Abstract
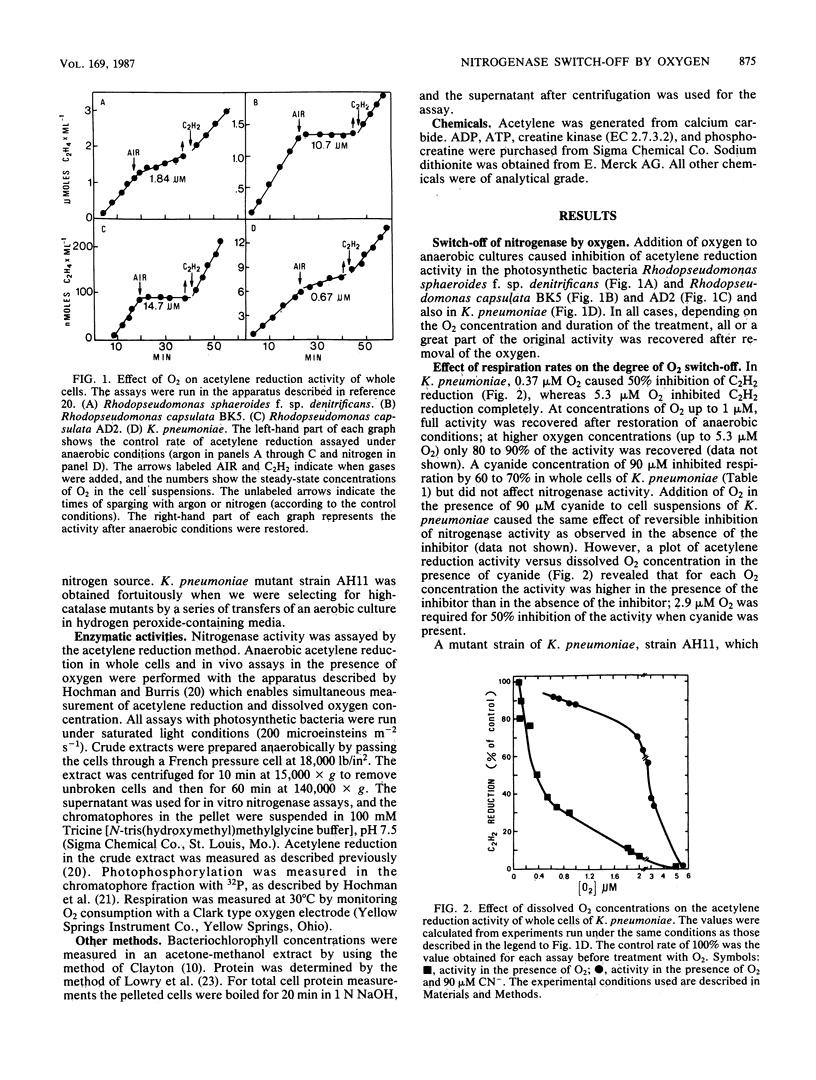
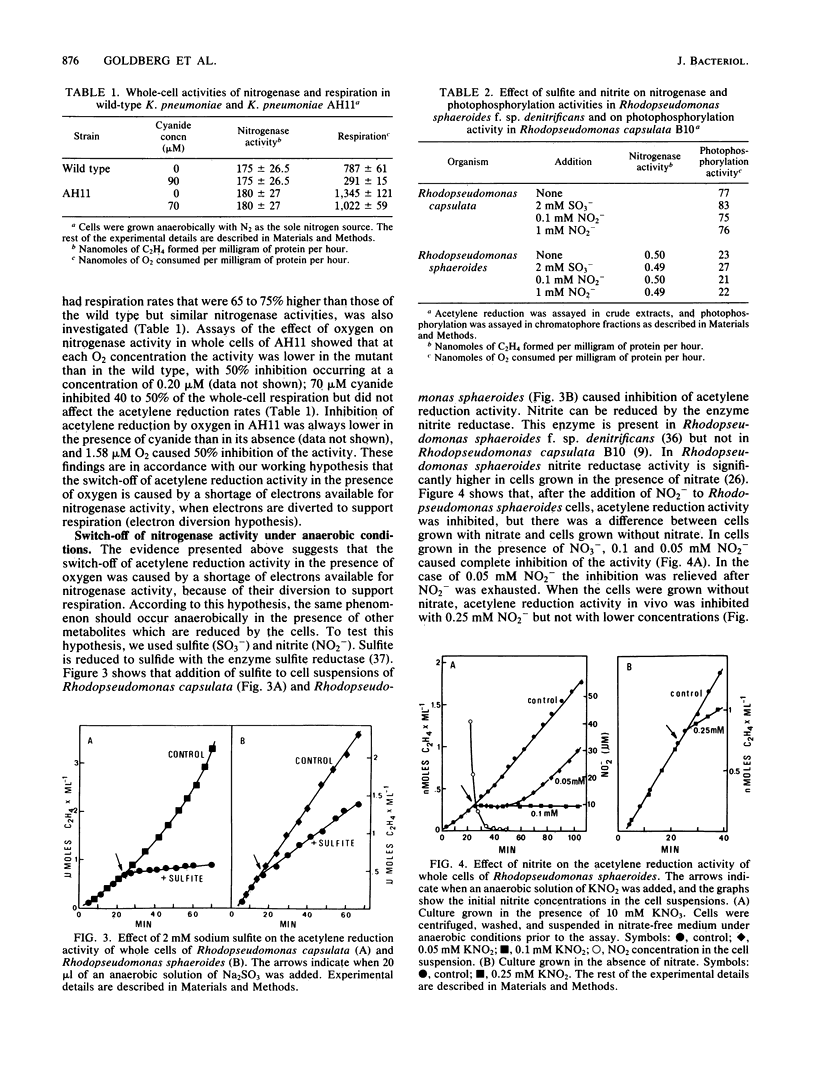
Oxygen caused a reversible inhibition (switch-off) of nitrogenase activity in whole cells of four strains of diazotrophs, the facultative anaerobe Klebsiella pneumoniae and three strains of photosynthetic bacteria (Rhodopseudomonas sphaeroides f. sp. denitrificans and Rhodopseudomonas capsulata strains AD2 and BK5). In K. pneumoniae 50% inhibition of acetylene reduction was attained at an O2 concentration of 0.37 microM. Cyanide (90 microM), which did not affect acetylene reduction but inhibited whole-cell respiration by 60 to 70%, shifted the O2 concentration that caused 50% inhibition of nitrogenase activity to 2.9 microM. A mutant strain of K. pneumoniae, strain AH11, has a respiration rate that is 65 to 75% higher than that of the wild type, but its nitrogenase activity is similar to wild-type activity. Acetylene reduction by whole cells of this mutant was inhibited 50% by 0.20 microM O2. Inhibition by CN- of 40 to 50% of the O2 uptake in the mutant shifted the O2 concentration that caused 50% inhibition of nitrogenase to 1.58 microM. Thus, when the respiration rates were lower, higher oxygen concentrations were required to inhibit nitrogenase. Reversible inhibition of nitrogenase activity in vivo was caused under anaerobic conditions by other electron acceptors. Addition of 2 mM sulfite to cell suspensions of R. capsulata B10 and R. sphaeroides inhibited nitrogenase activity. Nitrite also inhibited acetylene reduction in whole cells of the photodenitrifier R. sphaeroides but not in R. capsulata B10, which is not capable of enzymatic reduction of NO2-. Lower concentrations of NO2- were required to inhibit the activity in NO3- -grown cells, which have higher activities of nitrite reductase.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Kennedy C., Hill S. Influence of low oxygen concentration on derepression of nitrogenase in Klebsiella pneumoniae. J Gen Microbiol. 1982 May;128(5):909–915. doi: 10.1099/00221287-128-5-909. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Gibson A. H., Dudman W. F. Nitrogenase activity and respiration of cultures of Rhizobium spp. with special reference to concentrations of dissolved oxygen. Biochim Biophys Acta. 1976 Aug 24;444(1):164–174. doi: 10.1016/0304-4165(76)90233-6. [DOI] [PubMed] [Google Scholar]
- Biggins D. R., Kelly M., Postgate J. R. Resolution of nitrogenase of Mycobacterium flavum 30l into two components and cross reaction with nitrogenase components from other bacteria. Eur J Biochem. 1971 May 11;20(1):140–143. doi: 10.1111/j.1432-1033.1971.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Biggins D. R., Postgate J. R. Nitrogen fixation by cultures and cell-free extracts of Mycobacterium flavum 301. J Gen Microbiol. 1969 May;56(2):181–193. doi: 10.1099/00221287-56-2-181. [DOI] [PubMed] [Google Scholar]
- Biggins D. R., Postgate J. R. Nitrogen fixation by extracts of Mycobacterium flavum 301. Use of natural electron donors and oxygen-sensitivity of cell-free preparations. Eur J Biochem. 1971 Apr;19(3):408–415. doi: 10.1111/j.1432-1033.1971.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Dixon R. A., Postgate J. R., Primrose S. B. Chromosomal integration of Klebsiella nitrogen fixation genes in Escherichia coli. J Gen Microbiol. 1974 Jan;80(1):227–239. doi: 10.1099/00221287-80-1-227. [DOI] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Drozd J., Postgate J. R. Effects of oxygen on acetylene reduction, cytochrome content and respiratory activity of Azotobacter chroococcum. J Gen Microbiol. 1970 Sep;63(1):63–73. doi: 10.1099/00221287-63-1-63. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Burris R. H. Complementary functioning of the component proteins of nitrogenase from several bacteria. J Bacteriol. 1978 Jun;134(3):936–943. doi: 10.1128/jb.134.3.936-943.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Azotobacter vinelandii. Eur J Biochem. 1977 Jul 1;77(1):1–10. doi: 10.1111/j.1432-1033.1977.tb11634.x. [DOI] [PubMed] [Google Scholar]
- Hill S., Turner G. L., Bergersen F. J. Synthesis and activity of nitrogenase in Klebsiella pneumoniae exposed to low concentrations of oxygen. J Gen Microbiol. 1984 May;130(5):1061–1067. doi: 10.1099/00221287-130-5-1061. [DOI] [PubMed] [Google Scholar]
- Hochman A., Burris R. H. Effect of oxygen on acetylene reduction by photosynthetic bacteria. J Bacteriol. 1981 Aug;147(2):492–499. doi: 10.1128/jb.147.2.492-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman A., Fridberg I., Carmeli C. The location and function of cytochrome c2 in Rhodopseudomonas capsulate membranes. Eur J Biochem. 1975 Oct 1;58(1):65–72. doi: 10.1111/j.1432-1033.1975.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Kelly M. Some properties of purified nitrogenase of Azotobacter chroococcum. Biochim Biophys Acta. 1969 Jan 7;171(1):9–22. doi: 10.1016/0005-2744(69)90101-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- PARKER C. A., SCUTT P. B. The effect of oxygen on nitrogen fixation by Azotobacter. Biochim Biophys Acta. 1960 Feb 26;38:230–238. doi: 10.1016/0006-3002(60)91236-1. [DOI] [PubMed] [Google Scholar]
- Robson R. L. Characterization of an oxygen-stable nitrogenase complex isolated from Azotobacter chroococcum. Biochem J. 1979 Sep 1;181(3):569–575. doi: 10.1042/bj1810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Scherings G., Haaker H., Wassink H., Veeger C. On the formation of an oxygen-tolerant three-component nitrogenase complex from Azotobacter vinelandii. Eur J Biochem. 1983 Oct 3;135(3):591–599. doi: 10.1111/j.1432-1033.1983.tb07693.x. [DOI] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Shethna Y. I., DerVartanian D. V., Beinert H. Non heme (iron-sulfur) proteins of Azotobacter vinelandii. Biochem Biophys Res Commun. 1968 Jun 28;31(6):862–868. doi: 10.1016/0006-291x(68)90531-7. [DOI] [PubMed] [Google Scholar]
- St John R. T., Shah V. K., Brill W. J. Regulation of nitrogenase synthesis by oxygen in Klebsiella pneumoniae. J Bacteriol. 1974 Jul;119(1):266–269. doi: 10.1128/jb.119.1.266-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchant J. C., Rigaud J. Nitrite and nitric oxide as inhibitors of nitrogenase from soybean bacteroids. Appl Environ Microbiol. 1982 Dec;44(6):1385–1388. doi: 10.1128/aem.44.6.1385-1388.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G., Daniel R. M. Acetylene reduction with physiological electron donors by extracts and particulate fractions from nitrogen-fixing Azotobacter chroococcum. Biochim Biophys Acta. 1970 Mar 3;197(2):161–169. doi: 10.1016/0005-2728(70)90027-7. [DOI] [PubMed] [Google Scholar]
- Yates M. G. Effect of non-haem iron proteins and cytochrome C from Azotobacter upon the activity and oxygen sensitivity of Azobacter nitrogenase. FEBS Lett. 1970 Jun 27;8(5):281–285. doi: 10.1016/0014-5793(70)80287-3. [DOI] [PubMed] [Google Scholar]



