Abstract
IRAK4 is a member of IL-1 receptor (IL-1R)–associated kinase (IRAK) family and has been shown to play an essential role in Toll-like receptor (TLR)–mediated signaling. We recently generated IRAK4 kinase-inactive knock-in mice to examine the role of kinase activity of IRAK4 in TLR-mediated signaling pathways. The IRAK4 kinase–inactive knock-in mice were completely resistant to lipopolysaccharide (LPS)- and CpG-induced shock, due to impaired TLR-mediated induction of proinflammatory cytokines and chemokines. Although inactivation of IRAK4 kinase activity did not affect the levels of TLR/IL-1R–mediated nuclear factor κB activation, a reduction of LPS-, R848-, and IL-1–mediated mRNA stability contributed to the reduced cytokine and chemokine production in bone marrow–derived macrophages from IRAK4 kinase–inactive knock-in mice. Both TLR7- and TLR9-mediated type I interferon production was abolished in plasmacytoid dendritic cells isolated from IRAK4 knock-in mice. In addition, influenza virus–induced production of interferons in plasmacytoid DCs was also dependent on IRAK4 kinase activity. Collectively, our results indicate that IRAK4 kinase activity plays a critical role in TLR-dependent immune responses.
Innate immunity is the first line of defense against pathogenic microorganisms. Toll-like receptors (TLRs) (1–6) play a critical role in innate immune responses in mammals through the recognition of conserved molecular patterns associated with different microorganisms. Although TLR4 has been genetically identified as a signaling molecule essential for the responses to LPS, a component of gram-negative bacteria (7), TLR2 responds to mycobacteria, yeast, and gram-positive bacteria (8–11). TLR6 associates with TLR2 and recognizes lipoproteins from micoplasma. TLR5 and TLR9 mediates the induction of the immune response by bacterial flagellins (12) and bacterial DNA (5), respectively. Although TLR3 recognizes double-stranded RNA (13), single-stranded RNA is the natural ligand for TLR7/8 (14, 15). The natural ligands for TLR10 and TLR11 are still not known (6).
Upon binding of TLR ligands, all of the TLRs except TLR3 recruit the adaptor molecule MyD88 through the TIR domain, mediating the so-called MyD88-dependent pathway (16). MyD88 then recruits serine-threonine kinases IL-1R–associated kinase (IRAK)4 (17–19) and IRAK (20, 21). Although IRAK4 is the kinase that functions upstream of and phosphorylates IRAK, the phosphorylated IRAK mediates the recruitment of TRAF6 to the receptor complex (22). Upon phosphorylation of IRAK, the IRAK–TRAF6 complex dissociates from the receptor complex to interact with and activate TGF-β–activated kinase 1 (TAK1), a member of the mitogen-activated protein kinase kinase kinase family (23). The activation of TAK1 eventually leads to the activation of NF-κB and c-Jun NH2-terminal kinase (JNK) (24), resulting in induction of inflammatory cytokines and chemokines such as TNF-α, IL-1β, IL-6, and IL-8.
Recent studies have begun to unravel how a subset of TLRs, TLR7, TLR8, and TLR9 use a novel MyD88-dependent pathway to mediate the activation of transcription factors interferon regulatory factor (IRF)5 and IRF7 and subsequent induction of IFN-α. It has been recently reported that the transcription factor IRF7 interacts with MyD88 to form a complex in the cytoplasm, and this interaction resulted in activation of IFN-α–dependent promoters (25, 26). IRAK4, IRAK, and TRAF6 have also been implicated in this pathway. In addition, the ubiquitin ligase activity of TRAF6 has been shown to mediate IRF7 activation (25–29). However, the detailed molecular mechanisms for this novel TLR7-, TLR8-, and TLR9-mediated MyD88-dependent pathway are still unclear.
One important question for the TLR-induced MyD88-dependent pathway is the requirement of the kinase activity of IRAK4 in various signaling events. We have now generated IRAK4 kinase–inactive knock-in mice and found that the kinase activity of IRAK4 plays a critical role in TLR-mediated immune responses. Our data indicate that inactivation of IRAK4 kinase activity leads to reduced mRNA stability and diminished production of cytokines and chemokines in response to LPS stimulation. Also, both TLR7- and TLR9-mediated cytokine production was abolished in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice. In addition to induction of proinflammatory cytokines, TLR7- and TLR9-mediated signaling in plasmacytoid DCs (pDCs) has been shown to play a critical role in viral immunity through the efficient production of type I interferons (26, 30, 31). Importantly, TLR7-, TLR9-, and influenza virus–mediated type I IFN production was also impaired in pDCs from IRAK4 kinase–inactive knock-in mice. Collectively, our results indicate that IRAK4 kinase activity plays a critical role in TLR-dependent immune responses.
RESULTS
The IRAK4 kinase–inactive knock-in mice are resistant to LPS- and CpG-induced shock
To determine the role of the kinase activity of IRAK4 in TLR-mediated signaling, we generated IRAK4 kinase–inactive knock-in mice by mutating lysine 213 and 214 residues to methionines, which are part of the ATP-binding pocket of the kinase domain (Fig. 1, a–c). The inactivation of the kinase activity of the mutant IRAK4 from the knock-in mice was confirmed by immunoprecipitation kinase assay in vitro (Fig. 1 d).
Figure 1.
Generation of IRAK4 kinase–inactive knock-in mice. (a) Domains of mouse IRAK4 protein. DD, death domain; UD, undetermined domain. K213/K214 were mutated to methionines in kinase-inactive knock-in mice. (b) Structure of the mouse IRAK4 gene, the targeting vector, and the targeted allele. Pr1 and Pr2 are primers used for genomic DNA PCR as described in c. (c) PCR analysis of IRAK4 genomic DNA in wild-type and IRAK4 kinase–inactive knock-in mice with Pr1 and Pr2. WT, wild-type; HET, heterozygote; KI, IRAK4 kinase–inactive knock-in mice. (d) In the top panel, lysates from mouse embryo fibroblasts from either WT or KI mice were left untreated or treated with IL-1β for 5 min. IRAK4 was immunoprecipitated from whole cell lysates using a rabbit polyclonal antibody to full-length IRAK4 and assayed for its ability to phosphorylate recombinant kinase domain of IRAK1 (residues 182–546) by immunoprecipitation kinase assay as described in Materials and methods. Recombinant HIS-tagged IRAK4 protein (rIRAK4) was included as a control. In the bottom panel, Western blot analysis depicting IRAK4 expression levels in whole cell lysates described in Materials and methods.
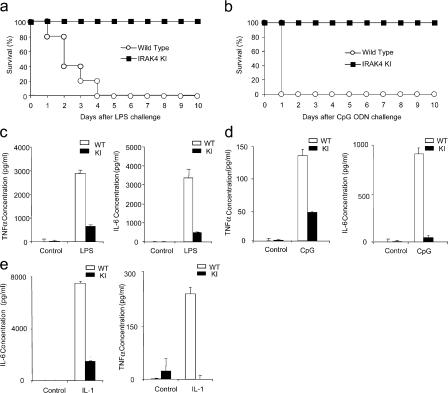
To examine the role of IRAK4 kinase activity in TLR-mediated pathways, we compared the wild-type and IRAK4 kinase–inactive knock-in mice for their susceptibility to LPS- and CpG-induced shock. IRAK4 kinase–inactive knock-in mice were completely resistant to LPS-induced septic shock (40 mg kg−1 body weight), whereas all of the wild-type mice died within 4 d of LPS challenge (Fig. 2 a). All wild-type mice injected intraperitoneally with a lethal dose of CpG (0.8 mg kg−1 and d-GalN 20 mg kg−1 body weight) died within 24 h of challenge, whereas IRAK4 kinase–inactive knock-in mice were completely resistant to CpG-induced shock (Fig. 2 b). Consistent with the lethality curve, high levels of TNF-α and IL-6 were produced in the sera of wild-type mice injected with LPS or CpG, whereas cytokine levels in the sera were significantly reduced in IRAK4 kinase–inactive knock-in mice in response to LPS and CpG stimulation (Fig. 2, c and d). Cytokine levels in the sera were also significantly reduced in IRAK4 kinase–inactive knock-in mice injected with IL-1 compared with that in wild-type mice (Fig. 2 e). Collectively, these results clearly indicate the critical role of the kinase activity of IRAK4 in IL-1R, TLR4, and TLR9 signaling in vivo.
Figure 2.
Resistance of IRAK4 kinase–inactive knock-in mice to LPS and CpG ODN-induced septic shock. (a) Age-matched wild-type (WT) and IRAK4 kinase–inactive knock-in (KI) mice (10 mice per group) were injected intraperitoneally with LPS (40 mg/kg body weight). Survival was monitored for 10 d. (b) Age-matched WT and KI mice (10 mice per group) were injected intraperitoneally with CpG ODN (0.8 mg/kg body weight) and d-GalN (20 mg). Survival was monitored for 10 d. (c) Serum concentration of TNF-α and IL-6 from WT and KI mice at 2 h after LPS injection was measured by ELISA. Results shown are the mean (± SD) of triplicate determinations. (d) Serum concentration of TNF-α and IL-6 from WT and KI mice at 2 h after CpG ODN injection was measured by ELISA. Results shown are the mean (± SD) of triplicate determinations. (e) Serum concentration of TNF-α and IL-6 from WT and KI mice at 2 h after IL-1β (40 μg/kg body weight) injection was measured by ELISA. Results shown are the mean (± SD) of triplicate determinations.
The kinase activity of IRAK4 is required for TLR-mediated cytokine and chemokine production
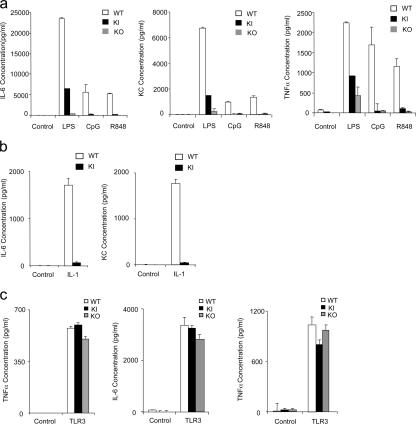
We then examined TLR-mediated cytokine production in primary cells from wild-type and IRAK4 kinase–inactive knock-in mice. Cytokine (IL-6 and TNF-α) and chemokine (KC) production were significantly reduced in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice in response to LPS, CpG, and R848 stimulation compared with that in wild-type cells (Fig. 3 a). IRAK4-deficient mice were used as a negative control. Although there was detectable residual cytokine and chemokine production in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice in response to LPS stimulation, LPS-induced TNF-α, IL-6, and KC production was almost completely abolished in BM-derived macrophages from IRAK4-deficient mice (Fig. 3 a). Furthermore, IL-1R–mediated cytokine and chemokine production were also severely impaired in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice (Fig. 3 b).
Figure 3.
Impaired TLR-mediated cytokine and chemokine production in macrophages from kinase–inactive IRAK4 knock-in mice. (a) Wild-type (WT), IRAK4 kinase–inactive knock-in (KI), or IRAK4 knock-out (KO) macrophages were treated with TLR ligands LPS (1 μg ml−1), R848 (1 μg ml−1), or CpG ODN (4 μg ml−1) for 24 h. IL-6, KC, and TNF-α concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. (b) WT or KI macrophages were treated with IL-1β (10 ng ml−1) for 24 h. IL-6 and KC concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. (c) WT, KI, or KO mDCs were treated with TLR3 ligand, PolyIC (1 μg ml−1) for 24 h. IFN-α, IL-6, and TNF-α concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations.
We also examined the impact of IRAK4 kinase activity on cytokine and chemokine production mediated by other TLRs. Interestingly, we found that TLR2- and TLR5-mediated cytokine and chemokine production was greatly reduced in BM-derived macrophages from IRAK4 knock-in mice compared with that in wild-type control cells, indicating that the kinase activity plays an important role in proinflammatory cytokine production mediated by all of the TLRs that use the MyD88-dependent pathway (unpublished data). TLR3 seems to be the only TLR that does not use the MyD88-dependent pathway (32–34). TLR3-mediated cytokine production was normal in IRAK4-deficient cells derived from human patients. TLR3-mediated cytokine production (IFN-α, IL-6, and TNF-α) was normal in myeloid DCs (mDCs) derived from IRAK4-deficient mice and IRAK4 kinase– inactive knock-in mice compared with that in wild-type mice (Fig. 3 c). However, it is important to point out that a certain defect in TLR3-mediated cytokine production was detected previously in IRAK4-deficient mice, suggesting a possible role of IRAK4 in TLR3 signaling directly or indirectly, possibly by affecting the expression levels of TLR3 in certain primary cells (18).
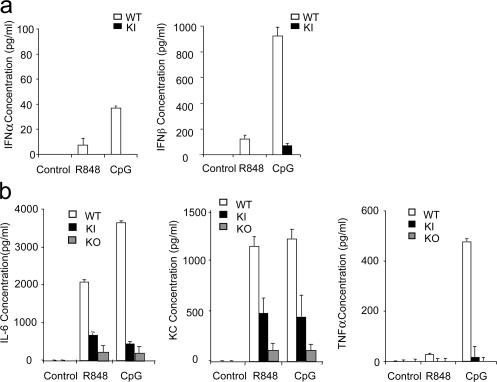
TLR7- and TLR9-mediated production of IFN-α/β was abolished in IRAK4 kinase–inactive pDCs
In addition to the induction of proinflammatory genes, TLR7- and TLR9-mediated signaling in pDCs has been shown to play a critical role in viral immunity through the efficient production of type I IFNs. In humans, it has been shown that TLR7-, TLR8-, and TLR9-mediated induction of IFN-α/β is strictly IRAK4 dependent. To examine the role of IRAK4 kinase activity in TLR7- and TLR9-mediated IFN-α/β production, we generated Flt3 ligand–driven pDCs from the BM of IRAK4 kinase–inactive knock-in and wild-type mice. Importantly, we found that TLR7- and TLR9-mediated IFN-α/β production was abolished in pDCs from IRAK4 knock-in mice, indicating that the kinase activity of IRAK4 is required for TLR7- and TLR9-mediated type I IFN production (Fig. 4 a). TLR7- and TLR9-mediated proinflammatory cytokine production (IL-6, KC, and TNF-α) was also greatly reduced in pDCs from kinase-inactive IRAK4 knock-in mice compared with that in wild-type pDCs (Fig. 4 b). It is important to note that TLR7- and TLR9-mediated proinflammatory cytokine production was completely abolished in IRAK4-deficient mice (Fig. 4 b), indicating that IRAK4 kinase–inactive mutant still retained partial ability to confer TLR signaling.
Figure 4.
Impaired TLR7- and TLR9-mediated type I IFN production in pDCs derived from IRAK4 kinase–inactive knock-in mice. (a) Wild-type (WT) or IRAK4 kinase–inactive knock-in (KI) pDCs were stimulated with R848 (1 μg ml−1) or CpG ODN (1 μg ml−1) for 24 h. IFN-α and IFN-β concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. (b) WT, KI or IRAK4 knock-out (KO) pDCs were stimulated with R848 (1 μg ml−1) or CpG DNA (1 μg ml−1) for 24 h. IL-6, KC, and TNF-α concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations.
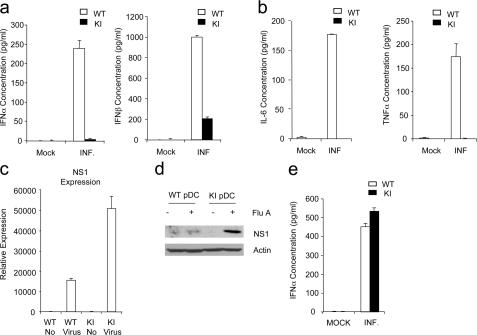
We then examined the impact of kinase activity of IRAK4 on viral-induced IFN production. pDCs were infected with influenza virus, and IFN levels were monitored 24 h after infection. In addition to the observed defect in TLR7- and TLR9-mediated induction of IFNs, virus-mediated type I IFN production was also impaired in pDCs from IRAK4 knock-in mice (Fig. 5 a). The production of IL-6 and TNF-α was also greatly reduced in the knock-in mice after infection with influenza virus (Fig. 5 b). The fact that we detected viral NS1 mRNA and protein in both wild-type and IRAK4 kinase–inactive knock-in pDCs infected with influenza virus indicates that infection was successful in these cells (Fig. 5, c and d). It is important to note that NS1 expression was much higher in IRAK4 kinase–inactive knock-in cells compared with that in wild-type cells, which might be because of the impact of IFN production on the exclusion of influenza virus in the wild-type cells (Fig. 5, c and d).
Figure 5.
Impaired responses to type A influenza virus in IRAK4 kinase–inactive knock-in pDCs and mDCs. (a) Wild-type (WT) or IRAK4 kinase–inactive knock-in (KI) pDCs were infected with type A influenza virus (MOI = 2) for 24 h. IFN-α and IFN-β concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. (b) WT or KI pDCs were infected with type A influenza virus (MOI = 2) for 24 h. IL-6 and TNF-α concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. (c and d) WT or KI pDCs were infected with type A influenza virus (MOI = 2) for 24 h. pDCs were harvested and analyzed by quantitative real-time PCR (c) or Western blot analysis (d) with antibody against influenza virus NS1 and actin. (e) WT or KI mDCs were infected with type A influenza virus (MOI = 2) for 24 h. IFN-α concentrations were measured by ELISA. Results shown are the means (± SD) of triplicate determinations. Mock, mock infected; INF, infected; ND, not detectable.
Interestingly, virus-induced IFN production in mDCs was independent of the IRAK4 kinase activity (Fig. 5 e). These results demonstrate that the kinase activity of IRAK4 is required for the TLR-mediated viral immune response in pDCs, but not involved in viral detection in mDCs, which is mediated by intracellular receptors RIG-I and MDA5 (35). Collectively, our results indicate that IRAK4 kinase activity plays a critical role in TLR-dependent immune responses, especially for TLR7- and TLR9-mediated production of type I IFN.
The IRAK4 kinase activity is partially required for TLR–IL-1R signaling
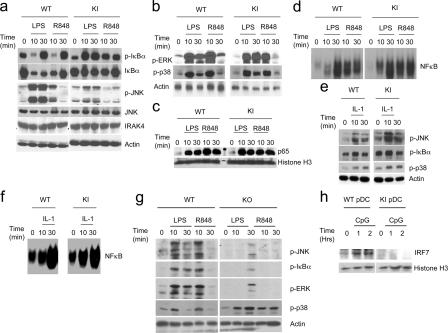
To study the direct impact of IRAK4 kinase activity on TLR-mediated signaling events, BM-derived macrophages from wild-type, IRAK4-deficient, and IRAK4 kinase– inactive knock-in mice were examined for TLR-mediated NF-κB and JNK activation. LPS- and R848-induced JNK activation was greatly reduced in BM-derived macrophages from IRAK4 kinase–inactive knock-in and IRAK4-deficient mice compared with that in wild-type control cells (Fig. 6, a and g). Interestingly, LPS and R848 induced phosphorylation of ErK and IκB and NF-κB activation (including NF-κB nuclear translocation and DNA binding activity) in both wild-type and IRAK4 kinase–inactive knock-in cells, despite the fact that these signaling events were greatly diminished in BM-derived macrophages from IRAK4-deficient mice (Fig. 6, a–d and g). However, it is important to point out that whereas LPS and R848 induced IκB phosphorylation and NF-κB activation in BM-derived macrophages from both wild-type and IRAK4 kinase–inactive knock-in mice, TLR-mediated IκB degradation was attenuated in IRAK4 kinase–inactive knock-in cells compared with that in wild-type cells (Fig. 6 a). The attenuated IκB degradation was probably responsible for the higher level of phosphorylated IκB detected in IRAK4 kinase–inactive knock-in cells 10 min after ligand stimulation compared with that in wild-type cells. We recently identified two TLR-mediated NF-κB activation pathways: TAK1-dependent and MEKK3-dependent (36). Although NF-κB is activated by IκB phosphorylation and degradation in the TAK1-dependent pathway, IκB is phosphorylated, dissociated from NF-κB but not degraded in the MEKK3-dependent pathway. We found that IRAK phosphorylation is required for IL-1–induced TAK1-dependent but not MEKK3-dependent NF-κB activation, indicating that these two pathways bifurcate at the level of IRAK modification (36). The fact that LPS and R848 stimulation led to IκB phosphorylation, NF-κB activation but with attenuated IκB degradation in IRAK4 kinase–inactive knock-in cells suggests that the kinase activity of IRAK4 might play a more critical role in TLR/IL- 1R–induced TAK1-dependent than in the MEKK3-dependent NF-κB activation pathway.
Figure 6.
Role of IRAK4 kinase activity in TLR-mediated signaling. (a) Cell lysates from BM-derived macrophages that were either untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for the indicated times were analyzed by Western blot analysis with antibodies against phospho–IκB-α, IκB-α, phospho-JNK, JNK, IRAK4, and actin. (b) Cell lysates from BM-derived macrophages that were either untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for the indicated times were analyzed by Western blot analysis with antibodies against phospho-ERK, phospho-p38. (c) Nuclear extracts prepared from BM-derived macrophages that were either untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for the indicated times were analyzed by Western blot analysis with antibodies against p65 and histone H3. (d) Cell lysates from BM-derived macrophages that were either untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for the indicated times were analyzed by electrophoretic mobility shift assay with a NF-κB–specific probe. (e) Cell lysates from BM-derived macrophages that were either untreated or treated with IL-1β (10 ng ml−1) for the indicated times were analyzed by Western blot analysis with antibodies against phospho-JNK, phospho–IκB-α, phosphor-p38, and actin. (f) Cell lysates from BM-derived macrophages that were either untreated or treated with IL-1β (10 ng ml−1) for the indicated times were analyzed electrophoretic mobility shift assay with a NF-κB–specific probe. (g) Cell lysates from BM-derived macrophages of wild-type (WT) or IRAK4 knock-out (KO) mice that were either untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for the indicated times were analyzed by Western blot analysis with antibodies against phospho-JNK, phospho–IκB-α, phospho-ERK, phospho-p38, and actin. (h) Nuclear extracts prepared from BM- derived pDCs that were either untreated or treated with CpG for the indicated times were analyzed by Western blot analysis with antibodies against IRF7 and histone H3.
LPS- and R848-induced p38 phosphorylation was normal in both IRAK4-deficient and IRAK4 kinase–inactive knock-in BM-derived macrophages, indicating that IRAK4 is not required for TLR-mediated p38 phosphorylation (Fig. 6, b and g). As a matter of fact, IRAK4-deficient cells had somewhat stronger LPS- and R848-mediated p38 activation. One possible explanation is that the loss of IRAK4 enhances TLR-signaling events independent of IRAK4, such as p38 phosphorylation. We previously reported that both wild-type IRAK4 and kinase-inactive IRAK4 mutant can restore IL-1–induced NF-κB and JNK activation in human IRAK4-deficient cells (37). Consistent with our previous findings, we showed that IL-1 stimulation led to similar levels of phosphorylation of JNK, IκB, and p38 and NF-κB activation in BM-derived macrophages from wild-type and IRAK4 kinase–inactive knock-in mice (Fig. 6, e and f).
We also examined IRF7 activation in pDCs in response to TLR9 ligand stimulation. As shown in Fig. 6 h, IRF7 translocated into the nucleus 1 h after stimulation with CpG in wild-type pDCs, whereas TLR9-induced nuclear translocation of IRF7 was abolished in IRAK4 kinase–inactive knock-in pDCs. These results indicate that the kinase activity of IRAK4 is required for TLR9-mediated IRF7 activation.
The IRAK4 kinase activity is required for a subset of cytokine and chemokine mRNA stability in response to LPS and R-848 stimulation
TLR–IL-1R–induced NF-κB and JNK activation were abolished in IRAK4-deficient cells, resulting in the failure of TLR–IL-1R–mediated up-regulation of cytokine/chemokine mRNA and protein (18, 19) (Fig. 3 a, Fig. 4 b, and Fig. 7 a). It is important to point out that TLR–IL-1R–induced cytokine/chemokine mRNA levels were significantly retained in IRAK4 kinase–inactive knock-in cells upon ligand stimulation compared with that in the wild-type cells (Fig. 7, b and f), which is consistent with the fact that the inactivation of IRAK4 kinase activity did not reduce LPS-, R848-, and IL-1–mediated NF-κB activation (probably because of the redundancy of TAK1- versus MEKK3-dependent NF-κB activation pathway). However, it was puzzling to note that LPS-, R848-, and IL-1–induced cytokine and chemokine production was greatly reduced in primary cells from IRAK4 kinase–inactive knock-in mice and that these mice were completely resistant to LPS-induced septic shock. Furthermore, in our previous study with human IRAK4-deficient cells, we reported that similar levels of IL-1–induced NF-κB and JNK activation and IL-1– induced IL-8 mRNA were detected in IRAK4-deficient cells reconstituted with IRAK4 kinase inactive mutant compared with that reconstituted with wild-type IRAK4. By ELISA analysis, we now found that IL-1–induced IL-8 and IL-6 production was actually reduced in IRAK4-deficient cells reconstituted in IRAK4 kinase–inactive mutant compared with that reconstituted with wild-type IRAK4 (unpublished data).
Figure 7.
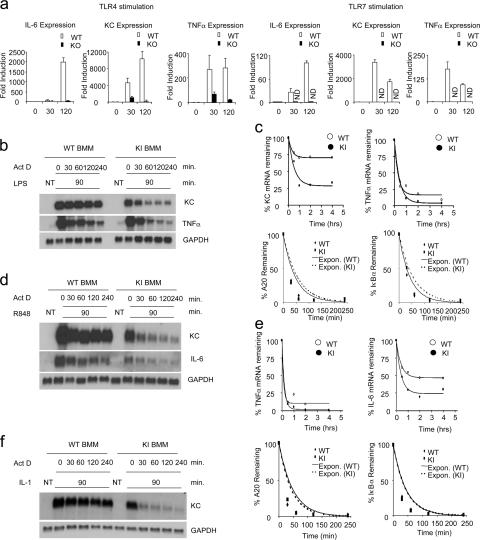
Impaired TLR-mediated mRNA stability in macrophages from kinase-inactive IRAK4 knock-in mice. (a) BM-derived macrophages from wild-type (WT) and IRAK4-deficient (KO) mice were untreated or treated with LPS (1 μg ml−1) or R848 (1 μg ml−1) for indicated times. Total RNA (3 μg) was subjected to RT-PCR analysis for the levels of IL-6, KC, and TNF-α mRNA. (b) BM-derived macrophages were untreated (NT) or treated with LPS (1 μg ml−1) for 90 min and then treated with actinomycin D (5 μg ml−1) and LPS (1 μg ml−1) for indicated times. Total RNA (10 μg) was subjected to Northern blot analysis to quantitate the levels of KC and TNF-α mRNA. (c) The autoradiographs in b were quantified for KC, TNF-α, and GAPDH mRNA levels using the NIH Image software package. Normalized levels of KC and TNF-α are depicted. Similar results were obtained in three separate experiments. A20 and IκBα mRNA from the same samples as described in b were also analyzed by quantitative RT-PCR, normalized by β-actin, and plotted. Similar results were obtained in three separate experiments. (d) BM-derived macrophages were untreated (NT) or treated with R-848 (1 μg ml−1) for 90 min and then treated with actinomycin D (5 μg ml−1) and R848 (1 μg ml−1) for indicated times. Total RNA (10 μg) was subjected to Northern blot analysis to quantitate the levels of KC and TNF-α mRNA. (e) The autoradiographs in d were quantified for KC, TNF-α, and GAPDH mRNA levels using the NIH Image software package. Normalized levels of KC and TNF-α are depicted. Similar results were obtained in three separate experiments. A20 and IκBα mRNA from the samples as described in d were also analyzed by quantitative RT-PCR, normalized by β-actin, and plotted. Similar results were obtained in three separate experiments. (f) BM-derived macrophages were untreated (NT) or treated with IL-1 for 90 min and then treated with actinomycin D (5 μg ml−1) and IL-1 (1 ng ml−1) for indicated times. Total RNA (10 μg) was subjected to Northern blot analysis to quantitate the levels of KC mRNA.
Whereas TLR-mediated cytokine production was abolished in BM-derived macrophages in IRAK4 kinase–inactive knock-in mice, TLR-induced NF-κB activation was normal. Therefore, we hypothesized that the kinase activity of IRAK4 might be involved in the regulation of cytokine production at a posttranscriptional level. To test this hypothesis, we designed experiments to measure the impact of IRAK4 kinase activity on cytokine and chemokine mRNA stability. BM-derived macrophages from wild-type and IRAK4 kinase–inactive knock-in mice were first treated with LPS for 1.5 h, followed by treatment with actinomycin D (to block transcription) and LPS (for mRNA stabilization) for 0.5–4 h. Although both TNF-α and KC mRNAs were induced to similar levels in BM-derived macrophages from wild-type and IRAK4 kinase–inactive mice after the initial treatment with LPS (Fig. 7 b), the decay rate of mRNA was accelerated and reached a lower plateau for both messages in BM-derived macrophages from IRAK4 kinase–inactive mice compared with wild-type cells (Fig. 7, b and c). The impact of IRAK4 kinase activity on mRNA stability was not limited to TLR4-mediated signaling. Although TLR7-mediated KC and IL-6 mRNA stability was abolished in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice (Fig. 7, d and e), IL-1–mediated TNF-α and KC mRNA stability was also abolished in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice (Fig. 7 f and unpublished data). As a control, we showed that LPS- and R848-induced mRNAs (A20 and IκBα) that are not regulated at the RNA stability levels decayed at the similar rate in macrophages from IRAK4 kinase–inactive knock-in mice to that in wild-type mice (Fig. 7, c and e). Collectively, these results indicate that the kinase activity of IRAK4 is required for a subset of TLR/IL-1R–mediated cytokine and chemokine mRNA stability.
DISCUSSION
Previous studies showed that IRAK4 deficiency leads to severe impairment of TLR signaling, indicating an essential role of IRAK4 in TLR-mediated pathways (18, 19). In this article, we report that the kinase activity of IRAK4 is critical for the functions of IRAK4 in TLR-mediated inflammatory and innate immune responses. It is clear that the kinase activity of IRAK4 is not only required for TLR (2, 4, 5, 7, and 9)-induced production of proinflammatory cytokines and chemokines but also necessary for TLR7- and TLR9-mediated induction of IFN-α/β production. Moreover, influenza virus–mediated type I IFNs production was totally abolished in pDCs from IRAK4 knock-in mice. Collectively, our results indicate that IRAK4 kinase activity plays an essential role in TLR-dependent immune responses.
Previous studies suggest that IRAK4 is required for the recruitment and activation of IRAK at the signaling complex. Interestingly, IRAK4 kinase–inactive mutant had similar ability as the wild-type IRAK4 in restoring IL-1–mediated NF-κB in human IRAK4-deficient cells. Only the impairment of the kinase activity of both IRAK and IRAK4 efficiently abolished the IL-1 pathway, suggesting that the kinase activity of IRAK and IRAK4 might be redundant for IL-1–mediated signaling. On the other hand, by reconstituting IRAK4-deficient mouse embryonic fibroblasts, Lye et al. showed that the kinase activity of mouse IRAK4 is required for the optimal transduction of IL-1–induced signals, although they found that IRAK4 is capable of mediating some NF-κB activation (38). In support of these previous findings, IL-1-, LPS-, and R848-induced NF-κB activation was not reduced in the BM-derived macrophages from IRAK4 kinase–inactive knock-in mice compared with that in the wild-type control cells. Therefore, the kinase activity of IRAK4 seems to be dispensable for TLR/IL-1R–mediated NF-κB activation.
It is interesting to note that although TLR-mediated IκB and NF-κB activation was not reduced in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice compared with that in wild-type mice, LPS- and R848-mediated IκB degradation was attenuated in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice, indicating that the requirement of IRAK4 kinase activity for certain aspects of TLR-mediated NF-κB activation. Such seemingly conflicting results could probably be explained by the complexity of the TLR–IL-1R–mediated NF-κB activation pathways. Through the analyses of IRAK modification mutants, we recently uncovered two parallel IL-1–mediated signaling pathways for NF-κB activation: TAK1-dependent and MEKK3-dependent, respectively (36). These two pathways bifurcate at the level of IRAK modification. The TAK1-dependent pathway leads to IκB kinase (IKK)-α/β phosphorylation and IKK-β activation, resulting in classical NF-κB activation through IκBα phosphorylation and degradation. The TAK1-independent MEKK3-dependent pathway involves IKK-γ phosphorylation and IKK-α activation, resulting in NF-κB activation through IκBα phosphorylation and subsequent dissociation from NF-κB but without IκBα degradation. These results provide new insight to our understanding of NF-κB activation data from the IRAK4 kinase–inactive knock-in cells. The fact that LPS and R848 stimulation led to IκB phosphorylation, NF-κB activation but with attenuated IκB degradation in IRAK4 kinase–inactive knock-in cells suggests that the kinase activity of IRAK4 is likely to play a more critical role in TLR–IL-1R–induced TAK1-dependent than in the MEKK3-dependent NF-κB activation pathway. In support of this, our preliminary results showed that TLR–IL-1R–induced IKK-α/β and TAK1 phosphorylation was indeed abolished in the absence of IRAK4 kinase activity (unpublished data). Future studies are required to further investigate the role of IRAK4 kinase activity in the TAK1-dependent NF-κB activation pathway.
It is intriguing that although IL-1R–mediated JNK activation was not reduced in the absence of IRAK4 kinase activity, TLR-mediated JNK activation was greatly reduced in IRAK4 kinase–inactive knock-in cells. One possible explanation is the differential signaling events mediated by TLRs versus IL-1R. Although both IL-1R and TLRs use MyD88–IRAK4–IRAK, the signaling outcomes are actually very different, indicating the involvement of different downstream components in IL-1R versus TLR signaling. For example, TLR7 can mediate IFN production through the induction IRF7 activation (25), whereas IL-1R cannot. The differential impact of IRAK4 kinase activity on TLR- versus IL-1R–mediated JNK activation implicates distinct signaling events derived from TLRs and IL-1R in leading to JNK activation.
TLR–IL-1R–induced NF-κB and JNK activation were abolished in IRAK4-deficient cells, resulting in the failure of TLR–IL-1R–mediated up-regulation of cytokine/chemokine mRNA and protein, probably because of a defect in gene transcription upon ligand stimulation (18, 19). Interestingly, we found that whereas inactivation of IRAK4 kinase activity did not reduce TLR–IL-1R–induced NF-κB activation and induction cytokine/chemokine mRNA, it did greatly diminish TLR–IL-1R–mediated induction of cytokines and chemokines. As a result, the IRAK4 kinase–inactive knock-in mice were completely resistant to LPS-induced septic shock. Importantly, whereas TLR–IL-1R induced similar levels of cytokine and chemokine mRNA in the absence of IRAK4 kinase activity, TLR–IL-1R–mediated cytokine and chemokine mRNA stability was reduced in BM-derived macrophages from IRAK4 kinase–inactive knock-in mice. These observations suggest that posttranscriptional regulation is at least one of the mechanisms responsible for the substantial reduction in TLR–IL-1R–mediated cytokine and chemokine production and the resistance to LPS-induced septic shock observed in these mice. One important question is whether the reduced JNK activation is linked to the reduction in mRNA stability in the absence of IRAK4 kinase activity. Interestingly, it has been reported that JNK plays a key role in IL-2 mRNA stability during T cell activation (39). However, whereas TLR–IL-1R–mediated JNK activation was completely abolished in TAK1-deficient mouse embryonic fibroblasts (MEFs), LPS- and IL-1–mediated KC mRNA stabilization was not reduced compared with that in wild-type MEFs, indicating that JNK activation is not required for TLR–IL-1R–mediated mRNA stability (unpublished data). Furthermore, although dominant-negative mutant of TAK1 blocked TLR–IL-1R–mediated JNK activation, it did not affect mRNA stability (unpublished data). These results suggest that the reduced TLR-mediated JNK activation may not be the cause for the abolished mRNA stability in IRAK4 kinase–inactive macrophages. Activation of p38 has been implicated in LPS-induced mRNA stability (40). However, we found that LPS-induced p38 phosphorylation was normal in IRAK4 kinase–inactive knock-in and even IRAK4-deficient cells. Future studies are required to investigate the intermediate signaling events leading to mRNA stability and what role the kinase activity of IRAK4 might play in this process.
Earlier studies showed that TLR7 and TLR9 are the receptors responsible for the detection of viral infection in pDCs and mediate production of type I IFNs (31). We found that TLR7 and TLR9-mediated IFN production was completely abolished in pDCs derived from IRAK4 kinase–inactive knock-in mice. The failure of influenza virus to induce type I IFN production in pDCs derived from IRAK4 knock-in mice indicates that the kinase activity of IRAK4 is required for TLR-mediated viral immunity.
MATERIALS AND METHODS
Reagents
Full-length HIS-tagged IRAK4 protein and kinase-dead IRAK1 (kinase domain) was expressed using bacculovirus and purified from SF9 cells. Recombinant human IL-1β was purchased from R&D Systems. CpG oligodeoxynucleotide (ODN) was purchased from Invivogen or IDEX. LPS (Escherichia coli 055:B5) was purchased from Sigma-Aldrich. R848 {1-(2-hydroxy-2-methylpropyl)-2-methyl-1H-imidazo[4,5-c]quinolin-4-amine} was commercially synthesized by GLSynthesis. PolyI:C was purchased from GE Healthcare. Antibodies against phosphorylated IκB-α (Ser [32]) and JNK were purchased from Cell Signaling. Antibody to β-actin was purchased from Sigma-Aldrich. Affinity-purified polyclonal antiserum to full-length IRAK4 used for immunoprecipitation was generated at Zymed Laboratories. IRAK4 antibody was also purchased from Upstate Biochemicals. Histone H3 (Cell Signaling, Technology, Inc.) and p65 (Cell Signaling, Technology, Inc.) were used to check the subcellular fractions.
Generation of the kinase-inactive IRAK4 knock-in mice
IRAK4 kinase–inactive knock-in mice were generated at InGenKO using C57BL/6 embryonic stem cells. A targeting construct containing lysines 213 and 214 (in exon 4) changed to methionines in the ATP binding pocket of the kinase domain was generated for this purpose (Fig. 1 b). The complete nucleotide sequence of the targeting construct is available upon request. Targeted embryonic stem cells were injected into mouse blastocysts to produce chimeric mice. The chimeric mice were bred to C57BL/6 (B6) mice to generate wild-type, heterozygous, and homozygous mice. IRAK4 knock-in mice and their age-matched wild-type littermates from these intercrosses were used for all experiments. The Cleveland Clinic Foundation Animal Research Committee approved all of the animal protocols used in this study.
Primary cell isolation
pDCs and mDCs.
BM-derived macrophages were obtained from the BM of tibia and femur by flushing with DMEM. BM cells were plated at 1 × 106 cells per ml in RPMI complete medium (10% FBS, 2 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 50 μM β-mercaptoethanol) containing 100 ng/ml of Flt3L (PeproTec) for pDCs or 200 IU of GM-CSF (R&D Systems) for mDCs, respectively. pDCs and mDCs were collected for experiments after 6 d of culture.
Macrophages.
BM-derived macrophages were obtained from the BM of tibia and femur by flushing with DMEM. The cells were cultured in DMEM supplemented with 20% FBS, and 30% L929 supernatant for 5 d.
For IL-1β treatment, the cells were cultured in DMEM supplemented with 20% FBS and 10 ng/ml of murine M-CSF (PeproTech) for 5 d.
Northern blot and quantitative real-time PCR
For Northern analysis, total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA (10 μg) was fractionated by electrophoresis on a formaldehyde gel and transferred to Hybond-N and probed with 32P-labeled gene-specific DNA probes, according to the procedures provided by GE Healthcare. Probe hybridization and washing were performed according to procedures provided by GE Healthcare, and signals were visualized by autoradiography.
For real-time PCR, total RNA was isolated using TRIzol reagent (Invitrogen). 3 μg of total RNA was then used for reverse transcription reaction using SuperScript-reverse transcriptase (Invitrogen). Quantitative PCR was performed in AB 7300 RealTime PCR System, and the gene expression of mouse A20, IκBα, β-actin, and human influenza virus NS1 was examined by SYBR GREEN PCR Master Mix (Applied Biosystems). PCR amplification was performed in triplicate, and water was used to replace cDNA in each run as a negative control. The reaction protocol included preincubation at 95°C to activate FastStart DNA polymerase for 10 min, amplification of 40 cycles that was set for 15 s at 95°C, and annealing for 60 s at 60°C. The results were normalized with the housekeeping gene mouse β-actin. The specific primer sequences used for mouse A20, mouse IκBα, mouse β-actin, mouse TNF-α, mouse IL-6, mouse KC, and influenza virus NS1 (available from GenBank/EMBL/DDBJ under accession no. CY009640) listed as follows: A20 (103 bp): 5′-CTGCAATGAAGTGCAGGAGT-3′ and 5′-GTGTGGCTGGCATTAATCTG-3′; IκBα (104 bp): 5′-ACTTTGGGTGCTGATGTCAA-3′ and 5′-TTCAACAAGAGCGAAACCAG-3′; β-actin (133 bp): 5′-GGTCATCACTATTGGCAACG-3′ and 5′-ACGGATGTCAACGTCACACT-3′; TNF-α (103 bp): 5′-CAAAGGGAGAGTGGTCAGGT-3′ and 5′-ATTGCACCTCAGGGAAGAGT-3′; IL-6 (127 bp): 5′-GGACCAAGACCATCCAATTC-3′ and 5′-ACCACAGTGAGGAATGTCCA-3′; KC (125 bp): 5′-TAGGGTGAGGACATGTGTGG-3′ and 5′AAATGTCCAAGGGAAGCGT-3′; influenza virus NS1 (125 bp): 5′-CTAAGGGCTTTCACCGAAGA-3′ and 5′-TTCCATTCAAGTCCTCCGAT-3′.
Western blot analysis
Cells stimulated as indicated were harvested, washed once with phosphate-buffered saline, and lysed for 30 min at 4°C in 1.0% NP-40, 100 mM Tris hydrochloride, pH 8.0, 20% glycerol, and 0.2 mM EDTA. Cellular debris was removed by centrifugation at 10,000 × g for 5 min. For immunoblotting, cell extracts were fractionated by sodium dodecyl sulfate-PAGE and transferred to Immobilon-P transfer membranes (Millipore), using a wet transfer apparatus (Bio-Rad Laboratories). Immunoblot analysis was performed and the bands were visualized with horseradish peroxidase–coupled goat anti–rabbit, goat anti–mouse, or donkey anti–goat immunoglobulin as appropriate (Rockland), using the ECL chemiluminescence Western blotting detection system (GE Healthcare). Protein levels were equilibrated with the Protein Assay Reagent (Bio-Rad Laboratories).
In vitro kinase assay
Lysates from MEF cells were prepared in a lysis buffer consisting of 20 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 2 mM EGTA, 2 mM DTT, 0.5% Triton X-100, 12.5 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, and protease inhibitors. IRAK4 was immunoprecipitated using affinity-purified rabbit polyclonal antibody to full-length IRAK4 and protein A agarose beads. The resulting immunoprecipitates were incubated with 2 μM bacterially purified kinase domain of IRAK1 (residues 182–546, predicted size 46 kd), in kinase buffer consisting of 50 mM HEPES, pH 7.4, 5 mM MgCl2, 0.005% Triton X-100, 2.0 mM DTT, 200 μM cold ATP, and 2 μCi of [γ32P]-labeled ATP (3,000 Ci/mmol) in a total reaction volume of 30 μl. Reactions were stopped by boiling and samples were analyzed by SDS-PAGE. Dried gels were analyzed by phosphorimaging.
Influenza viral infection
Infection of pDCs and mDCs with influenza A was performed as previously described (14). In brief, GM-CSF and FLT3 ligand–derived DCs were seeded on to 12-well plates at 5 × 105 cells/ml and infected with type A influenza virus (multiplicity of infection [MOI] = 2) for 24 h. IFNs, proinflammatory cytokine, nd chemokine production in the supernatants was measured by ELISA.
To check the infectivity of virus, influenza virus NS1 protein or mRNA was analyzed by Western blot sis or quantitative real-time PCR using pDCs from wild-type and IRAK4 knock-in mice.
ELISA assay
Blood samples were collected from wild-type and IRAK4 knock-in mice after intraperitoneal injections of LPS at the concentration and time indicated in Fig. 2 C. Cultures of pDCs and macrophages were stimulated with LPS (1 μg ml−1), CpD-ODN (1 μg ml−1), and R848 (1 μg ml−1) as indicated in Fig. 3 a, and collected after 24 h. IL-6, KC, and TNF-α production in culture medium was measured using ELISA kits obtained from R&D Systems, following manufacturer's instructions. The ELISA kits for mouse IFN-α and IFN-β were purchased from PBL Biomedical Laboratories. Total protein concentration was measured by BCA analysis (Pierce Chemical Co.).
Preparation and fractionation of nuclear extracts
BM-derived macrophage from wild-type and IRAK4 kinase–inactive knock-in mice were cultured as indicated. After appropriate stimulation, cells were harvested, cell pellets were washed with ice-cold PBS twice and resuspended in 6 volumes of buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 1 mM PMSF, 0.3 mM Na3VO4, 5 mM NaF) and allowed to sit on ice for 10 min. The cell suspension was transferred to the Dounce homogenizer, and the cells were disrupted with 30 strokes. Cytoplasmic extracts were obtained by centrifugation at 500 g for 1 min at 4°C. The nuclear pellet was washed with 1 ml Nuclei EZ Lysis buffer (Sigma Nuclei EZ Prep kit) followed by washing with 1 ml buffer A. Nuclei were recovered by centrifugation at 500 g for 1 min at 4°C and resuspended in 50 μl of buffer B (20 mM HEPES, pH 7.9, 500 mM NaCl, 1.5 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 1 mM PMSF, 0.3 mM Na3VO4, 5 mM NaF) and incubated on ice for 30 min. After centrifugation for 10 min at 14,000 g, the supernatant fraction was collected.
Acknowledgments
We would like to thank Dr. L. Herrero (Harvard University, Cambridge, MA) and S. Shoelson (Harvard University) for the generous supply of IRAK4−/− mice. We would also like to thank Jonathon Sedgwick for his helpful comments and support.
This work was supported by National Institutes of Health grant RO1 GM060020-06 (to X. Li) and PPG CA62220 (to G. Sen).
The authors have no conflicting financial interests.
Abbreviations used: IKK, IκB kinase; IRAK, IL-1 receptor–associated kinase; IRF, interferon regulatory factor; JNK, c-Jun NH2-terminal kinase; KC, chemokine; mDC, myeloid DC; MEF, mouse embryonic fibroblast; ODN, oligodeoxynucleotide; pDC, plasmacytoid DC; TAK1, TGF-β–activated kinase 1; TLR, Toll-like receptor.
T.W. Kim and K. Staschke contributed equally to this work.
References
- 1.Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. [DOI] [PubMed] [Google Scholar]
- 2.Rock, F.L., G. Hardiman, J.C. Timans, R.A. Kastelein, and J.F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA. 95:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi, O., T. Kawai, H. Sanjo, N.G. Copeland, D.J. Gilbert, N.A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding toll-like receptor family. Gene. 231:59–65. [DOI] [PubMed] [Google Scholar]
- 4.Chuang, T.H., and R.J. Ulevitch. 2000. Cloning and characterization of a sub-family of human Toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur. Cytokine Netw. 11:372–378. [PubMed] [Google Scholar]
- 5.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, D., G. Zhang, M.S. Hayden, M.B. Greenblatt, C. Bussey, R.A. Flavell, and S. Ghosh. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science. 303:1522–1526. [DOI] [PubMed] [Google Scholar]
- 7.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 11:443–451. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P.F. Muhlradt, and S. Akira. 2000. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554–557. [DOI] [PubMed] [Google Scholar]
- 10.Underhill, D.M., A. Ozinsky, A.M. Hajjar, A. Stevens, C.B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 401:811–815. [DOI] [PubMed] [Google Scholar]
- 11.Underhill, D.M., A. Ozinsky, K.D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA. 96:14459–14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi, F., K.D. Smith, A. Ozinsky, T.R. Hawn, E.C. Yi, D.R. Goodlett, J.K. Eng, S. Akira, D.M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 410:1099–1103. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 14.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 15.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 16.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 17.Li, S., A. Strelow, E.J. Fontana, and H. Wesche. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA. 99:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki, N., S. Suzuki, G.S. Duncan, D.G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, et al. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 416:750–756. [DOI] [PubMed] [Google Scholar]
- 19.Picard, C., A. Puel, M. Bonnet, C.L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, et al. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 299:2076–2079. [DOI] [PubMed] [Google Scholar]
- 20.Cao, Z., W.J. Henzel, and X. Gao. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science. 271:1128–1131. [DOI] [PubMed] [Google Scholar]
- 21.Li, X., M. Commane, C. Burns, K. Vithalani, Z. Cao, and G.R. Stark. 1999. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 19:4643–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D.V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature. 383:443–446. [DOI] [PubMed] [Google Scholar]
- 23.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z.J. Chen. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 103:351–361. [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 398:252–256. [DOI] [PubMed] [Google Scholar]
- 25.Kawai, T., S. Sato, K.J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068. [DOI] [PubMed] [Google Scholar]
- 26.Honda, K., H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W.C. Yeh, and T. Taniguchi. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA. 101:15416–15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uematsu, S., S. Sato, M. Yamamoto, T. Hirotani, H. Kato, F. Takeshita, M. Matsuda, C. Coban, K.J. Ishii, T. Kawai, et al. 2005. Interleukin-1 receptor–associated kinase-1 plays an essential role for Toll-like receptor (TLR)7– and TLR9-mediated interferon-{alpha} induction. J. Exp. Med. 201:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, K., A. Puel, S. Zhang, C. Eidenschenk, C.L. Ku, A. Casrouge, C. Picard, H. von Bernuth, B. Senechal, S. Plancoulaine, et al. 2005. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 23:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin, J., J. Yao, G. Cui, H. Xiao, T.W. Kim, J. Fraczek, P. Wightman, S. Sato, S. Akira, A. Puel, et al. 2006. TLR8-mediated NF-{kappa}B and JNK activation are TAK1-independent and MEKK3-dependent. J. Biol. Chem. 281:21013–21021. [DOI] [PubMed] [Google Scholar]
- 30.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti- viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200. [DOI] [PubMed] [Google Scholar]
- 31.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A.M. Silva, B.R. Williams, and X. Li. 2003. PolyI:C-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278:16713–16719. [DOI] [PubMed] [Google Scholar]
- 33.Meylan, E., and J. Tschopp. 2006. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 22:561–569. [DOI] [PubMed] [Google Scholar]
- 34.Liew, F.Y., D. Xu, E.K. Brint, and L.A. O'Neill. 2005. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5:446–458. [DOI] [PubMed] [Google Scholar]
- 35.Stetson, D.B., and R. Medzhitov. 2006. Antiviral defense: interferons and beyond. J. Exp. Med. 203:1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao, J., T.W. Kim, J. Qin, Z. Jiang, Y. Qian, H. Xiao, Y. Lu, W. Qian, M.F. Gulen, N. Sizemore, et al. 2007. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J. Biol. Chem. 282:6075–6089. [DOI] [PubMed] [Google Scholar]
- 37.Qin, J., Z. Jiang, Y. Qian, J.L. Casanova, and X. Li. 2004. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J. Biol. Chem. 279:26748–26753. [DOI] [PubMed] [Google Scholar]
- 38.Lye, E., C. Mirtsos, N. Suzuki, S. Suzuki, and W.C. Yeh. 2004. The role of interleukin 1 receptor-associated kinase-4 (IRAK-4) kinase activity in IRAK-4-mediated signaling. J. Biol. Chem. 279:40653–40658. [DOI] [PubMed] [Google Scholar]
- 39.Chen, C.Y., F. Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 280:1945–1949. [DOI] [PubMed] [Google Scholar]
- 40.Frevel, M.A., T. Bakheet, A.M. Silva, J.G. Hissong, K.S. Khabar, and B.R. Williams. 2003. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]