Abstract
T cell immunoglobulin and mucin domain–containing molecule-3 (Tim-3) is a surface molecule that is preferentially expressed on activated Th1 cells in comparison to Th2 cells. Blockade of Tim-3 has been shown to enhance Th1-driven pathology in vivo, suggesting that blockade of Tim-3 may improve the development of Th2-associated responses such as allergy. To examine the effects of Tim-3 blockade on the Th2 response in vivo, we administered anti–Tim-3 antibody during pulmonary inflammation induced by transfer of ovalbumin (OVA)-reactive Th2 cells, and subsequent aerosol challenge with OVA. In this model, anti–Tim-3 antibody treatment before each airway challenge significantly reduced airway hyperreactivity, with a concomitant decrease in eosinophils and Th2 cells in the lung. We examined Th1 and Th2 cytokine levels in the lung after allergen challenge and found that pulmonary expression of the Th2 cytokine IL-5 was significantly reduced, whereas IFN-γ levels were significantly increased by anti–Tim-3 antibody treatment. Thus, blocking Tim-3 function has a beneficial effect during pulmonary inflammation by skewing the Th2 response toward that of a Th1 type, suggesting an important role for Tim-3 in the regulation of allergic disease.
After activation, T cells differentiate into distinct effector populations according to the particular cytokine environment that surrounds them. High levels of IFN-γ generate a Th1 population that contributes to protection against bacteria and viruses, whereas an environment of IL-4 leads to development of Th2 cells that are protective against helminths (1). Allergic asthma is generally held to occur as a consequence of a dysregulated Th2 response to environmental allergens, as it is characterized by increased levels of the Th2 cytokines IL-4, -5, -9, and -13 (2–4). Manipulation of Th2 function has been proposed as a novel strategy for treatment of asthma, and enhancing Th1 responses in allergic individuals has been proposed as one method of such a strategy. In mice, transfer of Th1 cells has been shown to down-regulate pathology induced by transfer of Th2 cells alone, and this inhibition has been shown to be IFN-γ dependent (5).
T cell Ig and mucin domain–containing molecule-3 (Tim-3) was described as a transmembrane protein preferentially expressed on Th1 cells (6). In a Th1-mediated model of experimental allergic encephalomyelitis (EAE), in vivo neutralization of Tim-3 resulted in increased disease severity. Two further studies suggested that Tim-3 function was required for peripheral tolerance and acquisition of transplantation tolerance, respectively (7, 8). In addition, galectin-9 has recently been identified as the ligand for Tim-3, and it has been demonstrated that administration of galectin-9 induced selective death of Th1 cells and inhibited the development of EAE (9). Collectively, these studies implied that signaling through Tim-3 may negatively regulate Th1 responses, and thus suppression of Tim-3 may inhibit Th2 responses such as allergic disease through enhancement of a Th1 response.
Tim-3 belongs to a novel family of genes that map to a region of chromosome 11 termed T cell and airway phenotype regulator (Tapr), which confers reduced Th2 responsiveness and protects against airway hyperreactivity (AHR) (10). Experiments with mice have shown that the Tapr locus might regulate Th cell differentiation during primary antigen-specific responses (10). Currently, the Tim gene family has eight members, Tim-1–8, and genomic analysis has revealed that an equivalent Tim family of genes exists in humans (11). Polymorphisms in human Tim-1 and -3 have been associated with atopy, suggesting that the Tim family may have functional roles in human allergic diseases (12). In mouse, Tim-1–3 are reciprocally expressed by Th2 and Th1 cells during T cell differentiation, but their roles in the development of allergy and atopy have not yet been investigated. Therefore, we have used a monoclonal antibody to Tim-3 to determine the effect of Tim-3 blockade on development of allergen-induced airway pathology and AHR in mice.
RESULTS AND DISCUSSION
Administration of anti–Tim-3 antibody decreased airway inflammation induced by transfer of Th2 cells
Tim-3 has previously been found to be expressed by Th1 cells in vitro (6–8). Although allergen-induced airway inflammation is considered to be primarily a Th2-driven disease, Tim-3 expression on CD4 cells increased in both airway lumen and lung tissue after either allergen sensitization and challenge or transfer of allergen-reactive Th2-polarized cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062093/DC1).
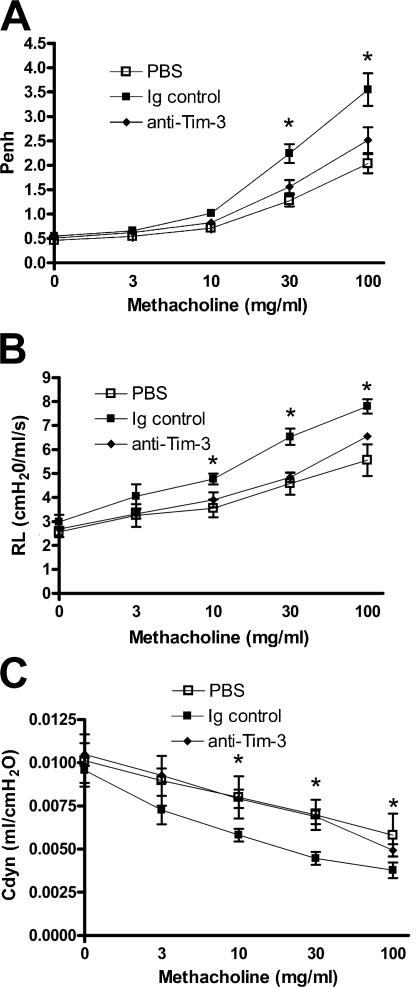
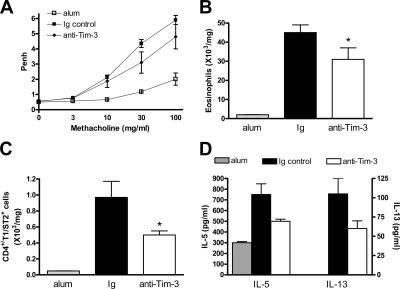
Because we detected increased Tim-3 expression in the lung during allergen-induced airway disease, we assessed the effect of anti–Tim-3 antibody treatment on inflammation induced by transferring OVA-reactive, Th2-polarized cells into naive mice, and challenging with OVA through the airways (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20062093/DC1). AHR was measured as changes in Penh, lung resistance, and compliance. Transfer of Th2 cells resulted in significantly increased AHR compared with mice that received PBS instead of cells (Fig. 1, A–C). Interestingly, this AHR response was significantly decreased by treatment with anti–Tim-3 antibody (Fig. 1, A–C).
Figure 1.
Administration of anti–Tim-3 antibody inhibits the development of AHR induced by transfer of polarized, OVA-reactive Th2 cells. AHR was measured 24 h after the final OVA challenge by changes in Penh (A). Penh results were confirmed by invasive measurements of resistance (B) and compliance (C) in anesthetized and tracheostomized mice. Results are expressed as the mean ± the SEM. n = 7–20 mice/group from 2–4 independent experiments. *, P < 0.05, in comparison to Ig-treated mice.
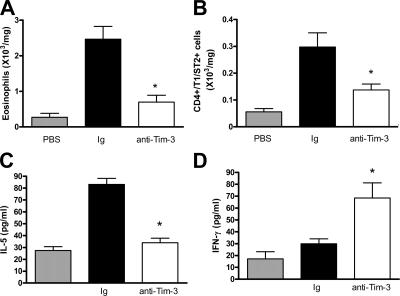
We also assessed the effect of anti–Tim-3 antibody on lung tissue eosinophilia. Administration of anti–Tim-3 significantly decreased eosinophil numbers in the lung tissue compared with mice that received control Ig (Fig. 2 A).
Figure 2.
Administration of anti–Tim-3 antibody decreases eosinophil and Th2 cell numbers in the lung tissue after transfer of polarized OVA-reactive Th2 cells. Lung tissue cells were isolated as described in Materials and methods. Eosinophil numbers (A) were determined by differential counting of Wright-Giemsa–stained cytospins. Th2 cell numbers (B) were determined by staining lung tissue digest cells with CD4 and the Th2 cell–specific marker T1/ST2. Pulmonary expression of IL-5 (C) and IFN-γ (D) was determined in BAL supernatant by ELISA. Data is expressed as the mean ± the SEM. n = 7–8 mice/group from 2 independent experiments. *, P < 0.05, in comparison to Ig-treated mice.
Because AHR and lung eosinophilia were reduced after anti–Tim-3 antibody treatment, we determined the magnitude of the lung Th2 response in the presence and absence of anti–Tim-3 by examining numbers of Th2 cells in the lung tissue. Anti–Tim-3 treatment significantly reduced Th2 cell numbers compared with mice that received control Ig (Fig. 2 B), suggesting that blockade of Tim-3 can suppress the Th2 response.
In addition, we quantified Th2 cytokine expression in bronchoalveolar lavage (BAL) supernatant after allergen challenge. Treatment of mice with anti–Tim-3 antibody significantly reduced lung expression of IL-5; however, levels of the Th1-associated cytokine IFN-γ were significantly increased (Fig. 2, C and D). We also measured cytokine levels in lung tissue homogenate, and found that administration of anti–Tim-3 reduced IL-5 and -13 levels (which were undetectable in BAL <0.5 pg/ml), although there was no substantial effect on IFN-γ (Fig. S3, A–C, available at http://www.jem.org/cgi/content/full/jem.20062093/DC1).
Anti–Tim-3 antibody decreases expression of proinflammatory chemokines after transfer of OVA-reactive Th2 cells
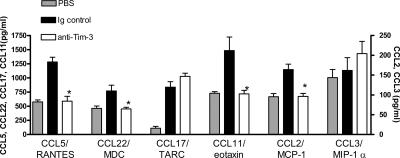
To determine whether the reduction in lung eosinophils and Th2 cells after anti–Tim-3 antibody treatment was caused by decreased recruitment of these leukocytes, lung levels of proinflammatory chemokines were determined. Administration of anti–Tim-3 antibody significantly decreased levels of CCL11/eotaxin, CCL22/MDC, CCL17/TARC, CCL2/MCP-1, CCL3/MIP-1α, and CCL5/RANTES compared with mice that received control Ig (Fig. 3). However, anti–Tim-3 treatment had no effect on levels of CCL17/TARC or CCL3/MIP-1α (Fig. 3). It is interesting that anti–Tim-3 treatment should reduce levels of CCL22/MDC, but not CCL17/TARC, as these chemokines both act on CCR4, which is postulated to play an important role in Th2 cell recruitment (13). This suggests that CCL22/MDC may be the more dominant of these two chemokines in this model.
Figure 3.
Effect of administration of anti–Tim-3 antibody on proinflammatory chemokine production. Lung tissue levels of CCL5/RANTES, CCL22/MDC, CCL17/TARC, CCL11/eotaxin, CCL2/MCP-1, and CCL3/MIP-1α were measured in lung tissue homogenate supernatant by ELISA in samples from mice treated with anti–Tim-3 antibody or Ig control. Data is expressed as the mean ± the SEM. n = 7–8 mice/group from 2 independent experiments. *, P < 0.05, in comparison to Ig-treated mice.
Monney et al. found that anti–Tim-3 treatment worsened the Th1-mediated disease EAE by increasing macrophage activation, suggesting that Tim-3 signaling may be able to modulate macrophage function (6). Macrophages are a source of many proinflammatory chemokines in the lung during allergic airway inflammation; thus, altering macrophage function could potentially affect recruitment of Th2 cells and eosinophils to the lung. However, although Monney et al. found that macrophage function was increased by anti–Tim-3 antibody, we found that the number of macrophages in the airway lumen and lung tissue are not changed by anti–Tim-3 antibody (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20062093/DC1), and that chemokine production in the lung is down-regulated by anti–Tim-3 (Fig. 3), pointing toward a decrease in macrophage function. This could be caused by differences in the cytokine environment in allergen-induced airway inflammation compared with EAE, or it could be that the suppression of lung inflammation by anti–Tim-3 antibody treatment is not caused by a macrophage-mediated effect. Indeed, a more recent study has suggested that the ligand for Tim-3, galectin-9, is expressed predominantly on CD4+ T cells, and only on a small subset of CD11b+ cells (7, 9, 11), suggesting that the macrophage activation by anti–Tim-3 antibody treatment may have been a secondary consequence in the EAE model.
Administration of anti–Tim-3 antibody skews the Th2 response to allergen toward a Th1 phenotype
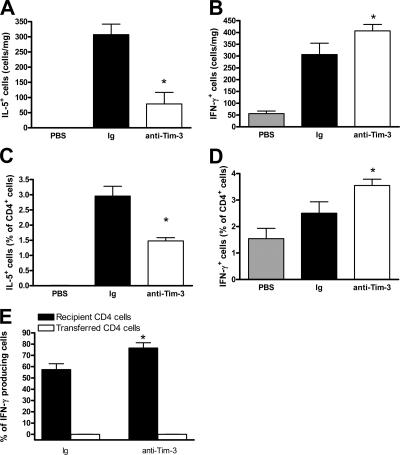
Because blockade of Tim-3 has previously been found to augment Th1 responses (6, 7, 11), and given the increased expression of IFN-γ in the BAL, we sought to further establish the effect of anti–Tim-3 antibody treatment on cytokine expression in the lung. To this end, we performed intracellular staining for IFN-γ and IL-5. The overall number of IL-5–producing cells in the lung was significantly decreased by anti–Tim-3 antibody treatment, whereas the number of IFN-γ–producing cells were significantly increased (Fig. 4, A and B). This reflects the decreased levels of IL-5 in the BAL and lung, as measured by ELISA. However, we found that although IFNγ levels were decreased in BAL, there was no change in the lung IFN-γ levels, as measured by ELISA (Fig. 2 D and Fig. S3 C). It is conceivable that the down-regulation of inflammation and AHR is caused by the increased BAL IFNγ levels in conjunction with increases in IFNγ-producing cells within local microenvironments in the lung. Cytokine production by individual cells within these tightly packed cellular microenvironments would induce changes in immunopathology, even though absolute values in the whole tissue, as measured by ELISA, are unchanged.
Figure 4.
Administration of anti–Tim-3 antibody reduces Th2 and enhances Th1 cytokine production in the lung. Percentages of IL-5+ (A), IFN-γ+ (B), CD4+IL-5+ (C), and CD4+IFN-γ+ (D) cells were determined in lung tissue digest by flow cytometric analysis. Data are expressed as the mean percentage of cells ± the SEM. n = 8–12 mice/group from 2 independent experiments. *, P < 0.05, in comparison to Ig-treated mice. (E) Percentage contribution to IL-5 and IFN-γ production was assessed in host and transferred CD4 cells (by fluorescent labeling of cells before injection) in lung tissue digest by flow cytometry. Data are expressed as the mean percentage of total cells producing IFN-γ ± the SEM. n = 6 mice/group. *, P < 0.05, in comparison to Ig-treated mice.
When cytokine-producing cells were defined by phenotype, the percentage of CD4 cells expressing IL-5 was significantly decreased in the lungs of mice that were treated with anti–Tim-3 antibody compared with Ig controls (Fig. 4 C). In contrast, anti–Tim-3 treatment significantly increased IFN-γ production from CD4 cells (Fig. 4 D). This increase in IFN-γ–producing CD4 cells could result from an induction in IFN-γ production from the host CD4 population or a switch to IFNγ production by the donor CD4 cells. To test this, we labeled the donor CD4 cells before transfer to the naive host mice. Although transferred CD4 cells produced IL-5, and this was unaffected by Tim-3 blockade, IFN-γ expression was not detected in donor cells either before or after in vivo treatment with anti–Tim-3 antibody (Fig. 4 E and not depicted). However, in contrast, there was an increase in host-derived CD4 cells producing IFN-γ after Tim-3 antibody treatment. These data suggest that Tim-3 blockade affects the Th2 response in the lung, resulting in a more pronounced Th1 phenotype in the bystander CD4 population rather than in the allergen-specific CD4 cells.
It has previously been shown that blockage of Tim-3 signaling induces Th1 cell hyperproliferation and cytokine release in vitro and worsens Th1-mediated diseases in vivo (7, 6, 11, 14). Although allergen-induced airway disease is considered to be a Th2-driven disease, it has also been shown that Th1 cells are recruited to the lung along with Th2 cells (15); indeed, we found an increase in Tim-3 expression in the lung after either allergen sensitization or adoptive transfer of Th2 cells (Fig. S1). As Th1 and Th2 cells can cross-regulate each other, anti–Tim-3 treatment may suppress Th2-mediated, allergen-induced inflammation by inducing hyperproliferation of Th1 cells in the lung and increased IFN-γ expression. In support of this, previous studies have shown that cotransfer of Th1 cells or IL-12 treatment can attenuate the airway inflammation induced by transfer of Th2 cells (5, 16, 17). Moreover, this attenuation was dependent on IFN-γ, rather than on direct suppression by Th1 cells, because airway inflammation could not be inhibited in IFN-γR–deficient mice or in the presence of an antibody against IFN-γ (5, 16). Indeed, a beneficial role of IFN-γ in allergen-induced airway inflammation has been demonstrated by several studies (18, 19). In support of this, we found significant decreases in the Th2 cytokines IL-5 and -13, and an increase in BAL expression of the Th1 cytokine IFN-γ after anti–Tim-3 antibody treatment. Moreover, anti–Tim-3 antibody increased the number of host-derived CD4+ T cells secreting IFNγ, implying that anti–Tim-3 treatment influences the host bystander T cells, rather than skewing the donor Th2 cells to a Th1 phenotype. Interestingly, it has previously been shown that blocking Tim-2, which is predominantly expressed on Th2 cells, has a beneficial effect on the Th1-mediated disease EAE (20). We show that blocking Tim-3 suppresses Th2-driven allergic inflammation, firmly implicating the Tim family in control of in vivo Th cell responses.
Anti–Tim-3 antibody treatment has no effect on expression of IL-10, TGF-β, or FoxP3 in the lung
Sanchez-Fueyo et al. demonstrated a role for Tim-3 signaling in the suppression of autoimmune disease mediated by CD4+CD25+ regulatory T cells (8). Previous studies have implicated IL-10 and TGF-β as important mediators in the regulation of immune responses by these cells (21–23). Therefore, we determined the levels of IL-10 and TGF-β in the lung after allergen challenge. We found no change in IL-10 (159 ± 19 pg/ml vs. 125 ± 10 pg/ml) or TGF-β (126 ± 15 pg/ml vs. 152 ± 23 pg/ml) expression in the presence or absence of anti–Tim-3 antibody after allergen challenge. We also determined expression of the regulatory T cell marker FoxP3 in the lung and found that the percentage of CD4+ cells expressing FoxP3 was unchanged by anti–Tim-3 antibody treatment (5.5 ± 0.43% vs. 6.1 ± 0.51%).
Our data suggests that Tim-3 may not be involved in the suppressive function of CD4+CD25+ regulatory T cells in this setting. We have previously shown that CD4+CD25+ T cells can reduce allergen-induced airway inflammation via induction of IL-10 in the lung (22). However, we found no difference in lung IL-10 expression, suggesting that CD4+CD25+ regulatory T cell function may not be affected. In addition, although we cannot discount IL-10 or TGF-β–independent mechanisms of regulation, it could be argued that if signaling through Tim-3 were needed for regulation of inflammation by CD4+CD25+ cells in this model, administration of anti–Tim-3 would worsen allergen-induced airway inflammation, rather than suppress it.
Administration of anti–Tim-3 antibody inhibited the development of allergen-induced airway inflammation
We also used an allergen challenge model of airway inflammation (Fig. S2 B) to determine the effect of blockade of Tim-3 on different aspects of allergen-induced lung pathophysiology. Although treatment with anti–Tim-3 had no significant effect on AHR, both lung tissue eosinophils and Th2 cells were significantly decreased in comparison with Ig control mice (Fig. 5, A–C). Despite this decrease in Th2 cells, lung expression of the Th2 cytokines IL-5 and -13 were only modestly decreased (Fig. 5 D), and there was no effect on IFN-γ (172 ± 35.7 pg/ml vs. 133 ± 16.6 pg/ml). These data indicate that a Tim-3 blockade can affect Th2 responses, even after active immunization with allergen. However, the observation that Tim-3 blockade is more effective in suppression of inflammation induced by transfer of Th2 cells compared with that induced by allergen sensitization suggests that, in the latter model, other factors may also contribute to the development of the allergic response.
Figure 5.
Administration of anti–Tim-3 antibody also reduces eosinophilia and Th2 cell numbers in an allergen sensitization and challenge model. Inflammation was induced by sensitization and challenge (Fig. S2 B). AHR was measured 24 h after the final OVA challenge by changes in Penh (A). Eosinophil (B) and Th2 cell numbers (C) were determined in lung tissue, as described in Materials and methods. Pulmonary expression of IL-5 and -13 (D) was determined in BAL supernatant by ELISA. Data is expressed as the mean ± the SEM. n = 7–8 mice/group. *, P < 0.05, in comparison to Ig-treated mice. Fig. S2 is available at http://www.jem.org/cgi/content/full/jem.20062093/DC1.
Modulation of Tim-3 function may be of therapeutic benefit in Th2-mediated diseases
This is the first report of a role for Tim-3 in a model of Th2-mediated immunity. We observed a decrease in allergen-induced airway disease by anti–Tim-3 antibody treatment, accompanied by a shift toward Th1-mediated immunity. We postulate that because blockade of Tim-3 has previously been demonstrated to worsen Th1-mediated diseases through an enhanced Th1 response, blockade of Tim-3 in a Th2-mediated disease such as allergic inflammation is beneficial because of cross-regulation of the Th2 response by Th1 cells. These data suggest that manipulation of Tim-3 function may represent a novel therapy for asthmatic patients.
MATERIALS AND METHODS
Animals
Female BALB/c mice (Harlan) were housed at Imperial College, London and used at 6–8 wk of age. UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986 were strictly observed.
Induction of airway inflammation by transfer of OVA-reactive, Th2-polarized cells
Airway inflammation was generated by transfer of OVA-specific, Th2-polarized cells in a modified version of a previously published method (24). OVA-reactive CD4+ T cells were derived from spleens of BALB/c mice that had been sensitized 7 d before by i.p. injection of 0.1 mg OVA in alum (SERVA Electrophoresis). Splenocytes were resuspended in RPMI with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, and 4 × 10−5% 2-mercaptoethanol (all Sigma-Aldrich), and cultured for 5 d in the presence of 100 μg/ml OVA, 10 ng/ml recombinant IL-4 (Roche), and 10 μg/ml anti–IL-12 (C17.8). Th2-polarized CD4 T cells were purified using a CD4 isolation kit (Miltenyi Biotech). In some experiments, Th2-polarized cells were labeled using a cell-labeling kit according to the manufacturer's protocol (Vybrant; Invitrogen). Purified Th2-polarized cells were transferred into naive BALB/c mice at 106 cells per mouse i.v. Control mice received an equal volume of PBS i.v. All mice were challenged with 5% OVA (aerosolized for 20 min) between days 1 and 7. 30 min before OVA challenge, mice were treated with 20 μg anti–Tim-3 antibody (8H7; a gift from Millennium Pharmaceuticals, Inc., Cambridge, MA) i.p. or with control rat Ig (Stratech Scientific), killed 24 h after the final OVA challenge, and analyzed (Fig. S2 A).
Induction of airway inflammation by OVA sensitization and challenge
Airway inflammation was generated as previously described (25). 30 min before OVA challenge, mice were treated with 20 μg anti–Tim-3 antibody i.p., killed 24 h after the final OVA challenge, and analyzed (Fig. S2 B).
AHR
AHR was measured in mice 24 h after the final OVA challenge by Penh, resistance, and compliance, as previously described (22).
Cell recovery
Airway lumen.
BAL was performed, and differential leukocyte counts were performed as previously described (22).
Lung parenchyma.
Lung tissue digests were prepared as previously described (22). Cytocentrifuge preparations were stained and counted as for BAL.
Flow cytometric analysis.
BAL and lung tissue digest cells were stained and analyzed, as previously described (22). Antibodies used were anti-CD4, -CD8, -IL-5, -IFN-γ (BD Biosciences), -CD68 (Serotec), and -T1/ST2 (Morwell Diagnostics). Tim-3 expression studies were performed by labeling anti–Tim-3 with a fluorescent protein labeling kit according to the manufacturer's protocol (Invitrogen).
ELISAs
Cytokines were analyzed in BAL supernatant and lung tissue homogenate supernatants. Paired antibodies for IFN-γ, TGF-β (BD Biosciences), IL-5 (Endogen), CCL11, CCL17, CCL22, CCL5, and CCL3 (R&D Systems) were used in sandwich ELISAs according to the manufacturer's protocol. IL-13 (R&D Systems), IL-10 (eBiosciences), and CCL2 (BD Biosciences) ELISA kits were used according to the manufacturer's protocol.
Data analysis
Graph generation and statistical analysis was performed by using Prism v4.00 software (GraphPad).
Online supplemental material
Fig. S1 shows Tim-3 expression in lung after allergen challenge and transfer of Th2 cells. Fig. S2 shows the protocol used to induce allergic airway inflammation. Fig. S3 shows that anti–Tim-3 antibody treatment reduces Th2 cytokine levels in lung tissue. Fig. S4 shows macrophage numbers in BAL and lung tissue. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062093/DC1.
Supplemental Material
Acknowledgments
This work was supported by the Wellcome Trust (ref #057704). J. Kearley was supported by a Biotechnology and Biological Sciences Research Council/Cooperative Awards in Science and Engineering studentship; C.M. Lloyd is supported by a Wellcome Senior Fellowship in Basic Biomedical Sciences.
The authors have no conflicting financial interests.
J. Kearley and S.J. McMillan contributed equally to this paper.
References
- 1.Mosmann, T.R., and R.L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173. [DOI] [PubMed] [Google Scholar]
- 2.Robinson, D.S., Q. Hamid, S. Ying, A. Tsicopoulos, J. Barkans, A.M. Bentley, C. Corrigan, S.R. Durham, and A.B. Kay. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298–304. [DOI] [PubMed] [Google Scholar]
- 3.Shimbara, A., P. Christodoulopoulos, A. Soussi-Gounni, R. Olivenstein, Y. Nakamura, R.C. Levitt, N.C. Nicolaides, K.J. Holroyd, A. Tsicopoulos, J.J. Lafitte, et al. 2000. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 105:108–115. [DOI] [PubMed] [Google Scholar]
- 4.Kroegel, C., P. Julius, H. Matthys, J.C. Virchow, and W. Luttmann. 1996. Endobronchial secretion of interleukin-13 following local allergen challenge in atopic asthma: relationship to interleukin-4 and eosinophil counts. Eur. Respir. J. 9:899–904. [DOI] [PubMed] [Google Scholar]
- 5.Cohn, L., R.J. Homer, N. Niu, and K. Bottomly. 1999. T helper 1 cells and interferon γ regulate allergic airway inflammation and mucus production. J. Exp. Med. 190:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monney, L., C.A. Sabatos, J.L. Gaglia, A. Ryu, H. Waldner, T. Chernova, S. Manning, E.A. Greenfield, A.J. Coyle, R.A. Sobel, et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541. [DOI] [PubMed] [Google Scholar]
- 7.Sabatos, C.A., S. Chakravarti, E. Cha, A. Schubart, A. Sanchez-Fueyo, X.X. Zheng, A.J. Coyle, T.B. Strom, G.J. Freeman, and V.K. Kuchroo. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102–1110. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Fueyo, A., J. Tian, D. Picarella, C. Domenig, X.X. Zheng, C.A. Sabatos, N. Manlongat, O. Bender, T. Kamradt, V.K. Kuchroo, et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093–1101. [DOI] [PubMed] [Google Scholar]
- 9.Zhu, C., A.C. Anderson, A. Schubart, H. Xiong, J. Imitola, S.J. Khoury, X.X. Zheng, T.B. Strom, and V.K. Kuchroo. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245–1252. [DOI] [PubMed] [Google Scholar]
- 10.McIntire, J.J., S.E. Umetsu, O. Akbari, M. Potter, V.K. Kuchroo, G.S. Barsh, G.J. Freeman, D.T. Umetsu, and R.H. DeKruyff. 2001. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2:1109–1116. [DOI] [PubMed] [Google Scholar]
- 11.Meyers, J.H., C.A. Sabatos, S. Chakravarti, and V.K. Kuchroo. 2005. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 11:362–369. [DOI] [PubMed] [Google Scholar]
- 12.Graves, P.E., V. Siroux, S. Guerra, W.T. Klimecki, and F.D. Martinez. 2005. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain–IL-2-inducible T-cell kinase gene cluster in chromosome 5q33. J. Allergy Clin. Immunol. 116:650–656. [DOI] [PubMed] [Google Scholar]
- 13.Bonecchi, R., G. Bianchi, P.P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P.A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of T helper type 1 cells (Th1s) and Th2s. J. Exp. Med. 187:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koguchi, K., D.E. Anderson, L. Yang, K.C. O'Connor, V.K. Kuchroo, and D.A. Hafler. 2006. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 203:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randolph, D.A., R. Stephens, C.J.L. Carruthers, and D.D. Chaplin. 1999. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Invest. 104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, T.J., P.A. MacAry, P. Eynott, A. Moussavi, K.C. Daniel, P.W. Askenase, D.M. Kemeny, and K.F. Chung. 2001. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J. Immunol. 166:207–217. [DOI] [PubMed] [Google Scholar]
- 17.Gavett, S.H., D.J. O'Hearn, X. Li, S.K. Huang, F.D. Finkelman, and M. Wills-Karp. 1995. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J. Exp. Med. 182:1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida, M., R. Leigh, K. Matsumoto, J. Wattie, R. Ellis, P.M. O'Byrne, and M.D. Inman. 2002. Effect of interferon-gamma on allergic airway responses in interferon-gamma-deficient mice. Am. J. Respir. Crit. Care Med. 166:451–456. [DOI] [PubMed] [Google Scholar]
- 19.Lack, G., H. Renz, J. Saloga, K.L. Bradley, J. Loader, D.Y. Leung, G. Larsen, and E.W. Gelfand. 1994. Nebulized but not parenteral IFN-gamma decreases IgE production and normalizes airways function in a murine model of allergen sensitization. J. Immunol. 152:2546–2554. [PubMed] [Google Scholar]
- 20.Chakravarti, S., C.A. Sabatos, S. Xiao, Z. Illes, E.K. Cha, R.A. Sobel, X.X. Zheng, T.B. Strom, and V.K. Kuchroo. 2005. Tim-2 regulates T helper type 2 responses and autoimmunity. J. Exp. Med. 202:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearley, J., J.E. Barker, D.S. Robinson, and C.M. Lloyd. 2005. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattes, J., M. Yang, A. Siqueira, K. Clark, J. MacKenzie, A.N.J. McKenzie, D.C. Webb, K.I. Matthaei, and P.S. Foster. 2001. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J. Immunol. 167:1683–1692. [DOI] [PubMed] [Google Scholar]
- 25.McMillan, S.J., B. Bishop, M.J. Townsend, A.N. McKenzie, and C.M. Lloyd. 2002. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 195:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.