Abstract
The ability of dendritic cells (DCs) to activate immunity is linked to their maturation status. In prior studies, we have shown that selective antibody-mediated blockade of inhibitory FcγRIIB receptor on human DCs in the presence of activating immunoglobulin (Ig) ligands leads to DC maturation and enhanced immunity to antibody-coated tumor cells. We show that Fcγ receptor (FcγR)–mediated activation of human monocytes and monocyte-derived DCs is associated with a distinct gene expression pattern, including several inflammation-associated chemokines, as well as type 1 interferon (IFN) response genes, including the activation of signal transducer and activator of transcription 1 (STAT1). FcγR-mediated STAT1 activation is rapid and requires activating FcγRs. However, this IFN response is observed without a detectable increase in the expression of type I IFNs themselves or the need to add exogenous IFNs. Induction of IFN response genes plays an important role in FcγR-mediated effects on DCs, as suppression of STAT1 by RNA interference inhibited FcγR-mediated DC maturation. These data suggest that the balance of activating/inhibitory FcγRs may regulate IFN signaling in myeloid cells. Manipulation of FcγR balance on DCs and monocytes may provide a novel approach to regulating IFN-mediated pathways in autoimmunity and human cancer.
The FcγR system comprises both activating and inhibitory receptors, and the balance of these two types of receptors determines the outcome of immune complex (IC)–mediated inflammation, immunity, and antibody-based immunotherapy (1). Altering this balance by using a selective blocking antibody against the human inhibitory FcγRIIB receptor in the presence of activating Ig ligands in human plasma leads to enhanced generation of antitumor T cell responses (2). Mice deficient in the inhibitory FcγR FcγRII also show enhanced T cell immunity to model antigens (3). However, the mechanisms by which activating FcγRs mediate maturation of human DCs and enhance adaptive immunity remain to be clarified.
IFNs are pleiotropic cytokines with potent antiviral, antitumor, growth suppressive, and immunomodulatory properties (4). The cellular effects of both type I (IFN-α and -β) and type II (IFN-γ) IFNs are mediated via activation of the STAT family of transcription factors and downstream activation of a distinct set of “IFN response genes” (IRGs) (5). IFNs play an important role in the regulation of both innate and adaptive immunity (6). For example, IFNs play a critical role in T cell–dependent antibody responses to antigens delivered with the classical complete Freund's adjuvant, DNA vaccines, and immunostimulatory DNA (7–9), and they promote the induction of cytotoxic T cells in vivo (10, 11). IFN-mediated signaling pathways also play an important role in immune surveillance and protection from tumors (12). Dysregulation of IFN signaling has been observed in patients with several autoimmune diseases (6, 13). Therefore, pathways that regulate IFN signaling in myeloid cells, particularly DCs, may have a major impact on immunity to tumors and pathogens, as well as autoimmunity. An important aspect of the biology of IFN signaling is that the level of constitutive signaling in the absence of pathogens determines the strength of IFN signaling in response to pathogens (14). Therefore, there is a need to identify the factors that regulate the level of this constitutive or basal IFN signaling.
We show that FcγR-mediated maturation of human DCs is associated with a distinct pattern of gene expression. This includes the expression of several inflammation-associated cytokines and chemokines, and the induction of several typical IRGs. These data suggest that the balance of activating/inhibitory FcγRs can regulate the IFN response program in human DCs and monocytes.
RESULTS
A distinct gene expression profile (GEP) of DCs treated with anti-FcγRIIB antibody
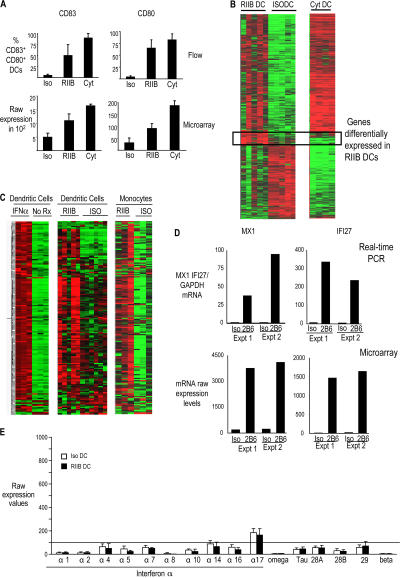
We have previously shown that treatment of monocyte-derived immature DCs (IDCs) with an anti-FcγRIIB–blocking antibody in the presence of Ig ligands in normal human plasma leads to DC maturation and enhancement of anti-tumor T cell immunity (2). To further characterize FcγR-mediated enhancement of DC function, we analyzed the GEPs of pure populations of monocyte-derived DCs (Mo-Dcs) from healthy donors (n = 5) using Affymetrix Human Genome U133 Plus 2.0 microarrays. IDCs cultured in 1% plasma were treated for 24 h with either anti-FcγRIIB or isotype control antibody. To test whether FcγR-mediated DC maturation was distinct from other maturation stimuli, we also compared DCs matured using the inflammatory cytokine cocktail (TNF-α, IL-1β, IL-6, and PGE2) that is commonly used in DC immunotherapy trials (15). To first validate the GEP data at the protein level, we compared the gene expression data for some of the genes associated with DC maturation (CD83 and CD80) with the detection of corresponding proteins by flow cytometry (Fig. 1 A). As expected, mRNA expression, as well as protein levels of CD80 and CD83, increased in DCs matured with the FcγRIIB blocking antibody and in cytokine-matured DCs compared with isotype-treated IDCs. Of the 24,296 expressed genes on the array, 1,801 were differentially regulated in DCs treated with anti-FcγRIIB antibody (RIIB DCs) versus those treated with isotype control antibody (Iso DCs). Most of these genes were also up-regulated in DCs matured with inflammatory cytokines (Cyt DCs), suggesting that these reflected changes in gene expression shared with other DC maturation stimuli. However, we also identified a distinct set of 95 genes that were differentially expressed only in RIIB DCs (Fig. 1 B and Table S1, available at http://www.jem.org/cgi/content/full/jem.20062545/DC1). Thus, the GEP of RIIB DCs includes genes shared with Cyt DCs, as well as a distinct subset of genes specifically overexpressed only in RIIB DCs.
Figure 1.
Changes in GEPs of human DCs treated for 24 h with isotype control antibody, anti-FcγRIIB antibody, or an inflammatory cytokine cocktail. (A) Validation of microarray. Immature monocyte–derived DCs from healthy donors (n = 4) were treated with 20 μg/ml mouse IgG1 (Iso), 20 μg/ml anti-FcγRIIB blocking antibody (RIIB), or inflammatory cytokine cocktail (IL-1β, TNF-β, PGE2, and IL-6; Cyt). 24 h later, some DCs from each of the three conditions were analyzed for the expression of DC maturation markers (CD83 and CD80) by flow cytometry. RNA was extracted from the rest of the DCs and gene expression was examined using Human Genome U133 Plus 2 Affymetrix chips. These graphs show the changes in the expression of DC maturation markers (CD80 and CD83) at the level of mRNA (by microarray) and protein (by flow cytometry). (B) Heatmap of 1,801 genes differentially expressed between Iso DC and RIIB DC. Day 5 immature Mo-DCs were treated with either 20 μg/ml mouse IgG1 (Iso DC), 20 μg/ml anti-FcγRIIB antibody (RIIB DC), or inflammatory cytokine cocktail (IL-1β, TNF-β, PGE2, and IL-6; Cyt DC). 24 h later, the DCs were harvested and RNA was extracted and analyzed using the Human Genome U133 Plus 2 Affymetrix gene array chips and the GeneSpring software. Genes that were marked as present and expressed above a raw level of 100 were included in the analysis. Iso DC, RIIB DC, and Cyt DC were analyzed to detect genes that were differentially expressed between the three groups using a parametric test with variance assumed equal (ANOVA) with a p-value cut off of 0.05, followed by the Benjamin and Hochberg false discovery rate multiple correction. 4,759 genes were found to be differentially expressed between Iso DC, RIIB DC, and Cyt DC. Of these 4,759 genes, 1,801 genes were found to be differentially expressed in RIIB DCs compared with Iso DCs (twofold difference; P < 0.05). (left) The expression of these 1,801 genes in RIIB DC and Iso DC. (right) The expression of the same 1,801 genes in DCs treated with inflammatory cytokines (Cyt DC). The box shows a subset of 95 genes that are overexpressed only in RIIB DCs compared with both Iso DCs and Cyt DCs. Details of these genes are noted in Table S1. (C) Heat map of IRGs in monocytes and DCs treated with anti-FcγRIIB antibody or isotype control antibody. Day 5 monocyte derived IDCs (n = 3) were treated with IFN-α2b (1,000 U/ml) or left untreated. 24 h later, RNA was extracted and analyzed using the Human Genome U133 Plus2 Affymetrix chips and the GeneSpring software (version 7.2). 167 genes were found to be up-regulated by more than fivefold in IFN-treated DCs compared with untreated DCs. (left) Expression of the 167 IFN-α–induced genes in DCs treated with IFN-α and untreated DCs (No Rx). Expression of the 167 IRGs was then compared in DCs (n = 5) and monocytes (n = 3) treated with anti-FcγRIIB mAb (RIIB) or isotype control antibody (Iso). (D) Expression of two IFN-induced genes (Mx1 and IFI27) in DCs treated with anti-FcγRIIB mAb or isotype control was analyzed by TaqMan, and expression data was compared with the data obtained by microarray analysis. RNA from two donors (used in the microarray analysis) was analyzed by TaqMan to verify the expression of two IFN-induced genes (Mx1 and IFI27). The figure shows the Taqman expression data compared with the expression data obtained by microarray analysis. (E) Expression of type I IFNs by microarray. Gene expression data obtained from DCs treated with either isotype antibody or anti-FcγRIIB antibody, as in Fig. 1 B, was analyzed for the expression of type I IFN genes. The histogram shows the mean ± the SD for the mRNA expression from four different donors. Table S1 is available at http://www.jem.org/cgi/content/full/jem.20062545/DC1.
Changes in type I IFN-induced genes after treatment with anti-FcγRIIB antibody
Interestingly, a majority of the genes expressed selectively in RIIB DCs were known to be induced by type I IFNs. To better characterize the IFN-responsive genes in DCs, we treated IDCs (n = 3) with 1,000 U/ml of IFN-α2b and identified 167 genes that were up-regulated by greater than fivefold at 24 h in IFN-treated DCs compared with untreated DCs (Fig. 1 C and Table S2, available at http://www.jem.org/cgi/content/full/jem.20062545/DC1). Indeed, 54 of 95 genes in the RIIB DC–specific signature were IFN-inducible genes (Table S1). Up-regulation of IFN-induced genes by anti-FcγRIIB antibody was not unique to DCs, but also observed in monocytes treated with this antibody (Fig. 1 C). The IFN-induced genes in these cells included several well-known type I IFN response signature genes, such as IFN-α inducible (IFI) protein 27, myxovirus resistance 1 (Mx1), Mx2, 2′-5′ oligoadenylate synthase, cig5, GIP3, IFI16, serpin E, IFI44, STAT1, and IRF-7 (Table S1). To validate the data for expression of IFN-induced genes in the microarray, the expression of two of these genes (IFI27 and Mx1) was also analyzed by real-time PCR, which confirmed the microarray findings (Fig. 1 D). Surprisingly, this increase in IRGs was not associated with an increase in the levels of any of the known type I IFNs themselves (IFN-α, -β, -ω, -τ, and including newer members of the family such as IL28A, IL-28B, and IL-29; Fig. 1 E) and only a modest increase in IFN-γ at the mRNA and protein level as analyzed in the supernatants using Luminex (Table I). It is notable that a low level signal for one of the type I IFN genes (IFN-α17) was detectable by microarray in DCs treated with isotype control mAb, and consistent with this, low levels of IFN-α were also detectable in the supernatants by Luminex (Fig. 1 E and Table I). Importantly, however, treatment with anti-FcγRIIB mAb did not lead to a change in the expression of any of the type I IFNs when compared with isotype mAb-treated cells. Therefore, a distinct component of FcγR-mediated activation of DCs is the strong and rapid activation of IFN-induced genes without a concurrent increase in the expression of type I IFNs themselves.
Table I.
Expression of cytokines and chemokines secreted by DCs by Luminex analysis
| Iso DC | RIIB DC | Cyt DC | RIIB DC versus Iso DC (p-value) |
RIIB DC versus Cyt DC (p-value) |
|
|---|---|---|---|---|---|
| IL-1α | 57.19 (29.4) | 699.5 (184.4) | 87.6 (80.8) | <0.001 | <0.001 |
| IL-1β | 11 (15.6) | 668.9 (780) | NE | 0.071 | NE |
| IL-2 | 10.2 (4.1) | 14.3 (4.7) | 21.9 (14) | 0.120 | 0.170 |
| IL-3 | 102.2 (16.2) | 176 (56.5) | 137.6 (35.5) | 0.023 | 0.147 |
| IL-5 | 0 (0) | 0.0 | 0 (0) | 0.000 | 0.000 |
| IL-6 | 229.6 (142.6) | 7941.8 (4116.4) | NE | 0.005 | NE |
| IL-7 | 0.1 (0.2) | 27 (3.8) | 16.4 (12.7) | 0.000 | 0.080 |
| IL-8 | 628.1 (408.1) | 10000 (0) | 5727 (4992.1) | <0.001 | 0.069 |
| IL-10 | 22.5 (2.8) | 1200.9 (987.5) | 108.6 (111.5) | 0.027 | 0.035 |
| IL-12p40 | 40.5 (28.4) | 4713.3 (5019.4) | 3191 (4483.3) | 0.056 | 0.333 |
| IL-12p70 | 15.9 (16.6) | 148.2 (148.5) | 29 (20.1) | 0.064 | 0.082 |
| IL-13 | 8.9 (3.4) | 28.9 (21.1) | 39.7 (47.2) | 0.055 | 0.345 |
| IL-15 | 3.8 (7.7) | 0.9 (1.8) | 0 (0) | 0.242 | 0.178 |
| IFN-γ | 116.5 (8.8) | 264.2 (61.1) | 198.3 (81.6) | 0.002 | 0.122 |
| TNF-α | 5.3 (6.3) | 1059.8 (911.3) | NE | 0.030 | NE |
| Eotaxin | 15.5 (19.2) | 35 (11) | 43.1 (14.5) | 0.065 | 0.204 |
| MCP1 | 1173.1 (1074) | 2776.34 (2655.6) | 1314.8 (1908.4) | 0.153 | 0.203 |
| Rantes | 27.8 (16.5) | 1531 (682) | 264.8 (356.3) | 0.002 | 0.008 |
| MIP1a | 710.6 (351.3) | 9746.9 (388) | 3067.8 (2733.7) | <0.001 | 0.001 |
| IP10 | 395.3 (185.4) | 8629.8 (2364.7) | 637.9 (664.9) | <0.001 | <0.001 |
| IFN-α | 10.2 (9.9) | 10.3 (12.5) | 28.9 (15.6) | 0.493 | 0.056 |
IDCs (n = 4) were treated with anti-FcγRIIB antibody (RIIB DC), inflammatory cytokine cocktail (Cyt DC), or isotype control antibody (Iso DC). 24 h later, the culture supernatant was collected and examined for cytokines and chemokines by Luminex assay. NE, not evaluable, as these cytokines were added exogenously to Cyt DCs.
Expression of inflammation-associated chemokines and cytokines in FcγR-matured DCs
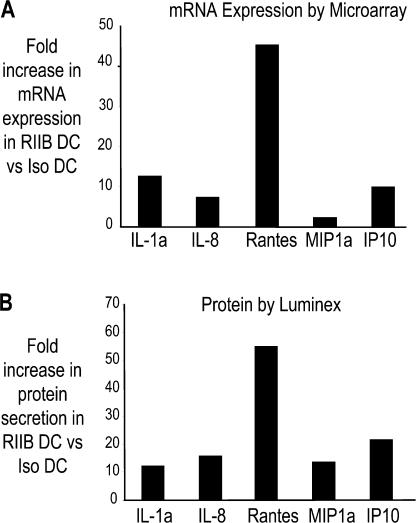
In addition to the IFN-inducible genes, several other genes involved in inflammation were also differentially up-regulated in RIIB DCs relative to Iso DCs or Cyt DCs. This included several CC chemokines (CCL2/MCP-1, CCL19, CCL3, CCL5/Rantes, and CCL4/MIP1β), cytokines (IL-1α), FcR, and complement-related genes. To validate the microarray data at the protein level, the DC culture supernatants were also analyzed for the presence of cytokines and chemokines by Luminex analysis. When compared with Iso DCs, RIIB DCs secreted greater amounts of MIP1α, IL1α, IL1β, IP10, IL-12p70, and CCL5/Rantes, which were also consistent with the microarray data (Table I and Fig. 2). Therefore, DC maturation by selective signaling via activating FcγRs is characterized by a distinct pattern of inflammation-associated genes relative to DCs matured using inflammatory cytokines.
Figure 2.
Up-regulation of chemokines/cytokines in FcγR-matured DCs. Validation of microarray data at the protein level. Monocyte-derived IDCs (four different donors) were treated with 20 μg/ml anti-FcγRIIB antibody (RIIB DC) or 20 μg/ml isotype control antibody (mouse IgG1; Iso DC). 24 h later, DCs were harvested and RNA was extracted and analyzed using the U133 Plus2 Affymetrix chips (as in Fig. 1). The culture supernatant was analyzed for the expression of cytokines/chemokines by Luminex analysis. The graphs show the fold increase in mRNA expression (A) or protein secretion (B) for the cytokines and chemokines in RIIB DC compared with Iso DC.
Mechanism of FcγR-mediated induction of type 1 IFN response
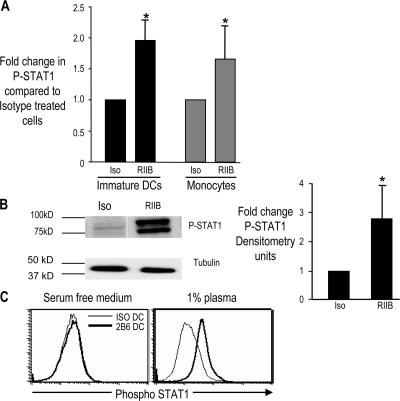
Because of the prominence of the IFN signature in RIIB DCs, we focused on further characterizing the mechanism for induction of these responses. Type 1 IFN-mediated activation of its receptor activates Jak protein tyrosine kinases, which in turn phosphorylate STAT proteins, including STAT1 (16). Therefore, we used the detection of phosphorylated STAT1 (P-STAT1) protein by flow cytometry as a marker for IFN-induced response at a single-cell level. Treatment of IDCs with the anti-FcγRIIB was associated with an increase in P-STAT1 detectable at 24 h after anti-FcγRIIB mAb treatment (Fig. 3 A). The increase in P-STAT1 observed in these experiments was also confirmed by Western blot analysis (Fig. 3 B). Anti-FcγRIIB–mediated up-regulation of P-STAT1 was not unique to DCs, but was also seen in monocytes (Fig. 3 A). In prior studies, we have shown that DC maturation induced by anti-FcγRIIB requires the presence of activating Ig ligands in normal human plasma (2). Consistent with this, anti-FcγRIIB enhanced P-STAT1 in DCs cultured in 1% plasma, but not those cultured in serum-free media (Fig. 3 C).
Figure 3.
Mechanism of FcγR-mediated induction of IFN response. (A) Up-regulation of P-STAT1 in DCs or monocytes treated with anti-FcγRIIB mAb versus isotype control. Immature Mo-DCs (n = 8) or freshly isolated monocytes (n = 7) were treated with anti-FcγRIIB antibody (RIIB) or isotype control antibody (Iso). 24 h later, P-STAT1 expression was examined by flow cytometry. The histogram shows fold change in expression of P-STAT1 in anti-FcγRIIB antibody–treated versus isotype control antibody–treated cells. *, P < 0.05. (B) Western blot confirmation of up-regulation of phosphorylated STAT1 in DCs treated with anti-FcγRIIB antibody versus isotype control antibody. Monocyte-derived IDCs were treated with isotype control mouse IgG1 antibody (Iso) or anti-FcγRIIB antibody (RIIB). 24 h later, the DCs were harvested and the protein was analyzed on a 7.5% polyacrylamide gel to detect the presence of phosphorylated STAT1. (left) Representative of four separate experiments. (right) The summary of data for quantitative densitometry. Value for P-STAT1 was first normalized against data for α-tubulin in that sample, before comparison between RIIB DCs and Iso DCs. Data shown are the summary of four separate experiments. *, P < 0.05. (C) Expression of phosphorylated STAT1 (P-STAT1) in DCs treated with anti-FcγRIIB antibody or isotype control antibody in serum-free medium compared with 1% plasma. Immature monocyte–derived DCs were treated with anti-FcγRIIB antibody (RIIB DC) or isotype control antibody (Iso DC) in serum-free medium or in medium supplemented with 1% human plasma. 24 h later, the expression of P-STAT1 was examined by flow cytometry. Data are representative of three similar experiments. Data represent the mean ± the SD.
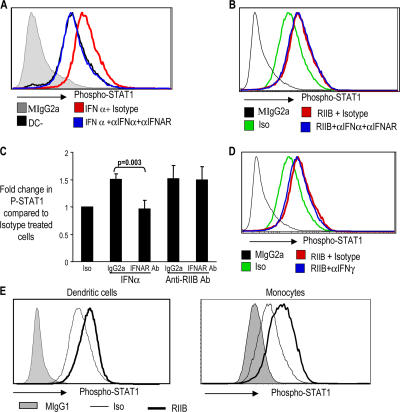
Next, we studied the effects of IFN-α blockade on anti-FcγRIIB–mediated STAT1 phosphorylation. As expected, the addition of IFN-α to IDCs led to an increase in P-STAT1, which was blocked by preincubation of DCs with IFN-α and IFN-α receptor (IFNAR) blocking antibodies (Fig. 4 A). However, preincubation with the IFN-α and IFNAR blocking antibodies did not abrogate the up-regulation of P-STAT1 in anti-FcγRIIB–treated DCs (Fig. 4 B). It is notable, however, that although these mAbs inhibit the increase in P-STAT1 in response to exogenous IFN-α, they do not abolish the constitutive levels of P-STAT1 (Fig. 4 A and not depicted). Similarly, pretreatment of monocytes with anti-IFNAR mAb inhibited the increase in P-STAT1 in response to exogenous IFN-α, but not after treatment with anti-FcγRIIB (Fig. 4 C).
Figure 4.
Induction of IFN response by anti-FcγRIIB antibody is not inhibited by blocking antibodies against IFN-α and IFN-γ. (A) Up-regulation of P-STAT1 by exogenous IFN-α is blocked by antibodies against IFN-α. Monocyte-derived IDCs were either left untreated (DC−) or treated with IFN-α2b (1,000 U/ml intron A). The DCs were either treated with a combination of blocking antibodies against IFN-α, as well as IFNAR (αIFNAR; both at 10 μg/ml) or with their isotype control antibodies (Isotype; mouse IgG1 and mouse IgG2a, respectively; both at 10 μg/ml) for 45 min before treatment with IFN-α. The DCs were analyzed for their expression of P-STAT1 by flow cytometry. Gray area of the histogram shows staining of the DCs with isotype control (Mouse IgG2a) for P-STAT1 antibody. The graph represents one of two similar experiments. (B) Effect of blocking antibodies against IFN-α on anti-FcγRIIB–mediated induction of P-STAT1. Monocyte-derived IDCs were treated with anti-FcγRIIB antibody (5 μg/ml RIIB) or mouse IgG1 isotype control antibody (Iso). DCs treated with anti-FcγRIIB antibody were either treated with blocking antibodies against IFN-α and IFNAR (10 μg/ml RIIB + αIFNa+αIFNAR) or isotype control antibody (RIIB + Isotype; 10 μg/ml mouse IgG2a and 10 μg/ml IgG1, respectively) for 45 min before the addition of anti-FcγRIIB antibody. 24 h later, flow cytometry was performed to examine the expression of P-STAT1. Some DCs were also stained with mouse IgG2a, which is isotype control for the P-STAT1 antibody. One of two similar experiments. (C) Effect of anti-IFNAR blocking antibody on anti-FcγRIIB–mediated induction of P-STAT1. Freshly isolated PBMCs were treated with IFN-α2b (1,000 U/ml intron A) or anti-FcγRIIB antibody and an isotype control antibody either in the presence of 20 μg/ml IFNAR antibody (IFNAR Ab) or isotype control antibody (mouse IgG2a). 1 h later, flow cytometry was performed to examine the expression of P-STAT1 on CD14+ monocytes. Data shown are the summary of three similar experiments. *, P < 0.05. Data represent the mean ± the SD. (D) IFNγ blocking antibodies do not inhibit the up-regulation of P-STAT1 by anti-FcγRIIB antibody. IDCs were treated with either anti-FcγRIIB antibody (5 μg/ml RIIB) or isotype control antibody (Iso). DCs treated with anti-FcγRIIB antibody were either treated with blocking antibodies against IFNγ and IFNAR (10 μg/ml RIIB + αIFNγ) or isotype control antibody (10 μg/ml RIIB + Isotype; mouse IgG1) for 45 min before the addition of anti-FcγRIIB antibody. 24 h later, flow cytometry was performed to examine the expression of P-STAT1. Some DCs were also stained with mouse IgG2a, which is isotype control for the P-STAT1 antibody. The graph shows one of two similar experiments. (E) Anti-FcγRIIB induced up-regulation of P-STAT1 is rapid. Immature monocyte derived DCs (left) or monocytes (right) were treated with 10 μg/ml anti-FcγRIIB antibody (RIIB) or mouse IgG1 isotype control antibody (Iso). 1 h later flow cytometry was performed to detect the expression of P-STAT1. Gray histogram shows staining with mouse IgG2a isotype control antibody for P-STAT1. The graphs show one of five similar experiments.
As there was a mild, but detectable, increase in IFN-γ in the DC supernatants, we also analyzed the effect of an IFNγ blocking mAb on anti-FcγRIIB–induced P-STAT1. The IFNγ blocking antibody was also unable to inhibit the up-regulation of P-STAT1 in anti-FcγRIIB–treated DCs (Fig. 4 D). Similar findings were observed in monocytes (unpublished data). Anti-FcγRIIB–mediated P-STAT1 up-regulation was rapid and detectable within an hour of mAb treatment in both monocytes and DCs (Fig. 4 E). Together, these data suggest that selective engagement of activating FcγRs leads to rapid activation of IFN signaling without a concurrent increase in the expression of type I IFNs themselves.
Role of activating FcγRs
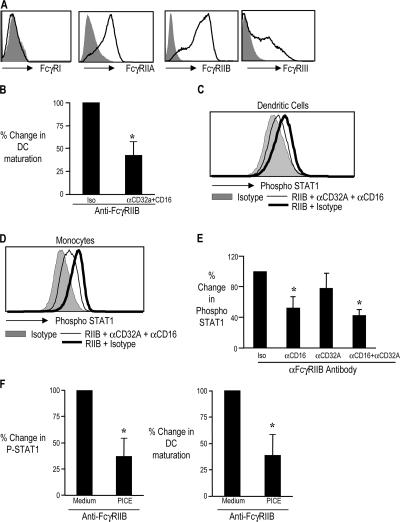
Induction of DC maturation by anti-FcγRIIB antibody requires the presence of activating Ig ligands present in normal human plasma (2). The Mo-DCs used in these experiments were generated from CD14+ monocytes purified using immunomagnetic beads and cultured in 1% plasma. These DCs express higher levels of CD32, as well as CD16, compared with DCs generated from plastic adherent monocytes (Fig. 5 A and not depicted) (17). To evaluate the role of specific activating FcγRs, the DCs were preincubated with antibodies against CD16 and CD32A before blockade of CD32B. Prior incubation with a cocktail of antibodies against activating FcγRs, FcγRIIA (CD32A), and FcγRIIIA (CD16) inhibited anti-FcγRIIB–mediated up-regulation of DC maturation markers (CD80 and CD83; Fig. 5 B).
Figure 5.
Role of activating FcγRs in the FcR-mediated DC maturation and induction of P-STAT1. (A) Expression of FcγRI, RIIA, RIIB, and RIIIA on DCs generated from CD14+ monocytes by flow cytometry. Day 5 monocyte-derived IDCs were examined for the expression of FcγR1, FcγRIIA, FcγRIIB, and FcγRIII by flow cytometry. The area in gray represents staining with isotype control antibodies. Figure represents one of six similar experiments. (B) Down-regulation of anti-FcγRIIB antibody induced DC maturation by blocking antibodies against activating FcγR. IDCs (n = 4) were treated with isotype control antibody or anti-FcγRIIB antibody either with blocking antibodies against CD32A and CD16 antibody (αCD32A + αCD16; clone IV.3 and 3G8, both at 10 μg/ml) or their isotype control antibodies (Isotype; mouse IgG2a and IgG1, respectively). 24 h later, the expression of CD80 and CD83 was monitored by flow cytometry, and the double-positive cells were used to assess DC maturation. Change in maturation between isotype-treated and anti-FcγRIIB–treated DCs was considered as 100%. The figure shows the percentage of decrease in maturation of DCs treated with blocking antibodies against the activating FcγR (CD32A and CD16). Data are a summary of four similar experiments. *, P < 0.05. (C and D) Down-regulation of anti-FcγRIIB antibody induced P-STAT1 by blocking antibodies against activating FcγRs. IDCs (C) or PBMCs (D) were treated with mouse IgG1 isotype control antibody (Isotype) or anti-FcγRIIB antibody (RIIB). Some of the DCs and PBMCs treated with anti-FcγRIIB antibody were pretreated with blocking antibodies against CD32A and CD16 (αCD32A + αCD16; clone IV.3 and clone 3G8, respectively, both 10 μg/ml) or isotype control antibodies for the CD32A and CD16 blocking antibodies (mouse IgG2a and mouse IgG1, respectively; 10 μg/ml) for 45 min. Expression of P-STAT1 on CD11c+ DCs or CD14+ monocytes was examined by flow cytometry. Data are representative of four similar experiments for DCs and three for monocytes. (E) PBMCs were treated with MIgG1 isotype control antibody or anti-FcγRIIB antibody. Some of the anti-FcγRIIB–treated PBMCs were pretreated with blocking antibody to CD16 (3G8), CD32A (IV.3), or a combination of blocking antibodies against CD16 and CD32A (3G8 + IV.3) or their isotype control antibodies (mouse IgG1 and mouse IgG2a, respectively; Iso). 1 h later, the expression of P-STAT1 in CD14+ monocytes was analyzed by flow cytometry. The change in expression of P-STAT1 between MIgG1-treated cells and anti-FcγRIIB–treated cells was considered as 100%. The figure shows the percentage of decrease in this P-STAT1 phosphorylation by blocking antibodies against CD16 (αCD16) and CD32A (αCD32A), either alone or in combination (αCD16 + αCD32A). The histogram shows the summary of experiments on three separate donors (*, P < 0.05). (F) Inhibition of Syk tyrosine kinase abrogates anti-FcγRIIB induced up-regulation of P-STAT1. Immature Mo-DCs were treated with medium alone (medium) or Syk tyrosine kinase inhibitor piceatannol (PICE) at 5 μmol concentration. 30 min later, the DCs were treated with either anti-FcγRIIB antibody or isotype control mouse IgG1 antibody (MIgG1). 24 h later, the DCs were analyzed for their P-STAT1 expression and maturation (CD80 and CD83) by flow cytometry. Change in expression of P-STAT1, as well as maturation between MIgG1-treated cells and anti-FcγRIIB–treated cells, was considered as 100%. The histogram on the left shows the percentage of decrease in this P-STAT1 phosphorylation by piceatannol treatment. The histogram on the right shows the percentage of decrease in DC maturation by piceatannol treatment. The graphs are a summary of experiments on three separate donors (*, P < 0.05). Data represent the mean ± the SD.
Preincubation of DCs with a cocktail of antibodies against both activating FcγRs (CD32a and CD16) inhibited anti-FcγRIIB–mediated induction of P-STAT1 (Fig. 5 C). These antibodies also blocked anti-FcγRIIB–mediated up-regulation of STAT1 in monocytes (Fig. 5 D). This was also associated with down-regulation of the IFN-induced genes on microarray analysis (unpublished data). To examine the relative contribution of specific activating FcγRs in anti-FcγRIIB mAb-mediated STAT1 up-regulation, we also evaluated the effect of individual blockade of FcγRIIIA and FcγRIIA on up-regulation of P-STAT1. Blockade of FcγRIIIA on monocytes with clone 3G8 led to greater inhibition of P-STAT1 up-regulation, compared with FcγRIIA blocking antibody (clone IV.3), suggesting that FcγRIIIA may be the major activating FcγR contributing to STAT1 phosphorylation in monocytes (Fig. 5 E). Aggregation of FcγRs bearing ITAM motifs leads to the activation of Syk family of tyrosine kinases (18, 19). Tassiulas et al. recently demonstrated that Syk and associated adaptor proteins can enhance IFN-α signaling by direct phosphorylation of STAT1 (20). Consistent with this, pretreatment of DCs with a Syk inhibitor, piceatannol, inhibited the up-regulation of P-STAT1, as well as maturation induced by anti-FcγRIIB (Fig. 5 F). Collectively, these data suggest that the signals via activating FcγRs contribute to the induction of P-STAT1 under these experimental conditions.
Role of STAT1 in FcγR-mediated DC maturation
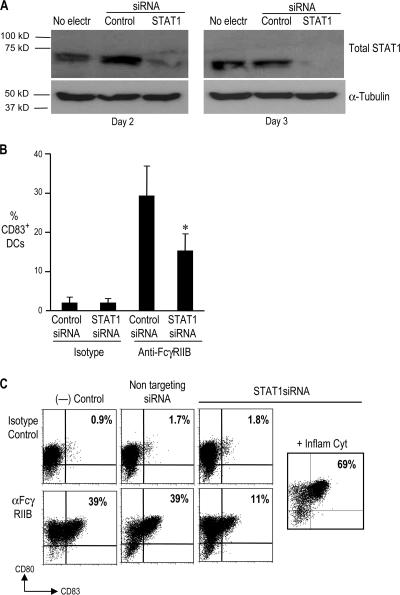
Prior studies have shown that type I IFNs can mediate phenotypic and functional activation of human DCs. We hypothesized that the activation of IFN pathway/STAT1 by anti-FcγRIIB mAb may be essential for FcγR-mediated DC maturation. To test this directly, we knocked down STAT1 protein in IDCs using RNAi. IDCs were electroporated with STAT1 siRNA or nontargeted control siRNA. The expression of STAT1 protein was markedly inhibited at 48 and 72 h in DCs treated with STAT1 siRNA, but not in nontargeted control siRNA (Fig. 6 A). At 72 h after treatment with siRNA, DCs were treated with anti-FcγRIIB or isotype control mAb. DCs treated with STAT1 siRNA showed decreased up-regulation of maturation markers (CD80 and CD83; Fig. 6, B and C). However, these DCs were capable of maturation in response to an inflammatory cytokines cocktail. Therefore, STAT1 plays an important role in FcγR-mediated induction of DC maturation.
Figure 6.
FcγR-mediated DC maturation can be inhibited by STAT1 knockdown. (A) Day 4 IDCs were electroporated with 10 μg of STAT1 siRNA (STAT1) or nontargeting siRNA (Control). Some DCs were cultured without electroporation (No electr). The DCs were harvested 48 and 72 h after electroporation, and a Western blot was performed to detect total STAT1 protein. Data are representative of two separate experiments. (B and C) DCs were harvested 72 h after electroporation with either STAT1 siRNA or nontargeting control siRNA. Some nonelectroporated DCs were also harvested. The DCs were treated with anti-FcγRIIB antibody or isotype control antibody. Some of the DCs electroporated with STAT1 siRNA were also treated with the inflammatory cytokine cocktail. 24 h later, DC maturation was determined by examining the expression of CD80, CD83, and CD11c by flow cytometry. (B) Summary of data in four independent experiments (mean ± the SD; *, P < 0.05). (C) Data from a representative experiment.
DISCUSSION
ICs and antibodies play an important role in autoimmune and allergic inflammation and in protection from infectious pathogens (21). The immunologic effects of ICs depend on the balance between activating and inhibitory FcγRs, including in DCs (3, 21). These data demonstrate that manipulating this balance via antibody-mediated blockade of inhibitory FcγRIIB in the presence of activating ligands in the human serum has major effects on gene expression and activation status of human monocytes and DCs. Signaling via activating FcγRs on DCs provides a distinct form of maturation stimulus that is characterized by the expression of P-STAT1 and several IRGs, as well as inflammation-associated chemokines/cytokines. Activation of P-STAT1 is critical to FcγR-mediated effects on DCs, because suppression of STAT1 inhibits FcγR- mediated DC maturation.
The finding that FcγR signaling can alter the expression of IRGs has implications for the regulation of IFN signaling in FcγR-expressing myeloid cells. Typically, induction of IFNs in response to viral pathogens is mediated by cytosolic receptors (e.g., RIG1 and MDA5) in infected cells, or endosomal receptors (e.g., Toll receptor family) in antigen-presenting cells (22). Downstream signaling in response to IFNs and activation of Jak–STAT pathway is also highly regulated (e.g., by the PIAS or SOCS family of proteins) (23). However, studies by Taniguchi et al. have shown that the strength of IFN response to pathogens depends on the level of constitutive signaling in the absence of pathogens (14). Our data suggest that the balance of activating/inhibitory FcγR signaling in DCs may have a direct impact on this basal level of IFN signaling in myeloid cells in the absence of pathogens. This balance may, in principle, be altered both in the context of specific FcγR polymorphisms, or the ability of ICs to bind activating versus inhibitory FcγRs.
Aggregation of FcγRs bearing ITAM motifs leads to the activation of Syk family of tyrosine kinases (18, 19). Tassiulas et al. recently showed that Syk and associated adaptor proteins can enhance IFN-α signaling by direct phosphorylation of STAT1 (20). Our data extend these observations and suggest a role for activating FcγRs on human monocytes and DCs in enhancing the expression of IRGs. Further studies are needed to evaluate this pathway.
Importantly, the FcγR-mediated induction of IRGs was not associated with a detectable increase in the expression of any of the type I IFNs, and it did not require the addition of exogenous IFNs to these cultures. Blocking mAbs against IFNAR and IFN-α, which block the increase in P-STAT in response to exogenous IFN-α, also fail to block this response. The induction of STAT1 phosphorylation by anti-FcγRIIB was rapid and detectable within 1 h of treatment with anti-FcγRIIB blocking antibodies, which also argues against the IFN signature being caused by synthesis of new IFNs. It is also important to emphasize that the IFN signature is not observed in DCs treated specifically with inflammatory cytokines (Cyt DCs). Collectively, these data suggest a novel link between FcγR signaling and IFN response in human DCs and monocytes. However, it is important to note that low levels of type I IFNs (IFN-α17) were expressed in control DCs and detectable in supernatants (although unchanged in response to anti-FcγRIIB mAb). Furthermore, the IFNAR blocking antibodies were unable to block the constitutive level of P-STAT1. Therefore, the observed effects are consistent with the ability of FcγR-mediated signals to modify the constitutive IFN signaling in these cells (14).
The nature of specific activating FcγRs and other signals that are important for regulating IFN signaling in DCs requires further study and may be cell/tissue type specific. In our experiments, the signal was delivered via both FcγRIIIA and FcγRIIA. Recent studies have shown that FcγRIIIA is expressed on distinct subsets of human monocytes and DCs (24, 25), although their functional significance remains to be clarified. Polymorphisms in both FcγRIIA and FCγRIIIA may therefore have a significant impact on immune activation mediated by ICs and antibody-coated tumor cells (26, 27).
Selective blockade of inhibitory FcγR signaling on DCs also leads to the up-regulation of several other genes not in the type I IFN pathway, but implicated in autoimmune and allergic inflammation. These include several CC chemokines, such as CCL5, CCL4, and CCL3, which were documented at both mRNA and protein level. Expression of these chemokines may allow FcγR-matured DCs to attract several immune cells, including T cells, monocytes, DCs, and eosinophils, which play an important role in inflammation (28).
These data also have several clinical implications. Dysregulation of FcγR signaling may contribute to the altered IFN signaling observed in several autoimmune states (13, 27). Therefore, either restoring the FcγR balance or inhibiting STAT1 may help suppress immune activation in lupus. Indeed, recent studies have shown that only small changes in inhibitory FcγR can have major effects on immunopathology in lupus (29). Altered IFN signaling and STAT1/STAT3 ratio in myeloid DCs and other myeloid cells has also been implicated in the loss of protective antitumor immunity and immune surveillance in several models (12, 30, 31). Interestingly, recent studies have also implicated a more direct role for ICs in promoting tumor growth (32). Our observations may serve to link these findings together. Manipulating FcγR balance on tumor-infiltrating DCs (e.g., via anti-FcγRIIB antibodies) may be of value as a general approach to regulate endogenous antitumor immunity. Modulation of IFN signaling may also contribute to the efficient induction of T cell immunity observed by selectively targeting ICs or antibody-coated tumor cells to activating FcγRs on human and murine DCs (2, 3, 33, 34); therefore, manipulating the FcγR balance may also be targeted for improving the efficacy of antibody-based therapy (35) or DC-based immunotherapy of cancer (36).
MATERIALS AND METHODS
Generation of DCs.
PBMCs were isolated from blood of healthy donors via density gradient centrifugation using Ficoll-Hypaque (GE Healthcare). CD14+ cells were obtained using CD14 microbeads and LS columns (Miltenyi Biotec) and used to generate DCs. DCs were generated by culturing CD14+ cells in RPMI-1640 medium with l-glutamine ( Mediatech) supplemented with 1% single donor plasma, gentamicin (20 μg/ml; BioWittaker), and 0.01 M Hepes (Cambrex). For some experiments, DCs were cultured in AIM-V serum free medium (Invitrogen). 20 ng/ml GM-CSF (Immunex) and 12.5 ng/ml IL-4 (R&D Systems) were added to the culture on days 0, 2, and 4. On day 5 of culture, IDCs were either treated with 10–20 μg/ml anti-FcγRIIB antibody (clone 2B6; MacroGenics), 10–20 μg/ml mouse IgG1 isotype control (Sigma-Aldrich), or inflammatory cytokine cocktail (10 ng/ml IL-1β, 1,000 U/ml IL-6, 10 ng/ml TNF-α (all from R&D Systems), and prostaglandin E2 (1 μg/ml, Sigma-Aldrich). As R/H polymorphisms of the activating receptor CD32A can potentially impact FcR-mediated DC maturation, they were monitored by flow cytometry using specific antibodies (17, 37). All donors in this study had HH/HR genotype (not depicted). Purity of the DC preparations was evaluated by flow cytometry and was >95%. The percentage of contaminating NK cells was <0.1%.
Microarray analysis.
IDCs on day 5 of culture (n = 5) or CD14+ monocytes isolated from immunomagnetic bead selection (n = 3) were treated with 20 μg/ml anti-FcγRIIB mAb, isotype control (mouse IgG1 mAb), or inflammatory cytokine cocktail. After 24 h of culture, cells were pelleted in RLT buffer. RNA was extracted from the DCs using the RNeasy kit (QIAGEN) as per the manufactures protocol. Total RNA (1–2 μg) was labeled and hybridized using the Enzo T7 labeling kit (Life Sciences) on Affymetrix Human Genome U133 Plus 2.0 microarray (Affymetrix). Washing and scanning was done with Fluidics Station 400 and GeneChip Scanner 3000 (both from Affymetrix) following the manufacturer's protocol. Affymetrix GCOS 1.2 software was used to obtain the raw signal and present/absent gene data. Further analysis of the data was performed using GeneSpring software version 7.2 (Silicon Genetics).
Data analysis of microarray data.
The methodology for analysis of microarray data was similar to that used by Napolitani et al. (38). The microarray data was normalized as follows. The signal values below 0.01 were set to 0.01. The percentile for all the measurements in each sample was calculated using the values for all genes not marked “absent.” Each measurement was divided by the 50th percentile of all measurements in that sample. Each gene was divided by the median of its measurements in all samples. Genes that were marked as present and expressed a raw level of >100 were included in the analysis. 29,499 genes passed this filter. Gene expressions in DCs treated with inflammatory cytokines, FcγRIIB blocking antibody, and isotype control antibody were compared, and genes with statistically different expression between the groups based on the values of the replicates were calculated using a parametric test with variance assumed equal (ANOVA) using a p-value cut off of 0.05, followed by Benjamin and Hochberg false discovery rate multiple-test correction. A gene list of 4,759 significant genes was obtained and used to determine fold changes in expression between the three different conditions. Of these 4,759 genes, 1,801 genes were differentially regulated between FcγRIIB antibody-treated DCs by twofold or higher, compared with isotype control antibody–treated DCs. Microarray data are available in the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nih.gov/geo/) under accession no. GSE7509.
Real time PCR (TaqMan) analysis.
RNA was isolated with RNeasy Mini kit (Qiagen) and RT-PCR was conducted with Assays-on-Demand primer-probe for IFI27 and Mx1 using the ABI PRISM 7700 sequence detection system (both Applied Biosystems). Expression of GAPDH was monitored as a housekeeping gene. Reactions were set up in triplicates using EZ PCR Core Reagents (Applied Biosystems) according to the manufacturer's instructions with 20 ng of total RNA. Relative expression of target genes was calculated using the comparative threshold cycle method.
Luminex assay.
Cell supernatants from 24-h DC cultures were analyzed for 20 cytokines and chemokines using the Protein Multiplex Immunoassay kit (Biosource International) as per the manufacturer's protocol. In brief, Multiplex beads (Biosource) were vortexed and sonicated for 30 s, and 25 μl was added to each well and washed two times with wash buffer. The samples were diluted 1:2 with assay diluent and loaded onto a Multiscreen BV 96-well filter plate (Millipore) with 50 μl of incubation buffer already added to each well. Serial dilutions of cytokine standards were prepared in parallel and added to the plate. Samples were then incubated on a plate shaker at 600 rpm in the dark at room temperature for 2 h. The plate was applied to a Multiscreen Vacuum Manifold (Millipore) and washed twice with 200 μl of wash buffer. 100 μl of biotinylated anti–human Multi-Cytokine Reporter (Biosource International) was added to each well. The plate was incubated on a plate shaker at 600 rpm in the dark at room temperature for 1 h. The plate was applied to a Multiscreen Vacuum Manifold (Millipore) and washed twice with 200 μl of wash buffer. Streptavidin-phycoerythrin was diluted 1:10 in wash buffer, and 100 μl was added directly to each well. The plate was incubated on a plate shaker at 600 rpm in the dark at room temperature for 30 min. The plate was then applied to the vacuum manifold, washed twice, and each well was resuspended in 100 μl wash buffer and shaken for 1 min. The assay plate was then transferred to the Bio-Plex Luminex 100 XYP instrument (Millipore) for analysis. Cytokine concentrations were calculated using Bio-Plex Manager 3.0 software with a 5-parameter curve-fitting algorithm applied for standard curve calculations.
Detection of STAT1 phosphorylation by flow cytometry.
The methodology for the detection of P-STAT1 by flow cytometry was adapted from that described by Lesinski et al. (39). Freshly isolated PBMCs, purified CD14+ monocytes, or monocyte-derived IDCs were treated with either mouse IgG1 isotype control antibody or the FcγRIIB blocking antibody. After a 1–24-h culture, the cells were labeled with the mouse anti–human phospho STAT1 antibody (clone pY701; BD Biosciences) as per the manufacturer's protocol. In some experiments, cells were treated with 1,000 IU/ml IFN-α 2b (Schering-Plough). In some experiments, DCs or monocytes were pretreated with 10 μg/ml anti-IFNAR mAb (Fitzgerald Industries), 10 μg/ml anti-IFN α antibody (clone MMHA-1; PBL Laboratories), 10 μg/ml anti–IFN-γ antibody (clone MMHG-1; PBL Laboratories), 10 μg/ml anti-CD16 (clone 3G8; BD Biosciences), and 10 μg/ml antiCD32A (clone IV.3) or isotype controls.
Blocking inhibitory FcγR on human Mo-DCs and evaluation of their maturation.
In brief, IDCs were harvested on day 5 of culture and treated with either 10 μg/ml anti–human FcγRIIB blocking antibody (clone 2B6) or 10 μg/ml mouse IgG1 isotype control antibody (Sigma-Aldrich). After overnight culture, DCs were harvested to assess DC maturation. In some experiments, DCs were pretreated with 10 μg/ml CD16 blocking antibody (clone 3G8; BD Biosciences), and 10 μg/ml CD32A blocking antibody (clone IV.3) or isotype controls for 1 h before treatment with anti–human FcγRIIB blocking antibody. The following antibodies were used for evaluating the surface changes associated with DC maturation: CD11c-APC, CD80-PE, CD83-FITC, CD86-PE, and HLA-DR-FITC (all obtained from Becton Dickinson).
Inhibition of STAT1 synthesis by RNA interference.
CD14+ monocytes were cultured in 1% plasma supplemented with IL4 and GMCSF on days 0 and 2, as described in Generation of DCs. On day 4, the IDCs were harvested, washed with Opti-MEM I medium without phenol red (Invitrogen) and resuspended in Opti-MEM I at a concentration of 2.5 × 107 cells/ml. 4 × 106 IDCs were electroporated with 10 μg of STAT1 (siRNA; siGENOME SMARTpool; Thermo Fisher Scientific) or nontargeting siRNA (siCONTROL Nontargeted siRNA; Thermo Fisher Scientific) in a 4-mm electroporation cuvette using the ECM830 Square Wave Electroporator (Harvard Apparatus). The pulse conditions were a square wave pulse of 500V and 0.5 ms. The electroporated DCs were immediately resuspended in complete medium (RPMI 1% plasma) supplemented with IL4 and GMCSF. Inhibition of STAT1 protein was examined by Western blot analysis 2 and 3 d after electroporation.
Western blot analysis to detect total and phosphorylated STAT1.
Nonelectroporated DCs, as well as DCs electroporated with either nontargeting RNAi or STAT1 RNAi, were harvested, washed in PBS, and lysed with the radioimmunoprecipitation assay buffer containing 150 mM NaCL, 10 mM Tris, pH 7.2, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, 100 mM sodium orthovanadate, and protease inhibitors (protease inhibitor cocktail tablet; Boehringer Mannheim). 25–50 μg of protein was resolved on a 7.5% polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were probed with total STAT1 antibody (rabbit anti–human STAT1, 1:1,000; Cell Signaling Technologies) overnight according to the manufacturers protocol. The membrane was treated with goat anti–rabbit horseradish peroxidase secondary antibody (1:5,000; Southern Biotechnology). Detection was done using the ECL plus Western blot detection reagents (GE Healthcare).
For the detection of phosphorylated STAT1 protein, IDCs were treated with mouse IgG1 isotype control antibody or anti-FcγRIIB antibody. 24 h later, the cells were harvested and lysed with the radioimmunoprecipitation assay buffer containing 150 mM NaCL, 10 mM Tris, pH 7.2, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, protease inhibitors, 10 mM sodium orthovanadate, and 10 mM β glycerol phosphate. Protein was resolved on a 7.5% polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were probed with mAb specific for phosphorylated STAT1 (rabbit anti–human phospho-STAT1 antibody; 1:1,000, Cell Signaling Technologies) overnight, according to the manufacturers protocol. The membrane was treated with goat anti–rabbit horseradish peroxidase secondary antibody (1:5,000; Southern Biotechnology). Detection was done using the ECL plus Western blot detection reagents (GE Healthcare), and analyzed by quantitative densitometry using ImageJ software (W.S. Rasband, National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). Data for P-STAT1 were first normalized against values for α-tubulin in that sample, before comparison to other samples.
Detection of DC maturation after STAT1 knockdown with the STAT1 RNAi.
Nonelectroporated DCs or DCs electroporated with either STAT1 siRNA or nontargeting control siRNA were harvested 72 h after electroporation. DCs were treated with anti-FcγR antibody or mouse IgG1 isotype control antibody. 24 h later, flow cytometry was performed to examine the surface expression of CD83, CD80, and CD11c.
Online supplemental material.
Table S1 shows a list of 95 genes that are differentially expressed in DCs treated with anti-FcγRIIB antibody. Table S2 shows a list of genes up-regulated by more than fivefold at 24 h in immature Mo-DCs cultured in the presence of IFN-α2b (1,000 U/ml), compared with untreated DCs. The MIAME checklist for microarray analysis is presented as supplemental text.
Supplemental Material
Acknowledgments
The authors thank Drs. Ezio Bonvini and Jeff Stavenhagen for critical review of the paper, Dr. Virginia Pascual for thoughtful discussions, and Judy Adams for help with figures.
This work was supported in part by funds from the National Institutes of Health (AI054375 to K.M. Dhodapkar; CA106802 and CA109465 to M.V. Dhodapkar; PO1-AI51573 to R.M. Steinman and J.V. Ravetch; and MO1-RR00102 to The Rockefeller University General Clinical Research Center), the Dana Foundation, the American Society of Clinical Oncology Career Development award, the Alexandrine and Alexander Sinsheimer award (to K.M. Dhodapkar), the Damon Runyon Eli Lilly Clinical Investigator Award (to M.V. Dhodapkar), and the Montreal General Hospital Foundation (D. Banerjee).
K.M. Dhodapkar, M.V. Dhodapkar, R.M. Steinman, and J.V. Ravetch are coinventors on a patent application that describes the processes used in this study. These processes are licensed by The Rockefeller University to Macrogenics, Inc. M.C. Veri is an employee of Macrogenics, Inc. J.V. Ravetch has a financial interest in Macrogenics, Inc. which is managed by the Rockefeller University's conflict of interest committee. The remaining authors declare no financial conflict of interest.
Abbreviations used: FcγR, Fcγ receptor; GEP, gene expression profile; IC, immune complex; IDC, immature DC; IFI, IFN-α inducible; IFNAR, IFN-α receptor; IRG, IFN response gene; STAT, signal transducer and activator of transcription.
References
- 1.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 2.Dhodapkar, K.M., J.L. Kaufman, M. Ehlers, D.K. Banerjee, E. Bonvini, S. Koenig, R.M. Steinman, J.V. Ravetch, and M.V. Dhodapkar. 2005. Selective blockade of inhibitory Fc gamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody- coated tumor cells. Proc. Natl. Acad. Sci. USA. 102:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalergis, A.M., and J.V. Ravetch. 2002. Inducing tumor immunity through the selective engagement of activating Fc receptors on dendritic cells. J. Exp. Med. 195:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pestka, S., C.D. Krause, and M.R. Walter. 2004. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202:8–32. [DOI] [PubMed] [Google Scholar]
- 5.Platanias, L.C. 2005. Mechanisms of type-I- and type-II-interferon- mediated signalling. Nat. Rev. Immunol. 5:375–386. [DOI] [PubMed] [Google Scholar]
- 6.Theofilopoulos, A.N., R. Baccala, B. Beutler, and D.H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307–336. [DOI] [PubMed] [Google Scholar]
- 7.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D.F. Tough. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470. [DOI] [PubMed] [Google Scholar]
- 8.Tudor, D., S. Riffault, C. Carrat, F. Lefevre, M. Bernoin, and B. Charley. 2001. Type I IFN modulates the immune response induced by DNA vaccination to pseudorabies virus glycoprotein C. Virology. 286:197–205. [DOI] [PubMed] [Google Scholar]
- 9.Van Uden, J.H., C.H. Tran, D.A. Carson, and E. Raz. 2001. Type I interferon is required to mount an adaptive response to immunostimulatory DNA. Eur. J. Immunol. 31:3281–3290. [DOI] [PubMed] [Google Scholar]
- 10.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D.F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 11.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, G.P., A.T. Bruce, K.C. Sheehan, V. Shankaran, R. Uppaluri, J.D. Bui, M.S. Diamond, C.M. Koebel, C. Arthur, J.M. White, and R.D. Schreiber. 2005. A critical function for type I interferons in cancer immunoediting. Nat. Immunol. 6:722–729. [DOI] [PubMed] [Google Scholar]
- 13.Blanco, P., A.K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 294:1540–1543. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi, T., and A. Takaoka. 2001. A weak signal for strong responses: interferon-alpha/beta revisited. Nat. Rev. Mol. Cell Biol. 2:378–386. [DOI] [PubMed] [Google Scholar]
- 15.Jonuleit, H., U. Kuhn, G. Muller, K. Steinbrink, L. Paragnik, E. Schmitt, J. Knop, and A.H. Enk. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142. [DOI] [PubMed] [Google Scholar]
- 16.Darnell, J.E., Jr., I.M. Kerr, and G.R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 264:1415–1421. [DOI] [PubMed] [Google Scholar]
- 17.Boruchov, A.M., G. Heller, M.C. Veri, E. Bonvini, J.V. Ravetch, and J.W. Young. 2005. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 115:2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daeron, M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234. [DOI] [PubMed] [Google Scholar]
- 19.Sedlik, C., D. Orbach, P. Veron, E. Schweighoffer, F. Colucci, R. Gamberale, A. Ioan-Facsinay, S. Verbeek, P. Ricciardi-Castagnoli, C. Bonnerot, et al. 2003. A critical role for Syk protein tyrosine kinase in Fc receptor-mediated antigen presentation and induction of dendritic cell maturation. J. Immunol. 170:846–852. [DOI] [PubMed] [Google Scholar]
- 20.Tassiulas, I., X. Hu, H. Ho, Y. Kashyap, P. Paik, Y. Hu, C.A. Lowell, and L.B. Ivashkiv. 2004. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat. Immunol. 5:1181–1189. [DOI] [PubMed] [Google Scholar]
- 21.Ravetch, J.V. 2002. A full complement of receptors in immune complex diseases. J. Clin. Invest. 110:1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stetson, D.B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity. 25:373–381. [DOI] [PubMed] [Google Scholar]
- 23.van Boxel-Dezaire, A.H., M.R. Rani, and G.R. Stark. 2006. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 25:361–372. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann, F., S. Jung, and D.R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19:71–82. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald, K.P., D.J. Munster, G.J. Clark, A. Dzionek, J. Schmitz, and D.N. Hart. 2002. Characterization of human blood dendritic cell subsets. Blood. 100:4512–4520. [DOI] [PubMed] [Google Scholar]
- 26.Weng, W.K., and R. Levy. 2003. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21:3940–3947. [DOI] [PubMed] [Google Scholar]
- 27.Wu, J., J.C. Edberg, P.B. Redecha, V. Bansal, P.M. Guyre, K. Coleman, J.E. Salmon, and R.P. Kimberly. 1997. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Invest. 100:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charo, I.F., and R.M. Ransohoff. 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354:610–621. [DOI] [PubMed] [Google Scholar]
- 29.McGaha, T.L., B. Sorrentino, and J.V. Ravetch. 2005. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 307:590–593. [DOI] [PubMed] [Google Scholar]
- 30.Kortylewski, M., M. Kujawski, T. Wang, S. Wei, S. Zhang, S. Pilon-Thomas, G. Niu, H. Kay, J. Mule, W.G. Kerr, et al. 2005. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11:1314–1321. [DOI] [PubMed] [Google Scholar]
- 31.Ho, H.H., and L.B. Ivashkiv. 2006. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J. Biol. Chem. 281:14111–14118. [DOI] [PubMed] [Google Scholar]
- 32.Tan, T.T., and L.M. Coussens. 2007. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 19:209–216. [DOI] [PubMed] [Google Scholar]
- 33.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhodapkar, K.M., J. Krasovsky, B. Williamson, and M.V. Dhodapkar. 2002. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiner, L.M., M.V. Dhodapkar, and S. Ferrone. 2007. Monoclonal antibodies for cancer immunotherapy. Lancet. In press. [DOI] [PMC free article] [PubMed]
- 36.Dhodapkar, K.M., and M.V. Dhodapkar. 2005. Recruiting dendritic cells to improve antibody therapy of cancer. Proc. Natl. Acad. Sci. USA. 102:6243–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micklem, K.J., W.P. Stross, A.C. Willis, J.L. Cordell, M. Jones, and D.Y. Mason. 1990. Different isoforms of human FcRII distinguished by CDw32 antibodies. J. Immunol. 144:2295–2303. [PubMed] [Google Scholar]
- 38.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesinski, G.B., S.V. Kondadasula, T. Crespin, L. Shen, K. Kendra, M. Walker, and W.E. Carson III. 2004. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J. Natl. Cancer Inst. 96:1331–1342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.