 secretion by neonatal B cells after CpG stimulation was cell dose dependent (Fig. 2 A). Thus, neonatal B cells receive signals from neonatal pDCs that enhance their regulatory role.
Figure 2.
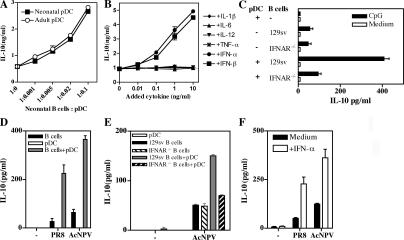
Type I IFNs enhance IL-10 production by neonatal B cells after stimulation with TLR-Ls or viruses. (A–F) Neonatal B cell responses to various stimuli were analyzed in vitro, under various conditions, in the presence or absence of splenic pDCs (A) or Flt3L-pDCs (C, D, and E), as indicated. IL-10 production was assessed by ELISA in supernatants at 48 h. B cells and pDCs from B6 neonates were used in all experiments except for (C) and (E), in which cells from 129sv neonates were used. Cell cultures were stimulated with 1 μg/ml CpG (A–C), 1,000 HAU/ml of influenza virus PR8 (D and F), or 107 PFU/ml AcNPV (D–F). In some experiments, exogenous cytokines were added as indicated (B and F), and under these conditions, no IL-10 was detected when B cells were cultured with cytokines alone. Data are presented as mean values ± SD.
Type I IFNs enhance IL-10 production by neonatal B cells in response to TLR-L or viral stimulation
We next explored the way in which pDCs increase the IL-10–producing capacity of neonatal B cells in response to CpG. We investigated whether cytokines secreted by pDCs could contribute to IL-10 secretion by neonatal B cells. IFN-α/β increased IL-10 production by neonatal B cells after CpG stimulation, whereas IL-1β, IL-6, IL-12, and TNF-α did not (Fig. 2 B). When type I IFN receptor–deficient (IFNAR−/−) neonatal B cells were co-cultured with neonatal pDCs and stimulated with CpG, no increase of IL-10 secretion was induced (Fig. 2 C), and IFNAR−/− B cells failed to inhibit IFN-α/β production by activated pDCs (Fig. 1 B).
We then analyzed the cross talk between pDCs and B cells stimulated with viruses. Baculovirus (Autographa californica nuclear polyhedrosis virus [AcNPV]), a DNA virus that activates innate cells through TLR9 (25), stimulated neonatal B cells to produce minute amounts of IL-10 (Fig. 2 D). However, the IL-10 response to AcNPV was much stronger in the presence of Flt3 ligand (Flt3L)–pDCs and depended on type I IFN receptor expression on B cells (Fig. 2 E). A sharp increase in IL-10 secretion was also observed if recombinant IFN-α was added to neonatal B cells stimulated with AcNPV (Fig. 2 F). Similar results were obtained with the heat-inactivated influenza virus PR8 (Fig. 2, D and F), an RNA virus targeting TLR7 (26), confirming that the regulatory activity of neonatal B cells can be triggered by viruses.
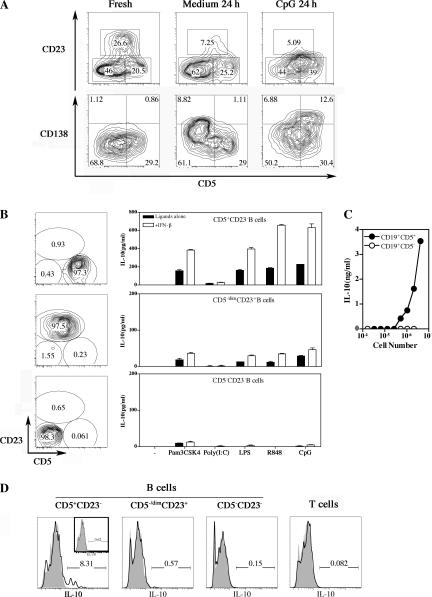
Neonatal mice have a higher percentage of splenic CD5+ B cells than adult mice (27), but neonatal B cells could be further divided into three subsets: CD5+CD23−[CD19high CD43+B220lowAA4.1−IgMhigh], CD5−/dimCD23+[CD19+ CD43−B220high AA4.1−IgM+], and CD5−CD23−[CD19+ CD43+/−B220+AA4.1+ IgM+/−] (Fig. 3 A and not depicted). CD23 expression was lost upon in vitro culture of neonatal B cells restricting subset analysis with total B cells, and upon CpG stimulation, CD138 expression was increased on CD5+ B cells showing ongoing plasma cell differentiation (Fig. 3 A). Therefore, we sorted these three B cell subsets and examined the IL-10 production after stimulation with various TLR ligands (TLR2, Pam3CSK4; TLR3, Poly(I:C); TLR4, LPS; TLR7, R848; and TLR9, CpG). CD5+CD23− B cells produced IL-10 in response to all TLR ligands tested, and IL-10 production was increased by type I IFNs (Fig. 3 B). CD5−/dimCD23+ B cells produced much less IL-10, and CD5−CD23− B cells did not produce any (Fig. 3 B). Similarly, after TLR7 and TLR9 ligand liter stimulation, the very few adult splenic CD5+CD23− B cells also produced IL-10, whereas the main CD5−CD23+ B cell population produced IL-6 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20062013/DC1). Therefore, the propensity of neonatal B cells to produce IL-10 reflects the hematopoietic developmental program that strongly favors the production of CD5+ B cells at the neonatal stage. After the activation of neonatal splenic B cells in vivo by CpG, only CD5+ B cells secreted a large amount of IL-10 (Fig. 3, C and D). We conclude that after stimulation with TLR-Ls or viruses, neonatal B cells produce IL-10 and that the level of IL-10 production is increased by type I IFNs.
Figure 3.
Analysis of IL-10 production by neonatal B cell subsets in response to various TLR agonists. (A) Phenotypic analysis of freshly isolated neonatal CD19+ B cells versus B cells cultured with or without CpG. (B) Neonatal splenic CD19+ B cells were sorted as CD5+CD23−, CD5−/dimCD23+, and CD5−CD23− subsets and stimulated with 1 μg/ml Pam3CSK4, 25 μg/ml Poly(I:C), 10 μg/ml LPS, 1 μg/ml R848, and 1 μg/ml CpG with or without 1 ng/ml IFN-β. Data are presented as mean values ± SD. (C and D) B6 neonates were injected i.p. with 100 μg CpG, and 3–6 h later, splenic B cells were either (C) sorted as CD19+CD5+ and CD19+CD5− cells and cultured for 48 h or (D) stained intracellularly with anti–IL-10. (D) Continuous lines and shaded histograms show anti–IL-10 and control isotype antibodies, respectively, for B cell subsets and T cells (inset shows staining of PBS-injected neonates). The numbers in the gates in A, B, and D represent cell percentages.
Neonatal B cells inhibit proinflammatory cytokine production by pDCs and cDCs in response to various TLR-Ls
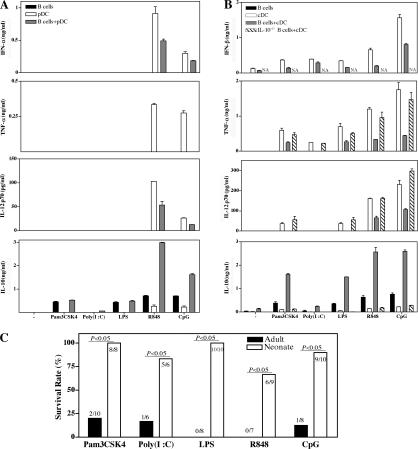
We next analyzed the regulatory potential of neonatal B cells on the inflammatory responses of pDCs and cDCs stimulated with various TLR-Ls. Consistent with the known expression of TLR7 and TLR9 in pDCs, only pDCs stimulated with R848 and CpG produced IFN-α, TNF-α, and IL-12p70. As seen with neonatal splenic pDCs, Flt3L-pDCs increased IL-10 production by neonatal B cells, whereas IFN-α, TNF-α, and IL-12p70 responses were weaker than those of pDCs alone (Fig. 4 A). As in pDCs, the production of IFN-β, TNF-α, and IL-12p70 by GMCSF-cDCs in response to several TLR-Ls (Pam3CSK4, Poly(I:C), LPS, R848, and CpG) was inhibited by WT neonatal B cells but not by IL-10−/− neonatal B cells (Fig. 4 B). Identical results were obtained with cDCs purified from neonatal spleen (Fig. S1 and unpublished data). cDCs also greatly increased the capacity of neonatal B cells to produce IL-10 (Fig. 4 B), with this increase being type I IFN dependent (not depicted).
Figure 4.
Neonatal B cells inhibit the production of proinflammatory cytokines in response to various TLR-Ls. (A and B) 5 × 105 B cells from B6 or IL-10−/− neonatal mice were cultured with 5 × 104 Flt3L-pDCs (A) or GMCSF-cDCs in the presence of 1 μg/ml Pam3CSK4, 25 μg/ml Poly(I:C), 10 μg/ml LPS, 1 μg/ml R848, and 1 μg/ml CpG for 48 h. (A) IFN-α, (B) IFN-β, and (A and B) TNF-α, IL-12p70, and IL-10 were detected in supernatants by ELISA. NA, not applicable. No detectable inflammatory cytokine was produced by pDCs in response to Pam3CSK4, Poly(I:C), and LPS. Data are presented as mean values ± SD. (C) B6 neonates and adults were injected with GalN, together with TLR-L, and survival was monitored for 3 d. The following doses were used: 4 mg/kg Pam3CSK4, 2 mg/kg Poly(I:C), 4 mg/kg LPS, 2 mg/kg R848, and 4 mg/kg CpG. 0.35 g/kg GalN was used for Poly(I:C)-, LPS-, and CpG-injected groups, and 1 g/kg GalN was used for Pam3CSK4- and R848-injected groups.
Collectively, these data show that neonatal CD5+ B cells are intrinsically prone to produce IL-10 after TLR-L and virus stimulation, enabling them to down-regulate the inflammatory responses of pDCs and cDCs. In addition, type I IFNs cooperate with B cells to increase IL-10 production, thereby restricting neonatal inflammatory responses to TLR triggering.
Impaired inflammation in neonatal mice after TLR triggering
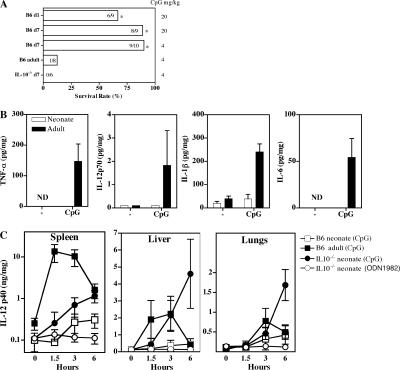
We next monitored the inflammatory responses induced in vivo by several TLR-Ls in neonates and adults, using d-galactosamine (GalN)–sensitized mice lethally susceptible to inflammation (28). Although adult mice succumbed to a lethal shock induced by all TLR-Ls tested, very few neonatal mice, if any, died, even after challenge with a high dose of CpG (Fig. 4 C and Fig. 5 A). We measured neonatal inflammatory responses by analyzing TNF-α, IL-1β, IL-6, and IL-12p70 responses in the spleen after CpG challenge. Under these conditions, CpG induced inflammatory cytokine production in the adult spleen, whereas no response was observed in neonates. Likewise, high levels of IL-12p40 were induced in the adult liver, spleen, and lungs, whereas IL-12p40 was barely detectable in neonatal organs (Fig. 5 C). We then investigated the potential role of IL-10 in curtailing neonatal inflammation in vivo. IL-10−/− neonates, like WT adults, died when challenged with a low dose of CpG (Fig. 5 A). Large amounts of IL-12p40 were induced in the IL-10−/− neonatal liver, spleen, and lungs, demonstrating the critical role of IL-10 in controlling neonatal inflammation (Fig. 5 C). Thus, WT neonates failed to develop an efficient inflammatory response, but adult-like inflammation can be induced in the absence of IL-10.
Figure 5.
Impaired neonatal inflammation in response to CpG is IL-10–dependent. (A) WT neonates (day 1 and 7), IL-10−/− neonates (day 7), and WT adults were injected i.p. with 0.35 g/kg GalN and the indicated dose of CpG, and survival was monitored for 3 d. *, P < 0.05 versus WT adults or IL-10−/− day 7 neonates. (B and C) WT or IL-10−/− neonates or WT adults were injected with 4 mg/kg CpG or ODN1982, and TNF-α, IL-1β, IL-6, IL-12p70, and IL-12p40 were detected by ELISA in the spleen (B and C), liver (C), and lungs (C), harvested at 1.5, 3, or 6 h. Four to nine mice were used for each time point. Data are presented as mean values ± SD. ND, not detectable.
CD5+ B cell–derived IL-10 can control lethal inflammation
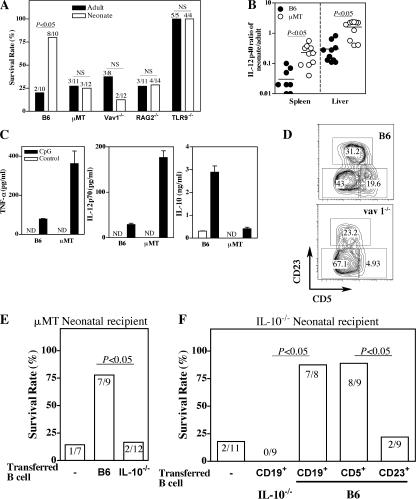
We next investigated the role of B cell–derived IL-10 in controlling neonatal inflammation after TLR9 ligation. We treated B cell–deficient (μMT) neonates, which lack the mature B cell compartment, with GalN and CpG. Unlike WT neonates, μMT neonates succumbed to CpG challenge, demonstrating the induction of an inflammatory response (Fig. 6 A). Accordingly, the IL-12p40 response in the liver and spleen was stronger in μMT than in WT neonates after CpG injection (Fig. 6 B). Similarly, spleen cells from μMT neonates injected with CpG produced larger amounts of TNF-α and IL-12p70, but lower amounts of IL-10, than WT spleen cells did (Fig. 6 C). RAG2−/− neonates, as well as Vav1−/− neonates, which lack the CD5+ B cell compartment (Fig. 6 D), were as susceptible to CpG challenge as μMT neonates (Fig. 6 A). In contrast to WT CBA/J neonates, CBA/Xid neonates, which also lack IgM+CD5+ B cells, produced much less IL-10 and larger amounts of IL-12p70 and were susceptible to GalN/CpG challenge (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20062013/DC1). Flt3L administration to neonatal mice selectively increased the numbers of pDCs and cDCs (29) but not of CD5+ B cells (Fig. S4). Flt3L-treated neonates were shown to be more resistant to Listeria infection (29), and we found that these neonates were susceptible to CpG challenge (Fig. S4), indicating that changes in the balance between CD5+ B cells and DCs increased neonatal inflammation. We next assessed the capacity of B cell–derived IL-10 to control neonatal inflammation. Transfer of WT, but not IL-10−/−, neonatal B cells provided protection to μMT neonates against GalN/CpG-induced death (Fig. 6 E). Identical results were obtained in IL-10−/− neonates, which develop lethal inflammation to CpG in the absence of GalN (Fig. 6 F). Among WT B cells, only CD5+CD23− B cells, but not CD5−/dimCD23+ B cells, were able to rescue IL-10−/− neonates. Collectively, these results show that CD5+ B cell–derived IL-10 is involved in the control of neonatal inflammation.
Figure 6.
Neonatal inflammation is restored in the absence of CD5+ B cells. (A) Neonatal and adult mice of the indicated strains were injected with 1 g/kg GalN and 4 mg/kg CpG, and survival was monitored for 3 d. Data are cumulative results for two to four experiments. (B) 3 h after a 4-mg/kg CpG injection, the IL-12p40 response was analyzed in the liver and spleen of B6 or μMT neonates and adult mice. Data from individual neonatal mice, obtained in three independent experiments, are expressed as the neonate/adult IL-12 response ratio (induced in the same experiment). Horizontal lines represent means. (C) B6 and μMT neonates were injected with CpG, and 3 h later, splenocytes were recovered and cultured for 24 h. TNF-α, IL-12p70, and IL-10 were detected in supernatants by ELISA. ND, not detectable. Data are presented as mean values ± SD. (D) CD5 expression by neonatal spleen B cells in WT and vav1−/− neonates. The numbers in the gates represent cell percentages. (E and F) μMT or IL-10−/− neonates received 8 × 106 CD19+ B cells from WT or IL-10−/− neonatal mice or were left untreated. μMT neonates were challenged with 1 g/kg GalN, plus 10 μg CpG and IL-10−/− with 30 μg CpG alone. (F) IL-10−/− neonates alternatively received 1.8–2 × 106 CD5−/dimCD23+ or 1.2–1.5 × 106 CD5+CD23− WT neonatal B cells before CpG challenge. Survival was monitored for 3 d.
Type I IFNs display pro- and antiinflammatory properties in adults and neonates, respectively
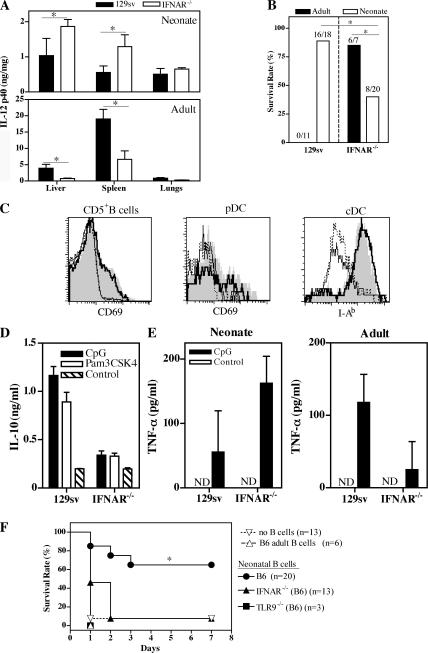
We next investigated the role of type I IFN in the regulation of inflammation. We detected IFN-α/β in sera from both WT and IFNAR−/− mice after the administration of 20 mg/kg CpG (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20062013/DC1). In adult sera, IL-12p70 and TNF-α were significantly lower in IFNAR−/− than in WT mice, and IL-10 was barely detectable in either strain. In contrast, no significant difference in serum TNF-α and IL-12p70 responses was observed between WT and IFNAR−/− neonates. The serum IL-10 response was much weaker in IFNAR−/− than in WT neonates (Fig. S5). We examined IL-12p40 responses in the liver, spleen, and lungs, after the injection of a low dose (4 mg/kg) of CpG, in both neonates and adults (IL-12p70, TNF-α, and IL-10 were not detectable under these conditions). The lack of an IFN-α/β receptor in adult mice resulted in a significantly weaker IL-12p40 response in the spleen and liver, with the opposite observed in neonates (Fig. 7 A). Consistently, IFNAR−/− neonates sensitized with GalN were more susceptible to CpG challenge than WT neonates, whereas IFNAR−/− adults were more resistant than their WT counterparts (Fig. 7 B). Thus, type I IFNs are proinflammatory in adults but decrease inflammation in neonates upon TLR9 signaling, with this effect being correlated with the effects on IL-10 production. Interestingly, pDCs and cDCs displayed similar levels of activation in IFNAR−/− and WT neonates after CpG injection (Fig. 7 C). CD5+ B cells were also similarly activated under these conditions (Fig. 7 C), and B cells from IFNAR−/− neonates produced much less IL-10 than WT B cells (Fig. 7 D), consistent with the lower serum IL-10 concentration in IFNAR−/− neonates (Fig. S5). Similar results were obtained after Pam3CSK4 injection (Fig. 7 D and not depicted), confirming that IFN-α/β are important for IL-10 production by neonatal B cells in vivo. Spleen cells from IFNAR−/− neonates injected with CpG produced more TNF-α than did WT spleen cells (Fig. 7 E). In contrast, IFNAR−/− adults displayed a weaker response than WT adults.
Figure 7.
Type I IFN signaling restricts the neonatal inflammation induced by CpG through IL-10–secreting B cells. (A) IL-12p40 was detected in the liver, spleen, and lungs of neonatal (n = 3) and adult (n = 4) 129sv or IFNAR−/− mice harvested 3 h after a 4-mg/kg CpG i.p. injection. The data shown are representative of three independent experiments. (B) WT and IFNAR−/− neonates and adults were injected i.p. with 1 g/kg GalN and 4 mg/kg CpG, and survival was monitored for 3 d. The cumulative results obtained in three to five experiments are shown. (C) WT and IFNAR−/− neonates were injected i.p. with 20 mg/kg CpG or left untreated. 3 h later, splenic CD5+ B cells, pDCs, and cDCs were analyzed for up-regulation of the CD69 and I-Ab activation markers. Nontreated: WT (thin line) and IFNAR−/− (dotted line); CpG: WT (shaded area) and IFNAR−/− (bolded line). (D) WT and IFNAR−/− neonates were injected i.p. with 20 mg/kg CpG, 4 mg/kg Pam3CSK4, or PBS, and CD19+ cells were purified 3 h later. IL-10 was detected in cell culture supernatants by ELISA. (E) WT and IFNAR−/− neonatal or adult mice were injected i.p. with 20 mg/kg CpG, and 3 h later, spleen cells were recovered and cultured for 24 h. TNF-α was detected in supernatants by ELISA. (F) IL-10−/− neonates received 8 × 106 neonatal B cells from WT, TLR9−/−, or IFNAR−/− mice or adult WT B cells, or were left untreated. They were then challenged with 30 μg CpG. Survival was monitored for 7 d. ND, not detectable. *, P < 0.05 versus the other groups.
WT, but not IFNAR−/−, neonatal B cells can rescue neonates from lethal inflammation
We therefore directly assessed the influence of IFN-α/β in the ability of B cell–derived IL-10 to control lethal inflammation to CpG in IL-10−/− neonates in the absence of GalN (Fig. 7 F). After the adoptive transfer of neonatal, but not adult, WT B cells, IL-10−/− neonates survived CpG challenge (Fig. 7 F). This protection was TLR9 dependent, as TLR9−/− neonatal B cells failed to rescue IL-10−/− neonates. IFNAR−/− neonatal B cells also failed to confer protection to IL-10−/− recipients after CpG challenge (Fig. 7 F). Intact type I IFN signaling in neonatal B cells is therefore required to complement the IL-10 deficiency of the recipient neonates in the context of CpG-induced inflammation. Thus, the negative regulatory role of type I IFNs in neonatal inflammation is linked to the positive effect of these molecules on IL-10 production by innately activated neonatal B cells.
DISCUSSION
In this paper, we show that both type I IFNs and B cells play a major role in restricting inflammation early in life. We found that neonatal B cells negatively regulated type I IFNs and the inflammatory responses of pDCs and cDCs by producing IL-10 in response to a large array of TLR-Ls and viruses. Maximal IL-10 production by neonatal B cells in vivo and the subsequent control of acute inflammation require a functional type I IFN system (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20062013/DC1). This regulatory mechanism can also protect against lethal inflammation.
The early postnatal period is characterized by a high level of susceptibility to various infections, to which the mechanism described in this paper may contribute. The balance between pro- and antiinflammatory cytokines is a critical factor in the control of infections. Neonatal Listeria infection is associated with a strong IL-10 production, and anti–IL-10 treatment protects neonatal, but not adult, mice against infection (30). CpG increases neonatal resistance to listeriosis in correlation with IL-12 production in vivo (31), but less efficiently in neonates than in adults. In this paper, we show a deficit in the inflammatory response elicited by CpG in neonatal mice. We were able to restore an adult-like inflammatory response to CpG in IL10−/− neonates, but these animals died within 24 h. IL-12 administration enhances the immune responses of neonatal mice to vaccines (32, 33) but may lead to growth retardation and death (34). Neonates are highly likely to encounter inflammatory stimuli because of their abrupt, massive exposure to microbes. Therefore, this transition period requires tight control over potentially immunopathophysiological mediators for optimal neonatal development. Accordingly, our study establishes that neonates develop much weaker systemic inflammatory responses than adults in response to various TLR-Ls because of an active regulatory mechanism mediated by the innate response of neonatal B cells to these stimuli.
TLR signaling is a critical component in the initiation of T cell immune responses through DC activation. Consistent with the large array of TLRs expressed by B cells (35), the generation of T cell–dependent antibodies also requires B cell activation via TLRs (36). We report that innate activation of neonatal CD5+ B cells by TLR-Ls and viruses may provide an inhibitory environment through the secretion of IL-10. CD5+ B cells are thought to originate in the fetal liver and to undergo self-renewal in the periphery (37). Neonatal livers display high levels of hematopoietic activity, probably contributing to the abundance of CD5+ B cells in the spleen, liver, and peripheral blood of neonatal mice (unpublished data) (14), a characteristic shared by human cord blood (38). The CD5+ B cells in adult mice are the B1a cells located primarily in the peritoneum and known to produce IL-10 (23). We show that CD5 expression does not define a single homogeneous B cell population in the neonatal spleen. A substantial number of conventional CD23+ B2 cells are CD5dim, whereas adult CD23+ B2 cells are negative for CD5. After TLR signaling, neonatal CD5−/dimCD23+ B cells innately produce IL-10, but much less than CD5+CD23− B cells. Conversely, adult CD23+ B2 cells produce large amounts of IL-6, but no IL-10, in response to TLR stimulation. The differences in cytokine production by neonatal and adult B cells after TLR triggering clearly require further investigation. However, the tendency for neonatal B cells to produce IL-10 and the high frequency of CD5+ B cells suggest that the systemic antiinflammatory role of these cells is developmentally controlled.
Type I IFNs play a key role in host defense, either directly or by modulating innate and adaptive immune responses. Type I IFNs may directly act on CD8+ T cells, B cells, NK cells, and DCs, enhancing their functions (39). The enhancement of neonatal B cell IL-10 production by type I IFNs raises questions about the role of type I IFNs in inflammation. Both proinflammatory cytokines and type I IFNs are produced after TLR triggering. Proinflammatory cytokines are mostly induced by activation of the NF-κB pathway and the mitogen-activated protein kinase pathway, whereas a different panel of downstream molecules are preferentially used for type I IFN production (40). IFN regulatory factor (IRF) 7 directly associates with MyD88 to initiate IFN-α/β transcription, whereas both IRF3 and IRF7 are important in the MyD88-independent pathway. IRF3 directs primary IFN-β induction, stimulating IRF7 production and forming a positive loop for IFN-α/β production (5). The biological effects of type I IFNs are principally through the IFNAR/STAT1-dependent pathway (39), but this signaling may be inhibited by IL-10 (41).
Our in vivo results show that type I IFNs have proinflammatory and antiinflammatory effects in adult mice and neonates, respectively, after CpG injection. Type I IFNs decrease inflammation by indirectly increasing the capacity of neonatal B cells to produce IL-10, and this regulatory loop is critical for control of the lethal inflammation induced by CpG. Opposite effects of type I IFNs on inflammation have been reported. Type I IFNs are indispensable in the mouse model of LPS-induced lethal shock (42). The potent proinflammatory effects of type I IFNs have been implicated in STAT1-dependent increases in the expression of inflammation genes (43, 44). A type I IFN autocrine–paracrine loop is required for maximal IL-12p70 and TNF-α secretion by GMCSF-generated DCs after TLR-L stimulation (44, 45). Antiinflammatory effects of type I IFNs have also been reported in experimental colitis (46), and a possible role for STAT3 in inhibiting the STAT1 pathway has been proposed (47). In vivo, type I IFNs block IL-12 production by splenic DCs during mouse CMV infection (48), and the selective depletion of pDCs in vivo markedly decreases IFN-α and increases IL-12p70 responses in the sera of mouse CMV–infected mice (49). Collectively, these results suggest that type I IFNs indirectly inhibit IL-12 production. The capacity of DCs to drive neonatal T cell responses was impaired for Th1, but not CTL, responses (14). Given the positive role of IL-10 in CTL responses (50) and the negative role of IL-10 in inhibiting type I IFNs, the production of other cytokines, and CpG-activated pDC survival (24), further work is required to determine the exact relationship between neonatal B cells and pDCs during viral infection in vivo.
In summary, we describe in this paper a new role for type I IFNs in the control of inflammation mediated by nonspecific activation of B cells. A previous study has shown that autoantigen-dependent secretion of IL-10 by B cells is important for inhibiting T cell–mediated pathogenesis (51). The regulatory role of innate B cells in controlling acute systemic inflammation in neonatal mice is demonstrated in this paper. This regulatory mechanism helps in providing an innocuous environment for the growth and development of neonates and may explain their high susceptibility to infections.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) mice were purchased from Janvier, and 129sv mice were purchased from Charles River Laboratories. CBA/J and CBA/Xid mice were purchased from Harlan. IL-10−/−, μMT (both obtained from A. Bandeira, Institut Pasteur, Paris, France), TLR9−/− (obtained from P. Viera, Institut Pasteur, Paris, France), vav1−/− (52), and RAG2−/− mice were all of the C57BL/6 background. IFNAR−/− mice were also of either the 129sv or C57BL/6 backgrounds. Pups were obtained from females mated and housed on site in specific pathogen-free conditions. Neonatal mice were 7 d old, unless specifically stated otherwise, and adult mice were 6–10 wk old. Similar B cell subsets were found in IL-10−/−, IFNAR−/−, TLR9−/−, and WT neonates. Animal studies were approved by the Institut Pasteur Safety Committee in accordance with French and European guidelines.
Culture medium and reagents.
Complete medium consisted of RPMI 1640 containing l-alanyl-l-glutamine supplemented with 5–10% FCS (MP Biomedicals), 5 × 10−5 M 2-Mercaptoethanol (Sigma-Aldrich), and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Invitrogen). In some experiments, cells were cultured in HL-1 serum-free medium (Bio Whittaker). TLR9 ligand type B CpG (CpG 1826, 5′-TCCATGACGTTCCTGACGTT-3′) and control (ODN 1982, 5′-TCCAGGACTTCTCTCAGGTT-3′) were synthesized by Proligo with phosphorothioated backbones. TLR2 ligand Pam3CSK4, TLR3 ligand Poly(I:C), TLR4 ligand LPS (from Escherichia coli 0111:B4), and TLR7 ligand R848 were purchased from Invivogen. Human influenza virus A/PR/8/34 (PR8 [H1N1]; Charles River Laboratories) was inactivated by heating at 56°C for 30 min before use. Baculovirus (AcNPV) was purchased from Agate. The antibodies used were anti-CD11c (HL3), anti-B220 (RA3-6B2), anti-CD19 (1D3), anti-IgM (II/41), anti-NK1.1 (PK136), anti-CD5 (53–7.3), anti–mPDCA-1, and anti-IL10 (JES5-16E3). All mAbs were obtained from BD Biosciences, except anti–mPDCA-1 (Miltenyi Biotec).
Cell purification and FACS analysis.
Spleens from neonatal mice were treated with collagenase D and DNase I (Roche Molecular Biochemicals) for 30 min and dissociated in Ca2+-free medium in the presence of EDTA. The preparation was enriched in DCs, using anti-CD11c (N418; Miltenyi Biotec) and anti–mPDCA-1 MicroBeads (Miltenyi Biotec), in an automated magnetic cell sorter (AutoMACS; Miltenyi Biotec). Neonatal cDCs and pDCs were further purified by flow cytometry to obtain cDCs (CD11chigh) and pDCs (CD11clow, B220+, NK1.1−, CD19−), using a FACSAria (BD Biosciences) or a MoFlo (DakoCytomation) apparatus. B cells were purified using anti-CD19 MicroBeads (Miltenyi Biotec) on an AutoMACS cell sorter. In some experiments, neonatal CD19+ cells were further sorted by flow cytometry to obtain CD5+CD23−, CD5−/dimCD23+, and CD5−CD23− B cell subsets. Each sorted cell population was 97–99% pure. Flt3L-pDCs and GMCSF-cDCs were obtained, as previously described, from bone marrow cells cultured with Flt3L (53) and GMCSF (54), respectively. Flt3L-pDCs and GMCSF-cDCs were purified using anti-B220 and anti-CD11c MicroBeads (Miltenyi Biotec), respectively. The preparations were >95% pure for Flt3L-pDCs and >99% pure for GMCSF-cDCs.
Cytokine determinations.
Neonatal cDCs, pDCs, and B cells were cultured in HL-1 serum-free medium and stimulated with various TLR-Ls or viruses for 48 h. In some experiments, recombinant IL-1β, IL-6, IL-12, TNF-α, IFN-α, and IFN-β (all obtained from R&D Systems) were added to the medium. IL-6, IL-10, IL-12p40, IL-12p70, and TNF-α were determined by standard sandwich ELISA, with appropriate antibodies (all obtained from BD Biosciences), or with a multiplex kit (Bio-Rad Laboratories) and a Luminex X-100 apparatus (Luminex Corporation). Mouse IFN-α/β were detected with the corresponding ELISA kits (PBL Biomedical Laboratories), used according to the manufacturer's instructions.
For direct cytokine detection in organs, neonatal and adult mice were injected with CpG1826 or ODN1982 (4 mg/kg of body weight). At various time points, liver, spleen, and lungs were collected and homogenized in RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor cocktail (Roche Molecular Biochemicals), using Tissue Lyser (QIAGEN). Samples were centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was collected and stored at −80°C. IL-12p40 was detected by ELISA. Plates were developed with streptavidin-alkaline phosphatase (BD Biosciences) and p-nitrophenylphosphate (Sigma-Aldrich).
Galactosamine-sensitized shock experiment.
Suckling pups from each litter were randomly assigned to experimental groups, marked, and kept with the mother until the completion of the experiments. Neonatal and adult mice were intraperitoneally injected with GalN (0.35 or 1 g/kg of body weight; Sigma-Aldrich) and various TLR-Ls. All materials were dissolved and diluted in pyrogen-free PBS. Injection volumes were 100 and 500 μl for neonatal and adult mice, respectively. Deaths were recorded every 24 h for 3 d.
B cell adoptive transfer experiments.
CD19+ B cells or B cell subsets were purified from B6 neonates, B6 adults, IL-10−/− neonates, IFNAR−/− neonates (B6 background), and TLR9−/− neonates and transferred into day 7 μMT or IL-10−/− neonates (each recipient received 8 × 106 B cells or 1–2 × 106 B cell subsets i.p. in 50 μl). Control litters received PBS. Neonates were challenged intraperitoneally, as indicated in the figures, with CpG1826 in 50 μl PBS. Survival was monitored for 3–7 d.
Statistics.
Student's t tests were used to compare groups (data are presented as mean values ± SD). Fisher's exact test was used to compare survival rates. Survival curves were analyzed with log-rank tests. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows inhibition of IL-12–cDC response by neonatal B cells after CpG stimulation. Fig. S2 shows cytokine response of adult splenic B cell subsets to TLR agonists. Fig. S3 shows restoration of inflammatory responses in Xid neonates. Fig. S4 shows lethal inflammation induced by CpG in Flt3L-treated neonates. Fig. S5 shows seric cytokines induced by CpG in IFNAR−/− and WT neonatal mice. Fig. S6 corresponds to a scheme showing the regulation of the neonatal acute inflammatory response by B cells and IFN-α/β. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20062013/DC1.
Supplemental Material
Acknowledgments
This work was supported by the Agence Nationale de la Recherche and the European Commission (grants CellPROM-NMP4-CT-2004-500039 and Theravac LSHB-2004-50832). X. Jiao was supported by grants from the 973 Project of China (2006CB504404) and the National Natural Science Foundation of China (30425031).
The authors have no conflicting financial interests.
Abbreviations used: AcNPV, Autographa californica nuclear polyhedrosis virus; cDC, conventional DC; Flt3L, Flt3 ligand; GalN, d-galactosamine; IFNAR−/−, type I IFN receptor deficient; IRF, IFN regulatory factor; μMT, B cell deficient; MyD88, myeloid differentiation factor 88; pDC, plasmacytoid DC; TLR, Toll-like receptor; TLR-L, TLR ligand.
References
- 1.Siegrist, C.A. 2001. Neonatal and early life vaccinology. Vaccine. 19:3331–3346. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 5.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 6.Lee, S.M., Y. Suen, L. Chang, V. Bruner, J. Qian, J. Indes, E. Knoppel, C. van de Ven, and M.S. Cairo. 1996. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood. 88:945–954. [PubMed] [Google Scholar]
- 7.Levy, O. 2005. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J. Endotoxin Res. 11:113–116. [DOI] [PubMed] [Google Scholar]
- 8.Upham, J.W., P.T. Lee, B.J. Holt, T. Heaton, S.L. Prescott, M.J. Sharp, P.D. Sly, and P.G. Holt. 2002. Development of interleukin-12-producing capacity throughout childhood. Infect. Immun. 70:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141–2146. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, H., C. Van De Ven, P. Satwani, L.V. Baxi, and M.S. Cairo. 2004. Differential gene expression patterns by oligonucleotide microarray of basal versus lipopolysaccharide-activated monocytes from cord blood versus adult peripheral blood. J. Immunol. 172:5870–5879. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun, C.M., L. Fiette, M. Tanguy, C. Leclerc, and R. Lo-Man. 2003. Ontogeny and innate properties of neonatal dendritic cells. Blood. 102:585–591. [DOI] [PubMed] [Google Scholar]
- 13.Dakic, A., Q.X. Shao, A. D'Amico, M. O'Keeffe, W.F. Chen, K. Shortman, and L. Wu. 2004. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 172:1018–1027. [DOI] [PubMed] [Google Scholar]
- 14.Sun, C.M., E. Deriaud, C. Leclerc, and R. Lo-Man. 2005. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 22:467–477. [DOI] [PubMed] [Google Scholar]
- 15.Levy, O., K.A. Zarember, R.M. Roy, C. Cywes, P.J. Godowski, and M.R. Wessels. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173:4627–4634. [DOI] [PubMed] [Google Scholar]
- 16.Berland, R., and H.H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253–300. [DOI] [PubMed] [Google Scholar]
- 17.Montecino-Rodriguez, E., H. Leathers, and K. Dorshkind. 2006. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7:293–301. [DOI] [PubMed] [Google Scholar]
- 18.Vosshenrich, C.A., A. Cumano, W. Muller, J.P. Di Santo, and P. Vieira. 2003. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 4:773–779. [DOI] [PubMed] [Google Scholar]
- 19.Vosshenrich, C.A., A. Cumano, W. Muller, J.P. Di Santo, and P. Vieira. 2004. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc. Natl. Acad. Sci. USA. 101:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briles, D.E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgarth, N., O.C. Herman, G.C. Jager, L.E. Brown, L.A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell–derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, F., A.M. Oliver, and J.F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629. [DOI] [PubMed] [Google Scholar]
- 23.O'Garra, A., R. Chang, N. Go, R. Hastings, G. Haughton, and M. Howard. 1992. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 22:711–717. [DOI] [PubMed] [Google Scholar]
- 24.Duramad, O., K.L. Fearon, J.H. Chan, H. Kanzler, J.D. Marshall, R.L. Coffman, and F.J. Barrat. 2003. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 102:4487–4492. [DOI] [PubMed] [Google Scholar]
- 25.Abe, T., H. Hemmi, H. Miyamoto, K. Moriishi, S. Tamura, H. Takaku, S. Akira, and Y. Matsuura. 2005. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 79:2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa, K., R.R. Hardy, D.R. Parks, and L.A. Herzenberg. 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 157:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galanos, C., M.A. Freudenberg, and W. Reutter. 1979. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc. Natl. Acad. Sci. USA. 76:5939–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollstedt, S., M. Franchini, H.P. Hefti, B. Odermatt, M. O'Keeffe, G. Alber, B. Glanzmann, M. Riesen, M. Ackermann, and M. Suter. 2003. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 197:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777–2782. [PubMed] [Google Scholar]
- 31.Ito, S., K.J. Ishii, M. Gursel, H. Shirotra, A. Ihata, and D.M. Klinman. 2005. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J. Immunol. 174:777–782. [DOI] [PubMed] [Google Scholar]
- 32.Arulanandam, B.P., J.N. Mittler, W.T. Lee, M. O'Toole, and D.W. Metzger. 2000. Neonatal administration of IL-12 enhances the protective efficacy of antiviral vaccines. J. Immunol. 164:3698–3704. [DOI] [PubMed] [Google Scholar]
- 33.Mancuso, G., V. Cusumano, F. Genovese, M. Gambuzza, C. Beninati, and G. Teti. 1997. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect. Immun. 65:3731–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovarik, J., X. Martinez, M. Pihlgren, P. Bozzotti, M.H. Tao, T.J. Kipps, T.F. Wild, P.H. Lambert, and C.A. Siegrist. 2000. Limitations of in vivo IL-12 supplementation strategies to induce Th1 early life responses to model viral and bacterial vaccine antigens. Virology. 268:122–131. [DOI] [PubMed] [Google Scholar]
- 35.Peng, S.L. 2005. Signaling in B cells via Toll-like receptors. Curr. Opin. Immunol. 17:230–236. [DOI] [PubMed] [Google Scholar]
- 36.Pasare, C., and R. Medzhitov. 2005. Control of B-cell responses by Toll-like receptors. Nature. 438:364–368. [DOI] [PubMed] [Google Scholar]
- 37.Hardy, R.R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621. [DOI] [PubMed] [Google Scholar]
- 38.Bhat, N.M., A.B. Kantor, M.M. Bieber, A.M. Stall, L.A. Herzenberg, and N.N. Teng. 1992. The ontogeny and functional characteristics of human B-1 (CD5+ B) cells. Int. Immunol. 4:243–252. [DOI] [PubMed] [Google Scholar]
- 39.Theofilopoulos, A.N., R. Baccala, B. Beutler, and D.H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307–336. [DOI] [PubMed] [Google Scholar]
- 40.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131–137. [DOI] [PubMed] [Google Scholar]
- 41.Ito, S., P. Ansari, M. Sakatsume, H. Dickensheets, N. Vazquez, R.P. Donnelly, A.C. Larner, and D.S. Finbloom. 1999. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 93:1456–1463. [PubMed] [Google Scholar]
- 42.Karaghiosoff, M., R. Steinborn, P. Kovarik, G. Kriegshauser, M. Baccarini, B. Donabauer, U. Reichart, T. Kolbe, C. Bogdan, T. Leanderson, et al. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 4:471–477. [DOI] [PubMed] [Google Scholar]
- 43.Tassiulas, I., X. Hu, H. Ho, Y. Kashyap, P. Paik, Y. Hu, C.A. Lowell, and L.B. Ivashkiv. 2004. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat. Immunol. 5:1181–1189. [DOI] [PubMed] [Google Scholar]
- 44.Gautier, G., M. Humbert, F. Deauvieau, M. Scuiller, J. Hiscott, E.E. Bates, G. Trinchieri, C. Caux, and P. Garrone. 2005. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor–induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollara, G., M.E. Handley, A. Kwan, B.M. Chain, and D.R. Katz. 2006. Autocrine type I interferon amplifies dendritic cell responses to lipopolysaccharide via the nuclear factor-kappaB/p38 pathways. Scand. J. Immunol. 63:151–154. [DOI] [PubMed] [Google Scholar]
- 46.Katakura, K., J. Lee, D. Rachmilewitz, G. Li, L. Eckmann, and E. Raz. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho, H.H., and L.B. Ivashkiv. 2006. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J. Biol. Chem. 281:14111–14118. [DOI] [PubMed] [Google Scholar]
- 48.Dalod, M., T.P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C.A. Biron. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krug, A., A.R. French, W. Barchet, J.A. Fischer, A. Dzionek, J.T. Pingel, M.M. Orihuela, S. Akira, W.M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 21:107–119. [DOI] [PubMed] [Google Scholar]
- 50.Fujii, S., K. Shimizu, T. Shimizu, and M.T. Lotze. 2001. Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood. 98:2143–2151. [DOI] [PubMed] [Google Scholar]
- 51.Fillatreau, S., C.H. Sweenie, M.J. McGeachy, D. Gray, and S.M. Anderton. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944–950. [DOI] [PubMed] [Google Scholar]
- 52.Turner, M., P.J. Mee, A.E. Walters, M.E. Quinn, A.L. Mellor, R. Zamoyska, and V.L. Tybulewicz. 1997. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 7:451–460. [DOI] [PubMed] [Google Scholar]
- 53.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X.L. Xu, G. Trinchieri, A. O'Garra, and Y.J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.