Abstract
We defined the function of type I interferons (IFNs) in defense against reovirus strain type 1 Lang (T1L), which is a double-stranded RNA virus that infects Peyer's patches (PPs) after peroral inoculation of mice. T1L induced expression of mRNA for IFN-α, IFN-β, and Mx-1 in PPs and caused localized intestinal infection that was cleared in 10 d. In contrast, T1L produced fatal systemic infection in IFNαR1 knockout (KO) mice with extensive cell loss in lymphoid tissues and necrosis of the intestinal mucosa. Studies of bone-marrow chimeric mice indicated an essential role for hematopoietic cells in IFN-dependent viral clearance. Dendritic cells (DCs), including conventional DCs (cDCs), were the major source of type I IFNs in PPs of reovirus-infected mice, whereas all cell types expressed the antiviral protein Mx-1. Neither NK cells nor signaling via Toll-like receptor 3 or MyD88 were essential for viral clearance. These data demonstrate a requirement for type I IFNs in the control of an intestinal viral infection and indicate that cDCs are a significant source of type I IFN production in vivo. Therefore, innate immunity in PPs is an essential component of host defense that limits systemic spread of pathogens that infect the intestinal mucosa.
Innate defenses at mucosal surfaces are likely of primary importance in protection against the large variety of potentially infectious pathogens to which animal hosts are constantly exposed (1). In the intestine, the first line of defense includes nonimmune factors, such as gastric acid, mucus, antimicrobial peptides, and commensal bacteria. A second line of defense occurs when pathogens contact or invade host cells and elicit the production of cytokines and chemokines, which in turn induce an influx of immune cells that effect pathogen clearance. Although much effort is currently being invested in understanding innate defenses against commensal and pathogenic bacteria, including how intestinal epithelial cells, underlying immune cells, and intercalating “intraepithelial” DCs sense and respond to these microbes, little is known about innate immune responses in the intestine evoked by viruses.
Type I interferons (IFNs) are critical mediators of innate immunity and limit disease caused by many viruses (2, 3). Type I IFNs consist of 15 subtypes of IFN-α, 1 subtype of IFN-β, and 1 subtype of IFN-ω, all sharing a common type I IFN receptor (4). Type I IFNs are produced by virtually all virus-infected cells in response to intracellular viral dsRNA, which is detected by the RNA helicases, retinoic acid–inducible gene (RIG) I, melanoma differentiation-associated gene 5 (Mda-5), and, possibly, protein kinase dependent on RNA (PKR) (5, 6). Plasmacytoid DCs (pDCs) produce type I IFNs in response to exogenous viral RNA or DNA signaling via Toll-like receptors (TLRs) TLR7 or TLR9 (6). Many cells, including conventional DCs (cDCs), are activated by viral RNA or the synthetic RNA analogue polyI:polyC to produce type I IFNs via TLR3-dependent and -independent mechanisms (6). Studies using genetically deficient strains of mice indicate that the contribution of each of these mechanisms (and those that are still unknown), as well as the cells producing type I IFNs during viral infections in vivo, depend on specific viral products and the pathogenesis of the particular viral infection (6).
Production of type I IFNs is dependent on an autocrine feedback mechanism involving signaling via the common type I IFN receptor that consists of the IFNαR1 and IFNαR2 chains (7). Signaling through this receptor induces the transcription of a broad array of type I IFN-stimulated genes (ISGs) (7), such as Mx-1, PKR, and the 2′-5′ oligoadenylate synthetases, which are important for inducing an antiviral state (4, 5, 7). Furthermore, type I IFNs can activate additional components of innate immunity by promoting cytotoxicity of NK cells (8), stimulating IL-15 production to induce NK cell proliferation (9), and, at low doses, enhancing release of IL-12 by DCs, which in turn can stimulate IFN-γ production by T cells and NK cells (10). Type I IFNs are produced in the intestine during certain viral infections (11); however, the extent to which type I IFNs are required for viral clearance at this site is not clear.
Reovirus is a nonenveloped, double-stranded RNA-containing virus that replicates in the cytoplasm of host cells (12). There are three reovirus serotypes that vary in certain pathogenic properties, including growth in the intestine, pathway of systemic spread, and end-organ tropism (13). Of the many reovirus strains characterized, strain type 1 Lang (T1L) is an ideal virus for use in studies of mucosal immune responses. After peroral inoculation of adult mice, reovirus T1L infects the follicular-associated epithelium (FAE) overlying the Peyer's patches (PPs) of the small intestine (14) and induces protective immunity via IgA secretion and generation of reovirus-specific CD4+ and CD8+ T cells (15–19). In adult mice, T1L is capable of spreading to mesenteric LNs (MLNs), but not to systemic sites. Intestinal infection of wild-type (WT) mice is cleared within 10 d.
In this study, we determined whether type I IFNs are capable of protecting adult mice against reovirus T1L infection. In contrast to WT mice, mice lacking IFNαR1 (2) developed lethal infection with T1L. In studies of BM chimeric mice, we uncovered an essential role for BM cells in mediating the type I IFN-dependent clearance of T1L infection and survival. Remarkably, DCs, including CD11chi cDCs, rather than infected epithelial cells, are the major producers of type I IFNs during reovirus infection. These findings provide the first evidence that type I IFNs are required for clearance of an intestinal viral infection and offer new insights into the role of innate immune response cytokines in control of viral dissemination from the site of entry into the host.
RESULTS
Type I IFNs are induced in PPs during reovirus infection
To determine whether type I IFNs are induced in PPs during intestinal infection by reovirus T1L, mRNAs encoding IFN-α, IFN-β, and Mx-1, which is a prototype ISG (20), were measured by reverse transcription (RT) and quantitative polymerase chain reaction (qPCR) from whole PPs isolated before and after peroral inoculation of adult WT mice with T1L. The mRNAs encoding IFN-α and -β were up-regulated 5- and 22-fold, respectively, by 20 h (Fig. 1), and these levels persisted for at least 96 h after infection (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061587/DC1). Mx-1 mRNA was also significantly elevated over this time course, indicating the functional production of type I IFNs in PPs after intestinal infection by T1L.
Figure 1.
Type I IFN production in PPs after infection with reovirus T1L. WT mice were inoculated perorally with T1L, PPs were resected at the indicated times, and total RNA was purified. IFN-α (open bars), IFN-β (closed bars), and Mx-1 (striped bars) mRNAs were quantified by RT-qPCR. Levels of target mRNAs were normalized to GAPDH mRNA as an endogenous control. Results are presented as the mean mRNA levels obtained from three mice at each time point. Error bars indicate the SD.
Type I IFNs mediate protection against fatal reovirus infection of adult mice
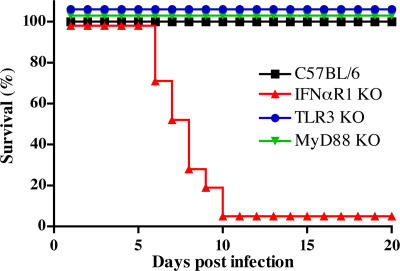
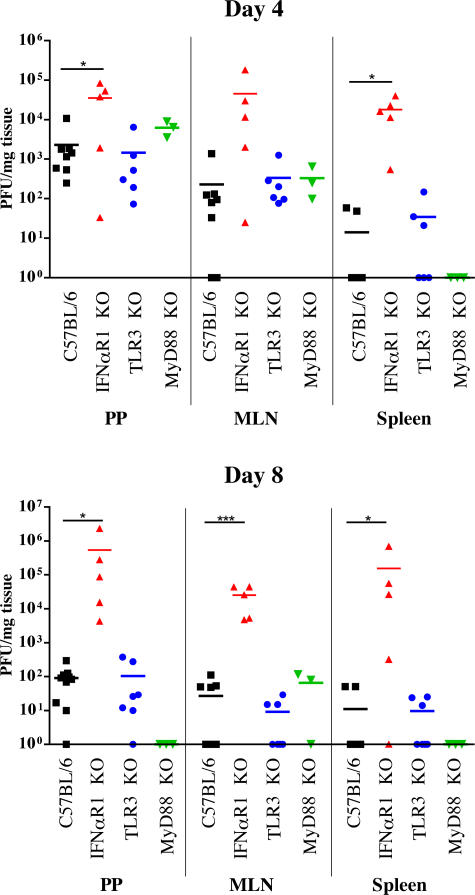
To determine whether type I IFNs function in viral clearance from intestinal sites, C57BL/6 WT and IFNαR1 KO mice (2) were inoculated perorally with reovirus T1L and monitored for survival and viral load. IFNαR1 KO mice succumbed to infection between 7–10 d post-inoculation, whereas WT mice survived (Fig. 2). Identical results were obtained after infection of WT and IFNαR1 KO mice on a 129 background (not depicted). Death of IFNαR1 KO mice was associated with overwhelming local and systemic infection (Fig. 3). On day 4 after inoculation, T1L was detected in PPs and MLNs of both WT and IFNαR1 KO mice, as well as in spleens of IFNαR1 KO mice. On day 8, WT mice had virtually cleared the infection, with only low titers remaining in mucosal tissues, whereas IFNαR1 KO mice had high viral titers in PPs, MLNs, and spleen (Fig. 3). On day 8, virus also was detected in heart, lung, liver, and brain of IFNαR1 KO, but not WT, mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061587/DC1).
Figure 2.
Survival after infection with reovirus T1L. C57BL/6 (black squares; n = 50), IFNαR1 KO (red triangles; n = 21), TLR3 KO (blue circles; n = 15), and MyD88 KO (green triangles; n = 15) mice were inoculated perorally with T1L and monitored daily for survival for 20 d. The difference in survival between C57BL/6 and IFNαR1 KO mice after reovirus T1L infection is highly significant (P < 0.0001) using a Wilcox log-rank test.
Figure 3.
Viral titers in mouse organs after reovirus T1L infection. C57BL/6 (black squares), IFNαR1 KO (red triangles), TLR3 KO (blue circles), and MyD88 KO (green triangles) mice were inoculated perorally with T1L and killed at the indicated times. Viral titers in homogenates of the organs shown were determined by plaque assay. Each data point represents viral titer in a tissue sample from a single mouse. Horizontal bars indicate mean viral titers. *, P < 0.05; ***, P < 0.001, as determined using a one-tailed Student's t test.
Examination of tissue sections from uninfected and infected WT and IFNαR1 KO mice revealed striking differences in end-organ damage between the two mouse strains. On day 8 after inoculation, all tissues from WT mice appeared normal and were indistinguishable from uninfected WT or IFNαR1 KO mice (Fig. 4 A and not depicted). In contrast, PPs and all other lymphoid organs examined from infected IFNαR1 KO mice at this interval showed severe diffuse necrosis with an overall pale appearance and loss of structural markings (Fig. 4 A). Nuclear staining was diminished in those tissues, suggesting elimination of cells, including lymphoid cells. Sections from liver showed patchy areas of moderate to severe necrotizing hepatitis containing a mixed inflammatory cell infiltrate. Areas of necrotizing enteritis were found in both small and large intestines, with blunted villi and degeneration of crypt epithelial cells. Interestingly, nonlymphoid organs, including heart, lung, and brain of IFNαR1 KO mice, appeared normal without substantial changes in tissue architecture or cellular infiltration, even though virus was detected at those sites (Fig. S2 and not depicted).
Figure 4.
Histopathology of tissues from IFNαR1 KO mice infected with reovirus T1L. IFNαR1 KO mice were either inoculated perorally with T1L or mock infected. 8 d after inoculation, PP, spleen, and liver were resected, sectioned, and stained with either hematoxylin and eosin (A) or mAb specific for the T1L σ1 protein (B, red). Cell nuclei in B were stained using Hoechst (blue). Bars: (white) 750 μm; (black) 150 μm.
In addition to the pathologic changes, large amounts of viral antigen were detected in lymphoid organs and within the inflammatory cell infiltrates in the liver of IFNαR1 KO mice (Fig. 4 B). Furthermore, we observed considerable tissue staining for the activated form of caspase-3 in lymphoid organs showing cell loss, indicating that at least some component of the cell death in those tissues was attributable to apoptosis (not depicted). Cultures of isolated splenic tissue from reovirus-infected IFNαR1 KO mice grew multiple bacterial species, including Proteus mirabilis and Enterococcus species, indicating systemic infection with enteric bacteria complicated initial infection with reovirus. Thus, the most likely cause of death in these mice was sepsis after necrotizing enterocolitis. These data demonstrate that type I IFNs are essential for control of intestinal infection with reovirus T1L and prevention of its spread to systemic sites.
Neither TLR3− nor MyD88-dependent signaling is crucial for clearance of reovirus T1L infection
TLRs are pattern-recognition receptors that engage discrete components of microbial organisms and activate innate immune response signaling pathways (21). Of particular relevance to this study is the observation that dsRNA is recognized by TLR3, which can signal to elicit production of type I IFNs (22). To determine whether TLR3 is responsible for type I IFN production during intestinal infection by reovirus T1L, TLR3 KO mice were inoculated perorally with T1L and monitored for survival and viral load. Like WT C57BL/6 mice, TLR3 KO mice survived T1L infection (Fig. 2). Concordantly, viral loads in PPs, MLNs, and spleen of TLR3 KO mice were similar to those in WT mice (Fig. 3), indicating that TLR3 is dispensable for protection against lethal T1L infection after peroral inoculation. Similarly, we found that mice lacking MyD88, which is an adaptor protein used by all known TLRs except TLR3 (23), survived reovirus T1L infection, with clearance of virus indistinguishable from that of WT or TLR3 KO mice (Figs. 2 and 3). We conclude that neither TLR3- nor MyD88-dependent TLR signaling pathways are required for innate protection against fatal infection by reovirus T1L. Furthermore, because MyD88 is required for IL-1 and IL-18 receptor signaling (24), these data also eliminate a nonredundant contribution from these receptors to reovirus clearance.
BM cells from WT mice reverse the susceptibility of IFNαR1 KO mice to reovirus T1L infection
Because reovirus T1L productively infects epithelial cells in the FAE overlying PPs (14), we hypothesized that production of type I IFNs by infected epithelial cells protects against the spread of virus within permissive cells in the epithelium. To test this hypothesis, we generated chimeric mice with BM cells derived from WT C57BL/6 mice transferred into irradiated IFNαR1 KO hosts and BM cells derived from IFNαR1 KO mice into irradiated WT C57BL/6 recipients. Chimeric mice were inoculated perorally with reovirus T1L and monitored for survival and viral load. Unexpectedly, IFNαR1 KO mice reconstituted with WT BM cells survived infection (Fig. 5). In sharp contrast, WT mice reconstituted with IFNαR1 KO BM cells were more susceptible to infection, with 85% of these mice succumbing by day 10 (Fig. 5). On day 8 after inoculation, viral loads in PPs and MLNs from WT mice reconstituted with IFNαR1 KO BM cells were similar to those in IFNαR1 KO mice (Fig. 6). In addition, reovirus T1L was detected in the spleen of these mice, indicating systemic spread identical to that observed after infection of intact IFNαR1 KO mice (Fig. 6). However, IFNαR1 KO mice reconstituted with WT BM cleared infection with kinetics similar to WT mice (Fig. 6). As controls, WT mice reconstituted with WT BM cells and IFNαR1 KO mice reconstituted with IFNαR1 KO BM cells displayed survival outcomes and viral loads similar to those after T1L infection of nonirradiated WT and IFNαR1 KO mice, respectively (Figs. 5 and 6). These results indicate that hematopoietic cells must be capable of responding to type I IFNs to control T1L infection. Moreover, type I IFN production and responsiveness of epithelial cells (or other stromal cells) is not sufficient to limit local and systemic spread of reovirus T1L.
Figure 5.
Survival of BM-chimeric mice after infection with reovirus T1L. C57BL/6 and IFNαR1 KO mice were irradiated and reconstituted with BM from either C57BL/6 or IFNαR1 KO mice. Chimeric mice were inoculated perorally with reovirus T1L and monitored daily for survival for 20 d. **, P < 0.01; ***, P < 0.0001, as determined using the log-rank test.
Figure 6.
Viral titers in organs of BM chimeric mice after reovirus T1L infection. C57BL/6 mice (open black squares), IFNαR1 KO mice (open red triangles), IFNαR1 KO mice reconstituted with C57BL/6 BM cells (open green circles), and C57BL/6 mice reconstituted with IFNαR1 KO BM cells (open blue diamonds) were inoculated perorally with T1L and killed at the indicated times. Viral titers in homogenates of the organs shown were determined by plaque assay. Each data point represents viral titer in a tissue sample from a single mouse. Horizontal bars indicate mean viral titers. *, P < 0.05; **, P < 0.001, as determined using a one-tailed Student's t test.
NK cells are not required for clearance of reovirus T1L
NK cells play an essential role in the early response to many, but not all viral infections (25). These cells are particularly important components of host defense against members of the herpesvirus family, several of which have developed strategies to attenuate NK cell killing (26). Given this central role for NK cells in innate immunity to viral infection and the capacity of type I IFNs to mediate NK cell activation, killing, and survival, we next sought to determine whether NK cells are involved in defense against reovirus T1L infection. We first quantified NK cells in PPs after peroral inoculation with T1L. In the absence of infection, PPs did not contain detectable numbers of NK cells (Fig. 7), similar to the paucity of NK cells in peripheral LNs observed in a previous study (27). However, after peroral inoculation with T1L, NK cells infiltrated PPs (Fig. 7, A and B). NK cell infiltration was similar in WT mice and IFNαR1 KO mice (Fig. 7), suggesting that NK cell recruitment is not dependent on type I IFNs.
Figure 7.
NK cells infiltrate PPs after infection with reovirus T1L. (A) Representative FACS plots showing CD3− cells from PPs of uninfected mice and mice 2 d after peroral inoculation with T1L. (B) CD3−NK1.1+DX5+ cells in PPs from C57BL/6 mice (closed bars) or IFNαR1 KO mice (striped bars) at the indicated times after inoculation were quantified using FACS analysis. Results are presented as the mean percentage of CD3−NK1.1+DX5+ cells in PPs from three independent experiments and at least three mice per group. Error bars indicate the SD. (C) Survival after infection with reovirus T1L. C57BL/6 mice (black squares), C57BL/6 mice depleted of NK cells (open circles, n = 6), and RAG KO mice (open triangles, n = 13) were inoculated perorally with T1L and monitored daily for survival for 20 d.
To determine whether NK cells serve a function in defense against reovirus T1L infection, we depleted NK cells in WT C57BL/6 mice by systemic injection of anti-asialo GM1 antibody (Ab) (28). Mice depleted of NK cells were not more susceptible to reovirus T1L than undepleted mice (Fig. 7 C). NK cell-depleted mice survived T1L infection, and viral loads in organs resected from NK cell-depleted mice did not differ from those in WT mice (not depicted). Therefore, activation of NK cells by type I IFNs is unlikely to mediate clearance of intestinal reovirus T1L infection and does not explain the requirement for BM-derived cells to respond to type I IFN in viral clearance. To evaluate a role for B and T cells in this process, RAG KO mice were infected with reovirus T1L and monitored for survival. These mice survived reovirus infection, indicating that it is unlikely that direct or indirect effects of type I IFN on B and T cells are sufficient for reovirus clearance (Fig. 7 C).
DCs produce type I IFNs, which induce expression of the antiviral protein Mx-1 in PPs
To identify the cellular source of type I IFNs, and to define the cells responding to type I IFNs in PPs during reovirus T1L infection, we quantified the expression of IFN-α, IFN-β, and Mx-1 mRNAs in cell populations isolated from PPs of infected mice. Surprisingly, epithelial cells isolated from PPs of infected mice did not express appreciable levels of IFN-α or -β mRNAs either before or after reovirus T1L infection (Fig. 8). In contrast, both IFN-α and -β mRNAs were found in the nonepithelial cell fraction (total cells), which contains 98% BM-derived cells (Fig. 8). Furthermore, after infection by T1L, both IFN-α and IFN-β mRNAs were highly expressed in PP cell populations enriched for CD11c+ cells, whereas cell populations depleted of CD11c+ cells did not express type I IFNs after infection (Fig. 8). To confirm these data, PP cells from infected and uninfected mice were purified to >97% homogeneity by flow cytometry. Both cDCs (7AAD−, CD11chigh, and B220−) and pDCs (7AAD−, CD11cint, and B220+) expressed high levels of IFN-α and -β mRNAs after infection (Fig. 8 B). In contrast, Mx-1 mRNA was found in all cell fractions, including epithelial cells (Fig. 8). Collectively, these data suggest that CD11c+ pDCs and cDCs are the primary producers of type I IFNs during reovirus infection, which in turn induce antiviral proteins in many cell types, including epithelial cells.
Figure 8.
Type I IFN production in PPs by DCs after infection with reovirus. PPs from naive (open bars) or reovirus-infected BALB/c mice (2 d after infection; striped bars) were resected and fractionated to yield epithelial cells, total PP cells, MACS-enriched CD11c+ cells, and cells depleted of CD11c+ (A) or epithelial cells (Epi), total PP cells, MACS-enriched CD11c+ cells, cells depleted of CD11c+, FACS-sorted cDCs (CD11c+, B220−), and FACS-sorted pDCs (CD11c+ B220+) (B). Total RNA was purified, and IFN-α, IFN-β, and Mx-1 mRNAs were quantified using RT-qPCR. Levels of target mRNAs were normalized to GAPDH mRNA as an endogenous control. Bars are the mean of two to three replicates, and dots represent each replicate. Results are representative of at least two independent experiments.
DISCUSSION
This study demonstrates that type I IFNs are required for control of intestinal infection by reovirus T1L. This control is not dependent on either TLR-dependent signaling pathways or activity of NK cells. DCs, including cDCs, are the primary producers of type I IFNs during reovirus infection. Epithelial cells or other stromal cells do not appear to be major contributors to type I IFN production, and responsiveness by these non–BM-derived cells is not sufficient for viral control. These data support the hypothesis that reovirus T1L induces non–TLR-dependent type I IFN production by DCs, which results in direct antiviral effects on a variety of cell types, including DCs, epithelial cells, and possibly other stromal cells.
Type I IFNs function in defense against many systemic viral infections in mice, including those caused by encephalomyocarditis virus, lymphocytic choriomeningitis virus, Semliki Forest virus, vaccinia virus, and vesicular stomatitis virus (2, 3). Type I IFNs also protect against myocarditis caused by some strains of reovirus (29, 30). In fact, the capacity of different reovirus strains to damage cardiac tissue in mice correlates inversely with the capacity to induce IFN-β in primary cultures of cardiac myocytes (29). In concordance with these observations, NF-κB–induced production of IFN-β in the murine heart during reovirus infection in vivo limits viral replication, apoptosis, and clinical disease (31).
Although reovirus T1L induces IFN-β in some epithelial cell lines (32), before this study it was not known whether type I IFNs are produced in intestinal tissues after reovirus infection in vivo or if type I IFNs contribute to host defense against any intestinal virus infection. Evidence accumulated thus far suggests that type I IFNs have a limited role in the intestine. For example, IFNαR1 KO mice are not more susceptible to oral infection by rotavirus (33), which is a member of the Reoviridae family that is closely related to reovirus, or murine norovirus 1 (34). Interestingly, some viruses that infect mucosal tissues have evolved mechanisms to inhibit either the production or effects of type I IFNs (20). For example, rotavirus nonstructural protein 1 (NSP1) blocks type I IFN production by binding to the IFN-inducing transcription factor, IRF3, and targeting it for degradation (35). Therefore, the requirement for type I IFNs for clearance of intestinal reovirus infection was not an expected finding.
Our experiments indicate that BM-derived cells must be capable of responding to type I IFNs to confer protection against intestinal reovirus T1L infection. These results are surprising for two reasons. First, reovirus T1L acutely infects only epithelial cells in the PP FAE (14) and, possibly, PP-associated villi (36). This strict tissue tropism may be attributable to the expression of junctional adhesion molecule-A, which serves as a receptor for reovirus binding and infection (37). Therefore, our finding that WT mice reconstituted with IFNαR1 KO BM do not clear infection indicates that innate control of reovirus infection does not occur solely at the level of the epithelium. Thus, autocrine effects of type I IFNs produced by infected epithelial cells do not appear to facilitate protection against spread of the virus to neighboring permissive, but uninfected, cells. Second, WT BM-derived cells can rescue IFNαR1 KO mice from lethal reovirus infection. This observation indicates that responsiveness to type I IFNs by BM-derived cells is sufficient for innate protection.
We considered two possible explanations for the essential role of BM cells in conferring type I IFN-mediated protection against fatal reovirus disease. First, type I IFNs produced in PPs during reovirus infection might act by recruiting and activating BM-derived effector cells (7, 20). To address this possibility, we performed experiments to determine whether type I IFNs act on NK cells to enhance their cytotoxicity as a potential mechanism of innate protection. NK cells activated by type I IFNs play a role in innate immunity against several viruses, such as murine cytomegalovirus (9, 38). In the absence of infection, LNs, including PPs, contain very few NK cells (Fig. 7 and reference (27)). After infection by reovirus T1L, NK cells were recruited to PPs of both WT and IFNαR1 KO mice. However, effective depletion of NK cells had no effect on viral clearance. Therefore, it seems unlikely that the primary effect of type I IFNs in PPs is mediated by activation of NK cell killing of infected cells. In addition, RAG KO mice survive peroral reovirus T1L infection, suggesting that B and T cells are not involved in the early clearance of reovirus (Fig. 7 C).
Second, BM-derived cells might be the primary source of type I IFNs and confer protection via autocrine type I IFN signaling and induction of antiviral ISGs. To address this possibility, we fractionated PP cells before and after infection and quantified type I IFN mRNA levels in different cell types by RT-qPCR. Surprisingly, we found that epithelial cells are not a substantial source of type I IFN during infection. In contrast, CD11c+ DCs, including both cDCs and pDCs, were responsible for the majority of type I IFNs produced. Although there is precedent for local expression of type I IFNs in a model of intestinal virus infection (11), the responsible cell type has not been identified. In addition, whereas pDCs are an important source of type I IFNs (6), there is little information about the capacity of cDCs to produce type I IFN during viral infections in vivo. Therefore, our data highlight a new role for DCs in intestinal lymphoid tissues in the production of type I IFNs during an intestinal virus infection. We find it noteworthy that pDCs from PPs produce little detectable type I IFNs after stimulation in vitro with either CpG oligodeoxynucleotides or influenza virus (unpublished data). Therefore, type I IFN production by pDCs in PPs is limited in steady-state conditions, but can be induced under conditions of viral infection, possibly by a change in the local cytokine environment, recruitment of new pDCs from the peripheral blood, or both. We have begun to determine the relative role for pDCs and cDCs in the production of type I IFN in reovirus infection. In preliminary studies, depletion of pDCs in C57BL/6 mice using 120G8 Ab (although only to 60% of untreated animals) did not affect viral clearance (unpublished data). Therefore, it is possible that type I IFN production by cDCs may be sufficient for protection, although this requires further study.
Our data suggest that type I IFNs produced largely by DCs have direct antiviral effects on surrounding cells. We found elevated levels of Mx-1 mRNA in all cell fractions from infected PPs, including epithelial cells. Moreover, in the absence of type I IFN receptor signaling, there was widespread reovirus antigen throughout lymphoid regions of PPs and spleen. Therefore, it is likely that a major effect of type I IFNs produced in PPs is to induce antiviral proteins in both BM-derived and stromal cells, resulting in limitation of local viral replication and systemic dissemination. Local production of type I IFNs might also activate innate effector cells, resulting in clearance of reovirus infection, or act on DCs to enhance cross-presentation of viral proteins to CD8+ T cells (39). Thus, our findings suggest that innate immune responses in PPs are important in containing replication and dissemination of microbial pathogens that use M cells to invade the host.
An important conclusion from our study is that TLR-mediated signaling is dispensable for clearance of intestinal reovirus T1L infection. TLRs are essential for protection against several viral pathogens (6, 40). Of the many TLRs identified, TLR3 is of particular interest to us, as this pattern-recognition receptor recognizes genomic dsRNA of reovirus T1L in vitro (22) and is required for type I IFN production during murine cytomegalovirus infection (38). In addition, TLR3 can promote cross priming during some viral infections in which apoptotic bodies containing dsRNA appear to activate DCs via TLR3 within endosomes (41). TLR3 uses the adaptor protein TRIF in downstream signaling (23), whereas other TLRs, particularly TLR7 and TLR9, which respond to viral nucleic acids by producing type I IFNs, use MyD88 as an essential signaling adaptor (21). We found that both TLR3 KO mice and MyD88 KO mice survived T1L infection and cleared virus with kinetics similar to that of WT mice, indicating that neither TLR3- nor MyD88-dependent TLRs are required for development of innate immunity to reovirus T1L in the intestine. Absence of a role for TLR3 in clearance of reovirus, which is a model dsRNA virus, is surprising. However, our results are consistent with a previous study in which TLR3 deficiency did not enhance the virulence of reovirus strain type 3 Dearing after intracranial inoculation of newborn mice (42). Although additional studies are necessary to elucidate the major pathogen-recognition mechanisms during reovirus infection, data from our experiments suggest that reovirus stimulates induction of innate immune responses by TLR-independent mechanisms, such as the detection of intracellular dsRNA by the RNA helicases RIG-I and Mda-5 (5, 6, 40).
Our findings indicate an essential function for type I IFNs in protection against reovirus infection of the murine intestine. Furthermore, they establish a primary role for hematopoietic cells in type I IFN-dependent innate immunity against this virus and demonstrate that DCs are the main producers of these antiviral cytokines. These observations challenge the notion that autocrine production and effects of type I IFNs by infected cells are sufficient for control of viral replication. Finally, we show that TLRs are not required for detection or clearance of intestinal infection by reovirus T1L. Together, these data enhance an understanding of mucosal immunity to viral infections and suggest that manipulating the type I IFN response at the level of the mucosa might augment strategies to prevent mucosal viral infections and diminish viral spread to systemic sites.
MATERIALS AND METHODS
Mice.
C57BL/6, BALB/c, and 129 mice were purchased from the National Cancer Institute. Congenic (CD45.1) C57BL6/SJL mice (used for the reconstitution experiments) and RAG KO mice were purchased from Taconic or Jackson ImmunoResearch Laboratories. IFNαR1 KO mice on either a C57BL/6 or a 129 background (2, 3), TLR3 KO mice (22) (a gift of R. Flavell, Yale University School of Medicine, New Haven, CT), and MyD88 KO mice (24) were bred and housed in the animal facility at the National Institutes of Health. All mice were maintained using pathogen-free conditions in accordance with institutional guidelines for animal welfare. Animals were used for infectivity studies between 7 and 12 wk of age.
Chimeric mice were obtained by irradiating mice with 950 RAD, followed by transplantation of 3 × 106 BM cells of the relevant donor strain on the same day. Mice were administered water supplemented with antibiotics (trimethoprim/sulfamethoxazole) for 5 wk after transplantation. Reconstitution of donor BM was monitored by analysis of congenic blood leukocytes 7–10 wk after transplantation and before use in infectivity experiments.
Virus and viral titers.
Reovirus T1L is a laboratory stock. Purified virion preparations were made using second-passage L-cell lysate stocks of twice plaque-purified reovirus, as previously described (14, 43). The concentration of viral particles was calculated from protein concentration (44), and the concentration of infectious virus was determined by plaque assay (45). Mice were inoculated perorally with 1–4 × 108 PFU of reovirus T1L in 200 μl borate-buffered saline (0.13 M NaCl, 0.25 mM CaCl2, 1.5 mM MgCl2 × 6H20, 20 mM H3BO3, and 0.15 mM Na2B4O7 × 10H2O) containing 5 g/L gelatin. Viral titers in organs from infected mice were determined from sonicated tissue samples by plaque assay (45). Weights of organs were measured before the assay, and PFU were calculated per mg of tissue.
RT-qPCR.
PPs were harvested at various times after inoculation and stored in RNAlater (Ambion). PPs were homogenized, and total RNA was extracted using an RNeasy mini kit (QIAGEN). Quality and quantity of RNA was assessed using a Bioanalyzer (Agilent Technologies, Inc.). RNA was reverse transcribed to cDNA using Superscript III first-strand synthesis (Invitrogen). PCR was performed using Taqman and an Applied Biosystems 7900HT. IFN-α mRNAs (all genes) were detected using SYBR GREEN RT-PCR (10). IFN-β and Mx-1 FAM-labeled probe and primers were obtained from Applied Biosystems. The mRNAs were detected by following the manufacturer's instructions (Assays-on-Demand). For quantitation, ΔCT was obtained by comparing cycle number (CT) required to reach a defined threshold value for target gene versus an endogenous control, GAPDH. The relative amount of mRNA was calculated as 1/(2ΔCT).
Cell preparation.
PPs were harvested and treated with 145 μg/ml DTT (Sigma-Aldrich) and 5 mM EDTA at 37°C for 10 min. Epithelial cells were removed by washing several times with HBSS. Single-cell suspensions were obtained by forcing the tissue through a cell strainer (Falcon). MACS enrichment was performed using CD11c (N418) microbeads (Miltenyi Biotech).
Antibodies, cell staining, and cell depletion.
Cells were incubated with 7AAD (Sigma-Aldrich) to facilitate detection of dead cells and anti–mouse CD16/CD32 Ab (2.4G2) to block Fc receptors (FcγRIII/II) before staining. Cell-surface staining was performed using anti-CD3ɛ (145-2C11), anti-NK1.1 (PK136), and anti-CD49b (DX5). Negative controls were performed using the corresponding isotype-matched Abs. All Abs were purchased from BD Biosciences. Stained cells were detected using a FACSCalibur flow cytometer (BD Biosciences). For FACS sorting experiments, anti-CD11c (HL3), anti-B220 (RA3-6B2) was used for staining, and stained cells were sorted using a FACSAria flow cytometer (BD Biosciences). The T1L σ1 structural protein was detected by immunofluorescence in tissue sections using murine mAb 5C6 (46). Biotinylated Ab specific for activated caspase 3 was purchased from BD Biosciences. NK cells were depleted by inoculating 50 μl of rabbit anti-asialo GM1 Ab (Wako Chemicals) i.p. every fourth day starting 2 d before peroral inoculation with virus. pDCs were depleted by inoculating 0.5 mg of Ab 120G8 (Schering-Plough Laboratory of Immunological Research, Dardilly, France) i.p. every second day during the course of the experiment.
Immunohistochemistry and tissue staining.
PPs, liver, and spleen were frozen in OCT embedding medium (Sakura Fineteck). Frozen sections (8 μm thick) were fixed in acetone at −20°C, and immunofluorescence staining was performed using the tyramide amplification method (Invitrogen; T20932, T20935) as previously described (47). Nuclei were identified by staining sections with Hoechst 33258 (Sigma-Aldrich) before mounting with Fluoromount G mounting media (Southern Biotechnology Associates, Inc.). Tissues were imaged using an Axioplan 2 fluorescence microscope (Carl Zeiss MicroImaging, Inc.). Sections also were stained with hematoxylin and eosin according to standard protocols.
Statistical analysis.
Statistical significance of differences was determined using unpaired, one-tailed, or two-tailed Student's t tests, using Prism 4 software (GraphPad Software, Inc.) or the log-rank test.
Online supplemental material.
A time course of induction of mRNA for IFN-α, IFN-β, and Mx-1 in PPs after reovirus T1L infection is shown in Fig. S1. The viral load in multiple organs from WT and IFNαR1 KO mice 4 and 8 d after reovirus T1L infection is shown in Fig. S2. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061587/DC1.
Supplemental Material
Acknowledgments
We thank members of our laboratories for many useful discussions and Dr. M. Eckhaus, National Institutes of Health, for assistance in interpreting the histopathology.
This research was supported by the Swedish Research Council (C. Johansson), Public Health Service award AI50080, and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt-Ingram Cancer Center and DK20593 for the Vanderbilt Diabetes Research and Training Center.
The authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; cDC, conventional DC; FAE, follicular-associated epithelium; ISG, IFN-stimulated gene; Mda, melanoma differentiation-associated gene; MLN, mesenteric LN; pDC, plasmacytoid DC; PKR, protein kinase dependent on RNA; PP, Peyer's patch; RIG-I, retinoic acid–inducible gene; T1L, type 1 Lang; TLR, Toll-like receptor.
C. Johansson's present address is Dept. of Respiratory Medicine, National Heart and Lung Institute, Imperial College, London, W2 1PG, England, UK.
References
- 1.Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. [DOI] [PubMed] [Google Scholar]
- 2.Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 3.Hwang, S.Y., P.J. Hertzog, K.A. Holland, S.H. Sumarsono, M.J. Tymms, J.A. Hamilton, G. Whitty, I. Bertoncello, and I. Kola. 1995. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc. Natl. Acad. Sci. USA. 92:11284–11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theofilopoulos, A.N., R. Baccala, B. Beutler, and D.H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307–336. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Sastre, A., and C.A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 312:879–882. [DOI] [PubMed] [Google Scholar]
- 6.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131–137. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, C.J., J.A. Trapani, and R.W. Johnstone. 2001. Mechanisms of interferon mediated anti-viral resistance. Curr. Drug Targets Immune Endocr. Metabol. Disord. 1:117–130. [PubMed] [Google Scholar]
- 8.Trinchieri, G., and D. Santoli. 1978. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 147:1314–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen, K.B., T.P. Salazar-Mather, M.Y. Dalod, J.B. Van Deusen, X.Q. Wei, F.Y. Liew, M.A. Caligiuri, J.E. Durbin, and C.A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279–4287. [DOI] [PubMed] [Google Scholar]
- 10.Gautier, G., M. Humbert, F. Deauvieau, M. Scuiller, J. Hiscott, E.E. Bates, G. Trinchieri, C. Caux, and P. Garrone. 2005. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201:1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riffault, S., C. Carrat, K. van Reeth, M. Pensaert, and B. Charley. 2001. Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet. Res. 32:71–79. [DOI] [PubMed] [Google Scholar]
- 12.Nibert, M.L., and L.A. Schiff. 2001. Reoviruses and their replication. In Fields Virology. D.M. Knipe, R. Lamb, and P. Howley, editors. Lippincott Williams & Wilkins, Philadelphia. 1679–1728.
- 13.Tyler, K.L. 2001. Mammalian reoviruses. In Fields Virology. D.M. Knipe, R. Lamb, and P. Howley, editors. Lippincott Williams & Wilkins, Philadelphia. 1729–1745.
- 14.Fleeton, M.N., N. Contractor, F. Leon, J.D. Wetzel, T.S. Dermody, and B.L. Kelsall. 2004. Peyer's patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J. Exp. Med. 200:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan, J.Y., C.S. Boyce, and C.F. Cuff. 1998. T-Helper 1 and T-helper 2 cytokine responses in gut-associated lymphoid tissue following enteric reovirus infection. Cell. Immunol. 188:55–63. [DOI] [PubMed] [Google Scholar]
- 16.Hutchings, A.B., A. Helander, K.J. Silvey, K. Chandran, W.T. Lucas, M.L. Nibert, and M.R. Neutra. 2004. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer's patches. J. Virol. 78:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.London, S.D., D.H. Rubin, and J.J. Cebra. 1987. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J. Exp. Med. 165:830–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Major, A.S., and C.F. Cuff. 1997. Enhanced mucosal and systemic immune responses to intestinal reovirus infection in beta2-microglobulin-deficient mice. J. Virol. 71:5782–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvey, K.J., A.B. Hutchings, M. Vajdy, M.M. Petzke, and M.R. Neutra. 2001. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer's patches. J. Virol. 75:10870–10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen, G.C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255–281. [DOI] [PubMed] [Google Scholar]
- 21.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 24.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 25.French, A.R., and W.M. Yokoyama. 2003. Natural killer cells and viral infections. Curr. Opin. Immunol. 15:45–51. [DOI] [PubMed] [Google Scholar]
- 26.Orange, J.S., M.S. Fassett, L.A. Koopman, J.E. Boyson, and J.L. Strominger. 2002. Viral evasion of natural killer cells. Nat. Immunol. 3:1006–1012. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Fontecha, A., L.L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265. [DOI] [PubMed] [Google Scholar]
- 28.Kasai, M., T. Yoneda, S. Habu, Y. Maruyama, K. Okumura, and T. Tokunaga. 1981. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 291:334–335. [DOI] [PubMed] [Google Scholar]
- 29.Stewart, M.J., K. Smoak, M.A. Blum, and B. Sherry. 2005. Basal and reovirus-induced beta interferon (IFN-beta) and IFN-beta-stimulated gene expression are cell type specific in the cardiac protective response. J. Virol. 79:2979–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzam-Smoak, K., D.L. Noah, M.J. Stewart, M.A. Blum, and B. Sherry. 2002. Interferon regulatory factor-1, interferon-beta, and reovirus-induced myocarditis. Virology. 298:20–29. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell, S.M., M.W. Hansberger, J.L. Connolly, J.D. Chappell, M.J. Watson, J.M. Pierce, J.D. Wetzel, W. Han, E.S. Barton, J.C. Forrest, et al. 2005. Organ-specific roles for transcription factor NF-kappaB in reovirus-induced apoptosis and disease. J. Clin. Invest. 115:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamamdzic, D., T. Phillips-Dorsett, S. Altman-Hamamdzic, S.D. London, and L. London. 2001. Reovirus triggers cell type-specific proinflammatory responses dependent on the autocrine action of IFN-beta. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L18–L29. [DOI] [PubMed] [Google Scholar]
- 33.Angel, J., M.A. Franco, H.B. Greenberg, and D. Bass. 1999. Lack of a role for type I and type II interferons in the resolution of rotavirus- induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19:655–659. [DOI] [PubMed] [Google Scholar]
- 34.Karst, S.M., C.E. Wobus, M. Lay, J. Davidson, and H.W. Virgin, IV. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 299:1575–1578. [DOI] [PubMed] [Google Scholar]
- 35.Barro, M., and J.T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. USA. 102:4114–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin, D.H., M.J. Kornstein, and A.O. Anderson. 1985. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J. Virol. 53:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton, E.S., J.C. Forrest, J.L. Connolly, J.D. Chappell, Y. Liu, F.J. Schnell, A. Nusrat, C.A. Parkos, and T.S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell. 104:441–451. [DOI] [PubMed] [Google Scholar]
- 38.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, et al. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 101:3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho, H.J., T. Hayashi, S.K. Datta, K. Takabayashi, J.H. Van Uden, A. Horner, M. Corr, and E. Raz. 2002. IFN-alpha beta promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 168:4907–4913. [DOI] [PubMed] [Google Scholar]
- 40.Harris, G., R. KuoLee, and W. Chen. 2006. Role of Toll-like receptors in health and diseases of gastrointestinal tract. World J. Gastroenterol. 12:2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz, O., S.S. Diebold, M. Chen, T.I. Naslund, M.A. Nolte, L. Alexopoulou, Y.T. Azuma, R.A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 433:887–892. [DOI] [PubMed] [Google Scholar]
- 42.Edelmann, K.H., S. Richardson-Burns, L. Alexopoulou, K.L. Tyler, R.A. Flavell, and M.B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 322:231–238. [DOI] [PubMed] [Google Scholar]
- 43.Furlong, D.B., M.L. Nibert, and B.N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, R.E., H.J. Zweerink, and W.K. Joklik. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 39:791–810. [DOI] [PubMed] [Google Scholar]
- 45.Virgin, H.W., 4th, R. Bassal-Duby, B.N. Fields, and K.N. Tyler. 1988. Antibody protects against lethal infection with neurally spreading reovirus type 3 (Dearing). J. Virol. 62:4594–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virgin, H.W., 4th, M.A. Mann, B.N. Fields, and K.N. Tyler. 1991. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J. Virol. 65:6772–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwasaki, A., and B.L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.