Abstract
Dendritic cell (DC) activation is a prerequisite for T cell priming. During infection, activation can ensue from signaling via pattern-recognition receptors after contact with pathogens or infected cells. Alternatively, it has been proposed that DCs can be activated indirectly by signals produced by infected tissues. To address the contribution of tissue-derived signals, we measured DC activation in a model in which radioresistant cells can or cannot respond to lipopolysaccharide (LPS). We report that recognition of LPS by the radioresistant compartment is sufficient to induce local and systemic inflammation characterized by high circulating levels of tumor necrosis factor (TNF) α, interleukin (IL) 1β, IL-6, and CC chemokine ligand 2. However, this is not sufficient to activate DCs, whether measured by migration, gene expression, phenotypic, or functional criteria, or to render DC refractory to subsequent stimulation with CpG-containing DNA. Similarly, acute or chronic exposure to proinflammatory cytokines such as TNF-α ± interferon α/β has marginal effects on DC phenotype in vivo when compared with LPS. In addition, DC activation and migration induced by LPS is unimpaired when radioresistant cells cannot respond to the stimulus. Thus, inflammatory mediators originating from nonhematopoietic tissues and from radioresistant hematopoietic cells are neither sufficient nor required for DC activation in vivo.
The ability to respond rapidly to invading pathogens is a prerequisite for effective immunity to infection. Many immune and nonimmune cells are equipped with specialized pattern-recognition receptors (PRRs), such as the vertebrate Toll-like receptors (TLRs), which allow them to sense a large variety of pathogen-associated molecular patterns (PAMPs) and initiate an inflammatory response (1, 2). In all organisms, the innate inflammatory response initiated by PRRs is essential to contain immediate pathogen spread and to promote tissue repair (3). But, in vertebrates, PRRs can additionally act to promote DC activation and translation of innate into adaptive immunity (4–6). DCs are bone marrow–derived cells scattered throughout lymphoid and nonlymphoid organs, where they act as immune sentinels by responding to invading pathogens (4–6). Direct stimulation of DCs via TLRs and some other classes of PRRs induces their transition from resting cells into an “activated” effector state in which they can direct the expansion and differentiation of naive T cells into effectors (4–7). This process is accompanied by processing and presentation of pathogen-derived material, up-regulation of co-stimulatory molecules, migration of DCs to the T cell areas of secondary lymphoid organs, and production of cytokines and chemokines, all of which are important for selecting and priming rare naive, pathogen-specific T cells (7). Thus, stimulation of DCs through their PRRs allows the coupling of an immediate, protective innate response to an antigen-specific and long-lasting adaptive response.
Inflammation can also be triggered in sterile conditions, arguing for the existence of PAMP-independent pathways of innate response. Some triggers, such as genomic DNA or self-RNA–protein complexes, appear to act only in pathological conditions, by mimicking viral PAMPs and triggering PRRs involved in antiviral defense (8). They may, therefore, not be representative of DC responsiveness to infection. However, other endogenous triggers have been proposed to have an important role in initiating self-defense. This has been most eloquently argued by Matzinger in the “danger” model, which suggests that aberrant exposure of normal cell constituents during necrotic cell death signifies a potentially hazardous situation that requires intervention by the adaptive immune system (9–12). Such danger signals and their putative receptors remain largely unidentified but may include heat shock proteins, high-mobility group box 1, hyaluronan fragments, or uric acid (13).
Although it is clear that endogenous triggers of inflammation can promote processes such as tissue repair (14), it is controversial whether they also trigger adaptive immunity. Indeed, attempts to demonstrate adjuvant effects or DC activation by putative danger signals such as heat shock proteins have been marred by the issue of possible contamination with PAMPs and by the fact that danger receptors remain largely unidentified and, therefore, loss-of-function experiments have yet to be performed (13). A more productive area has been the study of the products of the innate response itself and their effect on DCs. Proinflammatory cytokines such as TNF-α and type I IFNs induce many features of DC activation in vitro (10, 15–19), suggesting that they could substitute for PAMPs or signals from dying cells in DC activation and T cell priming. This has been ingeniously incorporated into the danger model by suggesting that tissues act as important controllers of DC activation, not only by releasing “constitutive” danger signals upon necrotic cell death but also by producing an additional class of “inducible” danger signals such as TNF-α and IFN-α/β, which effectively relay danger at a distance (9–12).
These inducible danger signals are known to be synthesized in response to PRR signaling by a variety of hematopoietic and nonhematopoietic cells. Best characterized in this respect is the response to LPS from the outer envelope of Gram-negative bacteria that is recognized by TLR4 (20–22). LPS can induce proinflammatory cytokine production in a large variety of cell types, including epithelial cells (23, 24), endothelial cells (25), adipocytes (26), keratinocytes (27), and smooth muscle cells (28). Epithelial cells, in particular, have been regarded as sensors of infection because of their strategic localization lining the outside world (29, 30). Their contribution has been shown to be important for resolving a bacterial infection with uropathogenic Escherichia coli (31) and for pulmonary responses to inhaled endotoxin (32). Furthermore, stromal cells are also able to respond to viral PAMPs and are essential for adequate protection from some viral infections (33). However, an important issue that remains to be addressed is whether nonhematopoietic tissue cells are sufficient to initiate an immune response by transmitting inducible danger signals to DCs or, rather, whether they amplify the response that has been initiated by direct DC activation via PRRs. We tested this by monitoring LPS-driven DC activation in chimeric mice in which either the radioresistant or the radiosensitive compartment was deficient in TLR4 signaling. We show that although radioresistant cells throughout the body react strongly to LPS and initiate a local and systemic inflammatory response, this is neither sufficient nor necessary to lead to changes in DC localization, phenotype, or T cell stimulatory function. We conclude that inducible signals from nonhematopoietic tissues and radioresistant hematopoietic cells do not play an essential role in DC activation.
RESULTS
In vivo response of radioresistant tissue cells to LPS
To address the extent to which stromal cells can drive an inflammatory response in vivo, we generated chimeric mice in which most hematopoietic cells cannot respond to LPS because of either a spontaneous mutation (TLR40/0 [20, 21]) or a deletion (TLR4−/− [22]) in the TLR4 gene, but nonhematopoietic tissues (and radioresistant leukocytes such as Langerhans cells [34]) are of WT origin and remain LPS responsive (Fig. 1 A). We initially examined the systemic response to LPS in the TLR4−/−→WT chimeras versus WT→WT control mice by analyzing expression of proinflammatory genes in different organs. 1 h after i.v. injection of the stimulus, TLR40/0→WT mice showed strong induction of GM-CSF, IL-1β, and TNF-α mRNA in the heart and kidney, similar to that seen in WT→WT mice (Fig. 1 B). LPS also induced substantial up-regulation of IL-1β and TNF-α in the liver and spleen of TLR40/0→WT mice (Fig. 1 B). Levels were lower than in WT→WT controls (Fig. 1 B), suggesting that radiosensitive cells contribute more significantly to inflammatory responses in the spleen and liver than in the heart and kidney, as might be expected from the relative leukocyte content of those organs. IFN-β was not induced in the heart and liver of TLR40/0→WT mice and only modestly in the kidney and spleen, which suggests that radiosensitive cells are the main in vivo source of this cytokine in response to LPS. CC chemokine ligand (CCL) 2 and CXC chemokine ligand (CXCL) 10, two inflammatory chemokines, were induced to equivalent levels in the two sets of chimeras (Fig. 1 B).
Figure 1.
TLR4 stimulation of radioresistant cells induces local organ inflammation. (A) Experimental design. (B) Relative mRNA expression of GM-CSF, IL-1β, IFN-β, TNF-α, CCL2, and CXCL10 in the kidney, heart, liver, and spleen of WT→WT mice or TLR40/0→WT mice 1 h after intravenous injection of 10 μg PBS or LPS per mouse. Expression of each gene was normalized to the expression of 18S rRNA and is shown as the fold induction of LPS over PBS. Data are the means ± SD of triplicate determinations. Expression of all genes was significantly up-regulated by LPS compared with PBS (P < 0.05 using the Student's t test), except for IFN-β in the liver of TLR40/0→WT mice (significant down-regulation) and for IFN-β in the hearts of TLR40/0→WT mice (not significant). Significant differences between WT→WT and TLR40/0→WT mice are indicated. * and **, P < 0.05 and 0.01, respectively, using the Student's t test.
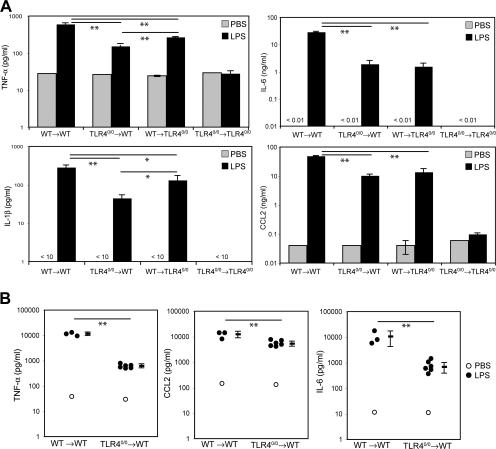
The activation of radioresistant cells by LPS also led to a marked systemic response, as indicated by the fact that high levels of proinflammatory mediators such as TNF-α, IL-6, IL-1β, and CCL2 could be detected in the serum of TLR40/0→WT mice 6 h after LPS injection (Fig. 2 A). These levels were not as high as in WT→WT mice, consistent with the fact that these cytokines are also produced by LPS-activated hematopoietic cells. This was evident in the serum from reciprocal chimeric mice in which the radiosensitive cells were of WT and the radioresistant cells of TLR40/0 origin: in such WT→TLR40/0 mice, LPS strongly induced TNF-α, IL-6, IL-1β, and CCL2 but, notably, without the participation of the radioresistant compartment, levels were also lower than in WT→WT mice (Fig. 2 A). No cytokine induction was detected in the serum of TLR40/0→TLR40/0 mice, which confirms the TLR4-restricted bioactivity of the LPS used in these experiments (Fig. 2 A).
Figure 2.
Both hematopoietic and nonhematopoietic cells contribute to LPS-induced systemic inflammation. (A) The concentration of TNF-α, IL-6, IL-1β, and CCL2 was determined in sera of WT→WT, TLR40/0→WT, WT→TLR40/0, and TLR40/0→TLR40/0 chimeric mice 6 h after injection of either PBS or LPS. (B) Similar analysis of TNF-α, IL-6, and CCL2 in serum from WT→WT and TLR40/0→WT at 1 h after injection. Data in A are the means ± SD of two to three mice. Each point in B represents an individual mouse, and the mean ± SD is also shown. Significant differences between groups are indicated. * and **, P < 0.05 and 0.01, respectively, using the Student's t test.
As LPS is known to elicit a very rapid cytokine response, in particular of TNF-α (35), we also examined the serum of chimeric mice at earlier time points. At 1 h after LPS injection, TNF-α reached very high levels in both WT→WT and TLR40/0→WT mice (up to 1 ng/ml in the latter). Levels of CCL2 and IL-6 were also high (Fig. 2 B), whereas IFN-β could be detected at only low levels (<100 pg/ml) in WT→WT mice but not in TLR40/0→WT mice (not depicted), in agreement with Fig. 1 B showing that it largely originates from radiosensitive cells. Collectively, these data indicate that radioresistant stromal cells throughout the body are rapidly activated by LPS and respond with the production of proinflammatory cytokines and chemokines, greatly contributing to both local and systemic inflammation.
Gene expression profile of stroma-stimulated DCs
To investigate the influence of systemic inflammation induced by radioresistant cells on the activation status of DCs, we analyzed the gene expression profile of DCs isolated from the spleen. Splenic DCs are strategically localized in close contact with the bloodstream and, therefore, are exposed to both local and systemic inflammatory mediators. Conventional CD11chiB220− DCs (i.e., excluding B220+ plasmacytoid DCs) were sorted from spleens of WT→WT and TLR40/0→WT mice 6 h after i.v. injection of either PBS or LPS, and the expression of several genes was analyzed by quantitative PCR. As shown in Table I, LPS induced the up-regulation of many activation-related genes in DCs from WT→WT mice but only few such genes were induced in DCs from TLR40/0→WT mice. DCs from TLR40/0→WT mice did not up-regulate expression of co-stimulatory molecules and proinflammatory cytokines upon LPS injection but, rather, expressed genes involved in downmodulating immune responses, such as IL-10 and suppressor of cytokine signaling (SOCS) 1 and 3 (Table I). In addition, they expressed mRNAs for a select set of chemokines (i.e., CCL17, CCL22, CXCL2, and CCL12), whereas those encoding several proinflammatory proteins, such as cyclooxygenase 2 (COX-2), TNF-α, and IL-12 p40, were significantly down-regulated (Table I). Expression of TGF-β and the mannose receptor (MRC-1) remained unaltered in TLR40/0→WT DCs, whereas these genes were strongly down-regulated in WT→WT DCs (Table I). Whether the observed differences in gene expression profile reflect the differential responsiveness of all DCs or is confounded by effects on particular subsets remains to be determined. Nevertheless, these data clearly show that LPS has very distinct effects on the conventional DC repertoire of TLR40/0→WT compared with WT→WT mice and that only a restricted and distinct set of genes is induced in DCs exposed to stromal-driven inflammation in vivo.
Table I.
Expression profile of genes induced by LPS in splenic DCs from WT→WT and TLR40/0→WT mice
LPS-activated stromal cells do not induce phenotypic DC maturation
A major component of DC activation is the acquisition of “maturation markers” (7). To determine to what extent stromal-driven inflammation leads to phenotypic maturation, we examined DC surface marker expression by flow cytometry. 6 h after LPS injection, splenic DCs from WT→WT control mice had increased their expression of CD40, CD80, CD86, CD95, and MHC class II when compared with PBS-injected controls (Fig. 3, A and B). Notably, no difference was seen in WT→TLR40/0 mice, which indicates that the activation of stromal cells is not required for DC maturation in vivo (Fig. 3, A and B). In contrast, no DC maturation was seen in TLR40/0→WT mice (Fig. 3, A and B) or TLR40/0→TLR40/0 control mice (Fig. 3 B). Even at 14 h after LPS injection, DCs from TLR40/0→WT mice displayed little sign of increased expression of maturation markers (Fig. 3 C). In addition, when DCs were sorted and cultured overnight in vitro, only DCs from LPS-injected WT→WT mice produced IL-6, another parameter associated with DC activation (Fig. 3 D). Control WT→WT and TLR40/0→TLR40/0 chimeric mice had comparable responses to WT and TLR40/0 mice, indicating that transplantation had no effect on responsiveness to LPS (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070325/DC1). Moreover, bone-marrow chimeras on a C57BL/6 background (TLR4−/−→WT) behaved similarly to those on the BALB/c background (TLR40/0→WT), as in both cases LPS induced high levels of proinflammatory cytokines without causing the activation of splenic DCs (see Fig. 6 and not depicted). This indicates that the observed nonresponsiveness of the DCs was not caused by the genetic background nor the nature of the TLR4 defect (spontaneous point mutation vs. induced deletion).
Figure 3.
LPS stimulation of radioresistant cells does not lead to DC activation. (A) Surface expression of CD40, CD80, CD86, CD95, and MHC class II (I-Ad) was determined on gated CD11chi cells from spleens isolated from WT→WT (top), WT→TLR40/0 (middle), and TLR40/0→WT (bottom) mice 6 h after injection with PBS (shaded histogram) or LPS (continuous line). (B) Geometric mean fluorescence intensity (GeoMFI) values were determined at 6 h after injection and expressed as a ratio of LPS/PBS for WT→WT, WT→TLR40/0, TLR40/0→WT, and TLR40/0→TLR40/0 mice. (C) Induction of surface expression of co-stimulatory molecules by LPS compared with PBS, gated on CD11chi cells from spleens isolated 6 or 14 h after injection into WT→WT (closed squares) or TLR40/0→WT (open squares) mice. (D) Concentration of IL-6 in overnight culture supernatant of CD11chiB220− DCs sorted from spleens of WT→WT or TLR40/0→WT mice 6 h after injection of PBS or LPS. Results indicate means ± SD of triplicate wells. (E) 1 μg PBS or LPS was injected into the hind footpads (left vs. right, respectively) of WT→WT or TLR4−/−→WT mice, and draining (popliteal) and nondraining (axillary) lymph nodes were isolated 6 h later. Surface expression of co-stimulatory molecules was determined on CD11chi cells and expressed as a ratio of LPS/PBS within one mouse. Results indicate means ± SD of two (WT→WT) or three (TLR4−/−→WT) mice. *, P < 0.05 between the indicated groups using the Student's t test.
Figure 6.
Increasing serum TNF-α levels is not sufficient to induce complete DC activation. (A) Serum concentration of TNF-α and IL-6 was determined in the sera of WT→WT and TLR4−/−→WT chimeric mice 1 h after injection with either PBS (open circles), LPS (closed circles), LPS plus 0.25 μg TNF-α (closed triangles), or LPS plus 1 μg TNF-α (closed squares). (B) Surface expression of co-stimulatory molecules on CD11chi cells from spleens isolated 6 h after injection of LPS ± TNF-α into WT→WT or TLR4−/−→WT mice. Data are expressed as a ratio of LPS/PBS. *, P < 0.05 using the Student's t test for LPS-injected WT→WT mice. (C) Surface expression of TNF-RI on the same DCs described in B, expressed as GeoMFI. Results (A–C) are means ± SD from two to three mice per group. *, P < 0.05 shows significantly lower TNF-RI expression compared with PBS injection.
To determine if the nonresponsiveness to stroma-derived inflammatory mediators was true outside the spleen, we also tested DCs in other lymphoid organs. Systemic LPS administration did not promote phenotypic changes in DCs from axillary, brachial, or mesenteric lymph nodes in TLR40/0→WT mice (unpublished data). Moreover, local administration of LPS gave the same results as systemic delivery, because LPS injection into the footpads of TLR4−/−→WT C57BL/6 mice did not activate DCs in draining popliteal lymph nodes (Fig. 3 E) even though it induced substantial paw inflammation, as determined by quantitative PCR analysis of proinflammatory mediators (not depicted). Collectively, these data indicate that stromal- derived inflammatory mediators alone are neither sufficient nor required for DC maturation in response to LPS administration.
LPS-activated stromal cells do not induce functional DC maturation
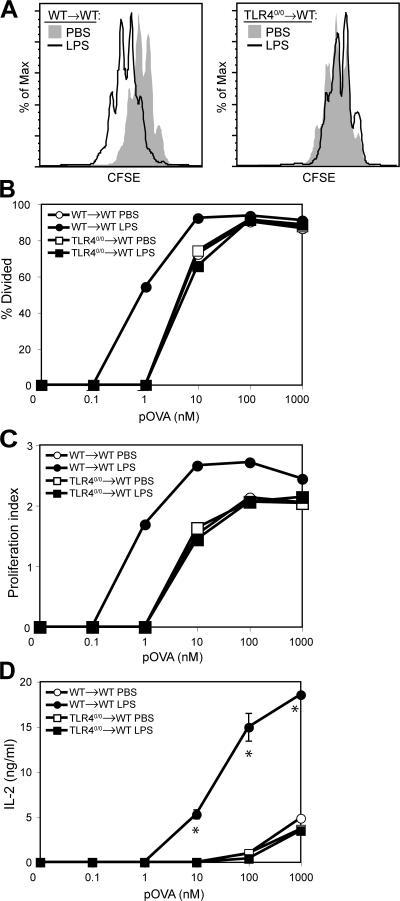
The phenotypic changes conventionally used as indicators of DC maturation correlate with an increased ability of the cells to stimulate T cell proliferation (7). To ask whether such functional maturation had occurred, splenic DCs were isolated from spleens of WT→WT or TLR40/0→WT mice 6 h after LPS injection, fixed in paraformaldehyde to prevent further changes, and used as stimulators for CFSE-labeled TCR-transgenic naive T cells in the presence of cognate peptide. DCs from LPS-injected WT→WT mice were 10-fold better T cell stimulators than DCs from TLR40/0→WT mice (Fig. 4 A), as confirmed by quantification of the CFSE profiles (Fig. 4, B and C) and by assessing IL-2 accumulation in culture supernatants (Fig. 4 D). In contrast, DCs from LPS-injected TLR40/0→WT mice were equivalent to DCs from PBS-injected control mice at stimulating T cell proliferation (Fig. 4, B–D). Furthermore, T cells primed by DCs from PBS-injected WT→WT or from PBS- or LPS-injected TLR40/0→WT mice all displayed a comparable cytokine production profile upon restimulation, which was markedly different from that of T cells primed by DCs from LPS-injected WT→WT mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070325/DC1). We therefore conclude that stromal recognition of LPS is not sufficient to induce functional maturation of DCs, despite creating a local and systemic inflammatory milieu.
Figure 4.
LPS activation of radioresistant cells does not result in functional DC maturation. CFSE-labeled DO11.10 T cells were stimulated with pOVA and paraformaldehyde-fixed CD11c-enriched cells from spleens isolated from chimeric mice 6 h after injection of PBS or LPS. CFSE profiles were analyzed after 96 h and gated for DAPI−CD4+KJ1-26+ cells. (A) CFSE profile of DO11.10 T cells stimulated with 10 nM pOVA and DCs from WT→WT mice (left) or TLR40/0→WT mice (right) that had been injected with PBS or LPS. (B) Percentage of T cells that divided expressed as a function of pOVA concentration. (C) Proliferation index (mean number of divisions undergone by dividing cells) expressed as a function of pOVA concentration. (D) IL-2 levels in culture supernatants after 96 h, expressed as a function of pOVA concentration. Data are the means ± SD of triplicate wells. *, P < 0.01 between groups using the Student's t test.
Activation of stromal cells is insufficient to induce splenic DC migration
Upon activation, splenic DCs migrate from the red pulp into the T cell area of the white pulp (also known as the periarteriolar lymphatic sheath [PALS]) (36, 37). Up-regulation of CC chemokine receptor (CCR) 7 and down-regulation of CCR6 correlates with DC migration (38, 39) and, consistent with this notion, considerably decreased levels of CCR6 and increased levels of CCR7 were seen in DCs from WT→WT mice after LPS injection. In contrast, no chemokine receptor usage switch was seen in DCs from TLR40/0→WT mice (Fig. 5 A), suggesting that signals from LPS-activated stromal cells might not be sufficient to induce migration of DCs into the T cell zone. To test this directly, we measured DC relocalization to the PALS. Marked relocalization was observed in WT→WT mice upon LPS injection (Fig. 5, B–D), as expected (36). In contrast, DC localization in TLR40/0→WT mice remained unaltered (Fig. 5, B–D). Notably, injection of LPS into the reciprocal WT→TLR40/0 mice was sufficient to induce DC migration to the T cell area in spleens (Fig. 5, B–D). These data indicate that the response of radioresistant stromal cells to LPS is neither sufficient nor required to induce migration of splenic DCs into the PALS.
Figure 5.
Activation of nonhematopoietic cells by LPS is not sufficient or required for migration of splenic DCs to the PALS. (A) Expression of mRNA for CCR6 and CCR7 in sorted splenic DCs from WT→WT mice or TLR40/0→WT mice at 6 h after injection of PBS or LPS. Values were normalized to the expression of 18S rRNA and expressed as the fold induction of LPS over PBS. Results indicate the means ± SD of triplicate wells. *, P < 0.01 for the expression level induced by LPS using the Student's t test. (B) Localization of CD11c+ cells (green) was determined in spleens of chimeric mice 6 h after injection of PBS (top) or LPS (bottom). White pulp area was delineated by MAdCAM-1+ sinus-lining cells in the marginal zone (red), while B cells areas were visualized by staining for B220 (blue). Bar, 100 μm. The percentage of CD11c+ staining present in the PALS (C) or in the red pulp (D) was determined in all four chimera groups after injection with PBS or LPS. Results indicate means ± SD from three to six analyzed wide-field images. *, P < 0.001 between PBS- and LPS-injected mice using the Student's t test.
Inflammatory cytokines induce limited DC maturation in vivo
Our experiments clearly indicate that DCs in secondary lymphoid organs do not become activated by local or systemic inflammatory signals produced naturally by stromal cells in response to LPS, even though these include cytokines such as TNF-α, known to provoke DC maturation in vitro (15, 40). However, TNF-α levels in the serum of LPS-treated TLR40/0→WT mice were still ∼10-fold lower than those in WT→WT mice (1 vs. 10 ng/ml; Fig. 2). To examine if this quantitative difference could explain the lack of responsiveness of DCs in TLR40/0→WT mice, we coinjected TNF-α with LPS into TLR4−/−→WT mice and examined the activation status of splenic DCs. Coinjection of 0.25 μg TNF-α with LPS into TLR4−/−→WT mice increased TNF-α and IL-6 serum levels to the level obtained with LPS alone in WT→WT mice (Fig. 6 A) and was accompanied by partial up-regulation of CD80, CD86, and MHC class II, but not CD40, in splenic DCs (Fig. 6 B). Injection of larger doses of TNF-α ± IFN-α/β induced very high levels of circulating TNF-α and led to greater up-regulation of maturation markers, although the degree of maturation was still markedly inferior to that achieved with LPS in WT→WT mice (Fig. 6, A and B; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070325/DC1). To determine if DCs in the spleen were actually exposed to the serum cytokines, membrane TNF receptor I (TNF-RI) expression was analyzed. We found that artificially increasing the levels of TNF-α in TLR4−/−→WT chimeras induced down-regulation of TNF-RI expression by splenic DCs, indicating receptor engagement (Fig. 6 C and Fig. S3). TNF-RI down-regulation in this setting was equivalent to that in LPS-treated WT→WT mice, yet it was not accompanied by an equivalent degree of DC maturation. It can therefore be concluded that resting DCs can respond to the presence of TNF-α in vivo and that, at high enough levels, this can induce some degree of maturation, albeit lower than that which is induced with LPS.
Finally, to determine whether chronic rather than acute exposure to TNF-α resulted in a different phenotype, we examined TNFΔARE mice, which have increased serum TNF-α levels caused by the deletion of regulatory AU-rich elements (ARE) in the TNF mRNA (41). DCs from these mice showed down-regulation of TNF-RI expression, indicating that they were sensitive to the increased levels of TNF-α (Fig. S4 A, available at http://www.jem.org/cgi/content/full/jem.20070325/DC1). However, expression of co-stimulatory molecules on DCs from TNFΔARE mice was comparable to that of control cells (Fig. S4 B). Thus, even chronic exposure to TNF-α does not induce substantial maturation of DCs in vivo.
Stroma-stimulated DCs respond normally to subsequent TLR stimulation
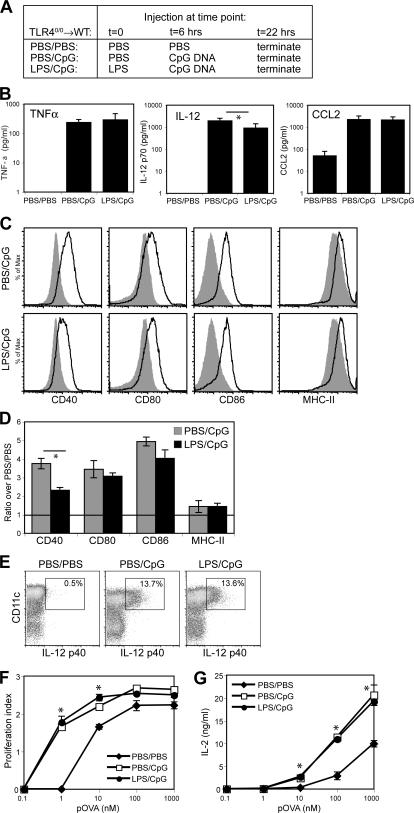
Further evidence that the unresponsiveness of DCs in LPS-injected TLR4−/−→WT mice did not simply reflect DC “ignorance” of the proinflammatory milieu comes from the fact that they up-regulated a select set of genes (Table I). Notably, many of these genes are associated with dampening of immunity, perhaps indicating a regulatory function (42–45). To examine whether this expression pattern could therefore reflect induction of an unresponsive state, we tested the ability of DCs to respond to subsequent direct TLR stimulation in vivo. TLR40/0→WT mice were initially injected with either PBS or LPS and 6 h later with CpG DNA, an agonist for TLR9, and were analyzed 16 h after the last injection (Fig. 7 A). CpG strongly induced TNF-α, IL-12p70, and CCL2, and the serum levels of these mediators were not substantially different when CpG challenge was preceded by LPS (Fig. 7 B). Analysis of splenic DCs showed that initial injection with LPS also did not affect CpG-induced maturation, as CD80, CD86, and MHC class II were expressed at similar levels on DCs from PBS/CpG and LPS/CpG mice (Fig. 7, C and D). LPS pretreatment did slightly decrease the CpG-induced up-regulation of CD40 expression on DCs (Fig. 7, C and D), but induction of IL-12 was identical in both groups, as determined by intracellular staining (Fig. 7 E). Finally, LPS/CpG DCs were comparable to PBS/CpG DCs at inducing naive T cell proliferation (Fig. 7 F) and IL-2 production (Fig. 7 G). It can therefore be concluded that systemic and local inflammation driven by LPS-induced activation of radioresistant cells does not render splenic DCs unresponsive to subsequent TLR stimulation.
Figure 7.
DCs exposed to stromal-driven inflammation respond normally to subsequent direct TLR stimulation. (A) Experimental setup. (B) Levels of TNF-α, IL-12 p70, and CCL2 in sera of PBS/PBS-, PBS/CpG-, and LPS/CpG-injected TLR40/0→WT mice. Results indicate means ± SD from four individual mice. (C) Surface expression profiles for CD40, CD80, CD86, and MHC class II (I-Ad) on CD11chi-gated splenocytes from PBS/PBS-injected mice (shaded histograms) overlaid with the profiles of PBS/CpG-injected mice (continuous line, top) or LPS/CpG-injected mice (continuous line, bottom). (D) As in C, but expressed as the ratio of GeoMFI values for PBS/CpG-injected mice or LPS/CpG-injected mice to PBS/PBS-injected mice. Results indicate means ± SD from two mice per group. Expression of all molecules was significantly up-regulated compared with PBS/PBS-injected mice (P < 0.05 using the Student's t test), except for MHC class II in PBS/CpG injected mice. (E) Intracellular IL-12 p40 staining on CD11c-enriched splenocytes from PBS/PBS- (left), PBS/CpG- (middle), and LPS/CpG-injected (right) mice. Numbers in boxes indicate the percentage of all cells shown that produce IL-12 p40. (F) Proliferation index (average number of divisions undergone by dividing cells) of CFSE-labeled DO11.10 T cells analyzed after 96 h of co-culture with pOVA and paraformaldehyde-fixed, CD11c-enriched cells from PBS/PBS-, PBS/CpG-, or LPS/CpG-injected mice. (G) IL-2 concentration in the culture supernatants from F, depicted as the mean ± SD of triplicate wells. *, P < 0.05 between indicated groups (B and D) or between PBS/PBS-injected mice and either PBS/CpG- or LPS/CpG-injected mice (F and G), using the Student's t test.
DISCUSSION
The widespread distribution of many TLRs on nonhematopoietic cells is important to allow tissue-resident cells to rapidly respond to early signs of infection and initiate a protective innate response. By secreting proinflammatory mediators, tissues have additionally been suggested to induce adaptive responses, transmitting inducible danger signals that promote DC activation (9–12, 46–48). The data presented in this paper show that signals from nonhematopoietic tissues and radioresistant hematopoietic cells such as Langerhans cells are not sufficient to perform this function in an endotoxemia model. Using chimeric mice in which only radioresistant cells respond to LPS, we found that DCs in the spleen, as well as in lymph nodes, remained immature with respect to their phenotype, function, and distribution. This is particularly surprising, because LPS induced high systemic levels of proinflammatory cytokines and chemokines in these mice, some of which act as stimuli for DC maturation in vitro. In particular, TNF-α and IL-1 have been described to activate DCs (15, 40, 49), and TNF-α is one of the most important cytokines driving the proinflammatory response during sepsis and endotoxemia (50, 51). Yet, we found that hematopoietic cells are also a major source of TNF-α, IL-1β, and even IFN-β (Fig. 1 and Fig. 2), which would suggest that activation of both hematopoietic and nonhematopoietic cells is required for optimal induction of these cytokines. Consistent with this notion, “spiking” LPS with TNF-α caused some up-regulation of co-stimulatory molecules on splenic DCs in TLR4−/−→WT chimeras (Fig. 6 and Fig. S3). This was further augmented by additional spiking with IFN-α/β consistent with reports that injection of IFN-α/β into mice can activate DCs in vivo (10, 18, 52). However, it is notable that the extent of maturation achieved in this setting was marginal compared with that achieved with LPS stimulation and out of proportion with the amount of circulating TNF-α and consequent down-regulation of the TNF-RI on DCs (Fig. 6 and Fig. S3). This leaves open the question of whether the observed lack of DC activation in TLR4−/−→WT chimeras is a quantitative effect, reflecting the fact that radioresistant cells can only provide suboptimal levels of inflammatory mediators, or a qualitative effect in which there is a requirement for a maturational signal uniquely originating from radiosensitive cells. Whichever the case, it does not alter the empirical conclusion that signals emanating from resident tissue cells are not sufficient to activate DCs in vivo, even in situations of massive systemic inflammation.
Apart from the enhanced ability to present antigen to and stimulate T cells, activated DCs also acquire the capacity to migrate to the T cell areas of secondary lymphoid organs. This migration is driven by the stroma-derived chemokine CCL21 and its cognate receptor CCR7 (for review see reference 53). DC maturation is characterized by a switch in chemokine receptor profile, and it has been suggested that the up-regulation of CCR7 and the down-regulation of chemokine receptors such as CCR1, CCR5, and CCR6 are required for the entry of maturing DCs into the T cell area (38, 39). Consistent with the fact that DCs exposed to stromal-driven inflammation remain immature, no chemokine receptor switch or DC relocalization to the PALS could be seen in LPS-injected TLR40/0→WT chimeras. Notably, a recent study has shown that maturation and migration of DCs can sometimes be uncoupled, as injection of CpG DNA in MyD88−/− mice and LPS in TRIF−/− mice induces migration of immature DCs into the PALS (54). As this indicates that DC activation is not a prerequisite for entry into the PALS, one possibility is that DC migration is also regulated at the stromal level, consistent with the fact that LPS or TNF-α increase expression of CCL21 on lymphatic endothelium and boost DC migration to draining lymph nodes (55). However, our experiments argue that activation of stromal cells is not required for the migration of splenic DCs, as LPS injection in WT→TLR40/0 mice was sufficient to induce entry of splenic DCs into the PALS (Fig. 5). Similarly, signals from radioresistant cells are dispensable for supporting direct DC activation, at least under optimal stimulatory conditions (Fig. 3, A and B).
We have previously shown in a mixed bone marrow chimera system that the presence of leukocytes responding to PAMPs in vivo causes bystander DC maturation, whether measured by phenotypic (expression of maturation markers) or functional (ability to stimulate T cell proliferation) criteria, whereas it fails to induce complete DC activation (production of IL-12), as well as priming of CD4+ Th effector cells (56). The data reported in this paper demonstrate that the ability to promote DC activation in trans is restricted to leukocytes and that there is no necessary role for radioresistant cells. This raises the question of whether nonhematopoietic tissues simply lack the operational ability to drive DC activation or are actively able to suppress it. The gene expression profile of DCs from LPS-injected TLR40/0→WT mice revealed a set of differentially expressed genes that could be linked to immune down-regulation. Indeed, IL-10 and SOCS-1 and -3 are involved in dampening the inflammatory response (43, 44), whereas CCL17 and CCL22 can attract regulatory T cells through the receptor CCR4 (42). Expression of these genes may be an indirect effect of the inflammatory milieu, as SOCS-3 can be induced by IL-6 and TNF-α (57, 58), CCL17 can be induced by CCL2 (59), and both CCL17 and CCL22 can be induced by TNF-α (60, 61). It has also been shown that TNF-α–conditioned “semimature” DCs can induce tolerance in vivo and protect from autoimmunity (62), whereas spleen-derived stromal cells are able to generate IL-10–producing regulatory DCs in vitro (63, 64). In addition, direct DC stimulation by TLR agonists leads to a state of “exhaustion” or “paralysis” characterized by the inability to respond to further stimulation (65, 66). However, we found little evidence to suggest that DCs from LPS-injected TLR40/0→WT mice had become unresponsive or suppressive, as they reacted normally to subsequent direct stimulation with CpG DNA (Fig. 7). We also found no altered cytokine profile in T cells stimulated by these DCs, which might have been noticed if the DCs had acquired a regulatory function (Fig. S2). Collectively, these data rather suggest that LPS-induced activation of nonhematopoietic cells is without major functional implications for DCs and does not lead to paralysis, although we cannot rule out a subtle modulatory effect that is not apparent in our assays.
In contrast to our data, several groups have shown that stromal cells are capable of inducing DC activation. Using a co-culture system, Rimoldi et al. described how gut epithelial cells exposed to bacteria could indirectly activate monocyte-derived DCs in a “noninflammatory” manner, whereas direct recognition of the bacteria by the DCs made them more inflammatory (67, 68). In another study, TLR signaling in both hematopoietic and stromal cells was shown to be required for adequate activation of mucosal DCs and antiviral immunity during vaginal infection with herpes simplex virus, although the stromal contribution in that instance may have been to sustain and amplify direct DC activation by the virus (33). Nevertheless, these studies focused on mucosal immunity, and it could well be that such interplay of epithelial cells with local DCs is unique for the mucosa. The ability of tissue-resident cells to convey inducible danger signals to DCs may thus be restricted to local responses.
The biology of DCs at the inflammatory site is an important issue for further investigation. The concentration of inducible tissue-derived signals in the microenvironment may reach levels high enough to surmount DC unresponsiveness or to activate DCs differentiated in situ from monocytes recruited to the inflamed tissue. Alternatively, tissue-resident DCs may respond to tissue-derived inflammatory signals in a qualitatively different manner from the secondary lymphoid tissue DCs studied in this paper. For example, skin Langerhans cells and dermal DCs may be especially tuned to inflammatory signals produced by surrounding keratinocytes (19). Unfortunately, we are unable to study the response of those DCs in our bone marrow chimera system because Langerhans cells are maintained by radioresistant precursors and are therefore not replenished by TLR4−/− donor bone marrow (34). Similarly, dermal DCs are only partially replaced by donor bone marrow–derived cells upon lethal mouse irradiation (69), which means that in our approach a substantial number are still able to respond directly to LPS (as it takes 12–24 h before dermal DCs reach the local draining lymph node, they do not affect our analysis of secondary lymphoid tissue DCs). Moreover, even if we were able to identify the subpopulation of dermal DCs that arises from radiosensitive precursors, any local activation of these cells in response to LPS could not be unequivocally ascribed to the effect of tissue-derived signals. It could have resulted from signals from other (radioresistant) DCs, consistent with our previous finding that directly activated DCs are able to indirectly activate other DCs (56). For all these reasons, the chimera system is not suitable for analyzing the response of locally resident DCs to tissue-derived inflammatory signals. Nevertheless, it allows us to conclusively demonstrate that DCs in secondary lymphoid organs cannot be stimulated by inflammatory signals from stromal cells in vivo but only by direct pathogen activation or, partially, by signals from cells of hematopoietic origin. This type of ignorant behavior might be relevant in situations where the pathogen has effectively been contained, but newly generated DCs become exposed to a residual proinflammatory environment. Finally, it could also explain clinical data indicating that autoinflammatory diseases do not necessarily lead to self- directed lymphocyte responses and are therefore quite distinct from autoimmunity (70).
MATERIALS AND METHODS
Mice.
C.C3-TLR4Lps-d/J BALB/c (TLR40/0) mice were obtained from the Jackson Laboratory. These mice carry the spontaneous mutation in the TLR4 gene found in the C3H/HeJ strain and have been backcrossed 20× onto BALB/c mice. Normal BALB/c mice were used as controls and as recipients for bone marrow transplantation experiments. DO11.10 mice on a BALB/c-SCID background carrying a transgenic Vα2/Vβ8 TCR specific for chicken ovalbumin (residues 323–339; pOVA) presented on I-Ad were originally a gift from P. Garside (University of Glasgow, Glasgow, UK). Bone marrow from C57BL/6 mice genetically deficient for the TLR4 gene (TLR4−/− mice [22]) was provided by J. Langhorne (National Institute for Medical Research, London, UK). Normal C57BL/6 mice were used as sources of control bone marrow, and congenic B6.SJL mice served as recipients, which allowed us to determine the degree of chimerism by staining cells for CD45.2 versus CD45.1. TNFΔARE mice (41) were made available by G. Kollias (Biomedical Sciences Research Center Alexander Fleming, Vari, Greece) and provided by J. Caamano (Institute for Biomedical Research, Birmingham, UK). All mice were bred and maintained at Cancer Research UK in specific pathogen-free conditions.
For the generation of chimeras, recipient mice were γ irradiated twice with 5 Gy and were reconstituted with 2 × 106 nucleated bone marrow cells of the relevant donor strain. Mice were sex- and age-matched within experiments. Importantly, all data reported in this paper were obtained with chimeras reconstituted no less than 4 mo before LPS challenge, when the hematopoietic system was completely donor derived (unpublished data); when younger chimeras were used, trans-DC maturation was sometimes observed, which correlated with the presence of donor-derived LPS-responsive hematopoietic cells (unpublished data).
All animal procedures and husbandry were performed under the authority of a project license in accordance with UK governmental regulations (Animals (Scientific Procedures) Act 1986).
Reagents.
A pure preparation of LPS from Salmonella abortus equi that selectively activates TLR4 was obtained from Qbiogene and used intravenously at a dose of 10 μg per mouse or subcutaneously at 1 μg per footpad. CpG oligonucleotide 1668 (CpG) was obtained from Sigma-Aldrich and used intravenously at a dose of 100 μg per mouse. Peptide derived from pOVA was synthesized and purified by high-performance liquid chromatography at Cancer Research UK. Mouse TNF-α (Sigma-Aldrich) was a gift from A. McDowall (Cancer Research UK, London, UK) and used at a dose of 0.25 or 1 μg per mouse in 1% FCS/PBS. IFNA/D, a hybrid human IFN-α active on mouse cells (71), was a gift from I. Kerr (Cancer Research UK, London, UK) and used at a dose of 105 U per mouse in 1% FCS/PBS. All reagents, except LPS, were free of endotoxin.
Cell purification.
DCs were purified from freshly isolated spleens after Liberase CI/DNase digestion (Roche Diagnostics) by preincubation with anti-CD11c MACS beads (Miltenyi Biotec) and subsequent immunomagnetic sorting using an autoMACS (Miltenyi Biotec). If required, splenic DCs were further purified by flow cytometric sorting (CD11chiCD45R/B220−) with a MoFlo cytometer (DakoCytomation) or a FACSAria (Becton Dickinson). Sorted cell preparations were routinely >99% pure and viable. To measure cytokine production, 5 × 105 sorted DCs/ml were cultured overnight in complete medium (RPMI 1640 with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 0.292 mg/ml l-glutamine, and 50 μM 2-mercaptoethanol; Invitrogen).
Naive CD4+ T cells from spleens and lymph nodes of DO11.10 donor mice were purified by negative selection with MACS beads (Miltenyi Biotec). The resulting cell preparations were routinely 80–90% pure and free of APCs. Purified T cells were subsequently labeled with 2 μM CFSE (Invitrogen) for 12 min at 37°C.
RNA isolation and quantitative real-time PCR.
Upon isolation, mouse organs were stored in RNAlater RNA stabilization reagent (QIAGEN) at 4°C. Stabilized tissue was homogenized in RNA lysis buffer (QIAGEN) using a rotor-stator homogenizer, whereas sorted DCs were homogenized in RNA lysis buffer using a QIAshredder spin column (QIAGEN). Total RNA was isolated using the RNeasy mini kit (QIAGEN) with on-column DNase digestion. Single-stranded cDNA was synthesized from total RNA with random hexamers and Superscript II reverse transcriptase (Invitrogen). Real-time PCR was performed in triplicate wells using the ABI PRISM 7700 sequence detection system (Applied Biosystems). Primers (Table S1, available at http://www.jem.org/cgi/content/full/jem.20070325/DC1) were obtained from Sigma-Aldrich and tested for quality by serial dilution of template cDNA and melt-curve analysis. Data normalization was performed using 18S rRNA as a reference gene. Quantification of IL-12 p35 and IL-12 p40 was done by Taqman PCR, using primer-probe combinations from Applied Biosystems.
Ex vivo DC stimulatory capacity assay.
CD11c-enriched cells from chimeric mice were fixed with 1% paraformaldehyde for 15 min at room temperature. After washing with PBS, residual paraformaldehyde was quenched by incubation in 0.1 mM glycine for 30 min at room temperature. 5 × 104 fixed and washed DCs were cultured in duplicate in complete medium in round-bottom 96-well plates together with 5 × 104 CFSE-labeled DO11.10 CD4+ T cells plus graded doses of pOVA. The CFSE profile of DO11.10 T cells was assessed by flow cytometry after 96 h of culture.
Flow cytometry.
Cell suspensions were stained in ice-cold FACS buffer (PBS supplemented with 2 mM EDTA, 1% heat-inactivated FCS, and 0.02% sodium azide). Monoclonal antibodies (conjugated to various fluorochromes or biotin) and fluorescence-labeled streptavidin were obtained from BD Biosciences or Caltag. Purified 2.4G2 (anti-FcγRIII/II; produced at Cancer Research UK) was used to block unspecific antibody binding. Where applicable, DAPI was used as a DNA-binding dye for live versus dead discrimination (Invitrogen). Intracellular staining for IL-12 was performed on CD11c-enriched cells using the Fix & Perm kit (Caltag) and allophycocyanin-labeled anti-IL12 p40/p70 (BD Biosciences). Data were collected on FACSCalibur or LSR II cytometers (Becton Dickinson) and were analyzed with FlowJo software (Treestar, Inc.). For analysis of cytokines in serum or culture supernatants, the Cytometric Bead Array (CBA) Mouse Inflammation kit (Becton Dickinson) was used according to the manufacturer's instructions. Data were analyzed using the CBA software (Becton Dickinson).
ELISA.
Levels of IL-1β in serum and culture supernatants were analyzed by a standard sandwich ELISA using a monoclonal rat anti–mouse IL-1β capture antibody (clone 30311; R&D Systems) and polyclonal biotinylated rabbit anti–mouse IL-1β detection antibody (R&D Systems). A serial dilution of purified mouse IL-1β (R&D Systems) was included to calculate the absolute concentration in the test samples. Levels of IFN-β were determined using a commercial IFN-β ELISA (PBL Biomedical Laboratories).
Immunohistochemistry.
For immunohistochemical analysis, isolated spleens were frozen in Tissue-Tek (Sakura Finetek), and 6-μm cryostat sections were prepared. Splenic sections were fixed in 100% acetone for 10 min, air dried, and rehydrated with 2.5% FCS in PBS plus 0.02% NaN3 (staining buffer). The sections were incubated with biotinylated hamster anti–mouse CD11c (clone HL3; BD Biosciences) and unconjugated rat anti–mouse MAdCAM-1 (hybridoma supernatant of clone MECA-367; a gift from R. Mebius, Free University Medical Centre, Amsterdam, Netherlands). The primary antibodies were detected using Alexa Fluor 546–conjugated streptavidin and Alexa Fluor 488–conjugated anti–rat IgG, respectively (Invitrogen). Next, sections were blocked for 5 min with 25 μg/ml rat IgG and subsequently incubated with allophycocyanin-labeled rat anti–mouse CD45R/B220 (clone RA3-6B2; BD Biosciences). All antibodies were diluted in staining buffer to the appropriate concentration, and stainings were performed for 45 min at room temperature. Slides were mounted in Mowiol and analyzed using a laser scanning confocal microscope (Axioplan 2; Carl Zeiss MicroImaging, Inc.), using a 10× Plan-Apochromat NA 0.45 objective. Image analysis was performed using LSM 510 software (Carl Zeiss MicroImaging, Inc.), and quantification of DC distribution in the spleen was performed using ImageJ software (National Institutes of Health).
Online supplemental material.
Fig. S1 shows the extent of maturation induced in splenic DCs by LPS in WT→WT and TLR40/0→TLR40/0 chimeric mice compared with WT and TLR40/0 mice. Fig. S2 shows the cytokine production potential of T cells co-cultured for 7 d with fixed DCs isolated from spleens of PBS- or LPS-injected WT→WT or TLR40/0→WT mice. Fig. S3 shows serum cytokine levels in TLR40/0→WT or WT→WT mice injected with PBS or LPS ± TNF-α and IFN and shows the extent of maturation and level of TNFRI expression in DCs taken from the spleens of the same mice. Fig. S4 shows the extent of maturation and the expression of TNFRI in DCs from the spleens and lymph nodes of WT or TNFΔARE mice. Table S1 lists the primers used in this study for quantitative PCR (SYBR Green). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070325/DC1.
Supplemental Material
Acknowledgments
We thank members of the Immunobiology Laboratory, Cancer Research UK, for advice and critical review of the manuscript and Richard Siegel at the National Institutes of Health for discussions.
This work was funded by Cancer Research UK. M.A. Nolte and S. LeibundGut-Landmann were each funded by an European Molecular Biology Organization long-term fellowship.
The authors have no conflicting financial interests.
Abbreviations used: ARE, AU-rich elements; CCL, CC chemokine ligand; CCR, CC chemokine receptor; CXCL, CXC chemokine ligand; GeoMFI, geometric mean fluorescence intensity; PALS, periarteriolar lymphatic sheath; PAMP, pathogen-associated molecular pattern; PRR, pattern-recognition receptor; SOCS, suppressor of cytokine signaling; TLR, Toll-like receptor.
M.A. Nolte's present address is Dept. of Experimental Immunology, Academic Medical Centre, 1105 AZ Amsterdam, Netherlands.
References
- 1.Janeway, C.A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54:1–13. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801. [DOI] [PubMed] [Google Scholar]
- 3.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 5.Reis e Sousa, C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16:21–25. [DOI] [PubMed] [Google Scholar]
- 6.Hoebe, K., E. Janssen, and B. Beutler. 2004. The interface between innate and adaptive immunity. Nat. Immunol. 5:971–974. [DOI] [PubMed] [Google Scholar]
- 7.Reis e Sousa, C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476–483. [DOI] [PubMed] [Google Scholar]
- 8.Rifkin, I.R., E.A. Leadbetter, L. Busconi, G. Viglianti, and A. Marshak-Rothstein. 2005. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 204:27–42. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger, P. 1994. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12:991–1045. [DOI] [PubMed] [Google Scholar]
- 10.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 11.Gallucci, S., and P. Matzinger. 2001. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13:114–119. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger, P. 2002. The danger model: a renewed sense of self. Science. 296:301–305. [DOI] [PubMed] [Google Scholar]
- 13.Rock, K.L., A. Hearn, C.J. Chen, and Y. Shi. 2005. Natural endogenous adjuvants. Springer Semin. Immunopathol. 26:231–246. [DOI] [PubMed] [Google Scholar]
- 14.Jiang, D., J. Liang, J. Fan, S. Yu, S. Chen, Y. Luo, G.D. Prestwich, M.M. Mascarenhas, H.G. Garg, D.A. Quinn, et al. 2005. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11:1173–1179. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: down-regulation by cytokines and bacterial products. J. Exp. Med. 182:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winzler, C., P. Rovere, M. Rescigno, F. Granucci, G. Penna, L. Adorini, V.S. Zimmermann, J. Davoust, and P. Ricciardi-Castagnoli. 1997. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 185:317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luft, T., K.C. Pang, E. Thomas, P. Hertzog, D.N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947–1953. [PubMed] [Google Scholar]
- 18.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D.F. Tough. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470. [DOI] [PubMed] [Google Scholar]
- 19.Lebre, M.C., J.C. Antons, P. Kalinski, J.H. Schuitemaker, T.M. van Capel, M.L. Kapsenberg, and E.C. De Jong. 2003. Double-stranded RNA-exposed human keratinocytes promote Th1 responses by inducing a Type-1 polarized phenotype in dendritic cells: role of keratinocyte-derived tumor necrosis factor alpha, type I interferons, and interleukin-18. J. Invest. Dermatol. 120:990–997. [DOI] [PubMed] [Google Scholar]
- 20.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi, S.T., L. Lariviere, G. Leveque, S. Clermont, K.J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (TLR4). J. Exp. Med. 189:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752. [PubMed] [Google Scholar]
- 23.Cario, E., I.M. Rosenberg, S.L. Brandwein, P.L. Beck, H.C. Reinecker, and D.K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966–972. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong, L., A.R. Medford, K.M. Uppington, J. Robertson, I.R. Witherden, T.D. Tetley, and A.B. Millar. 2004. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 31:241–245. [DOI] [PubMed] [Google Scholar]
- 25.Faure, E., O. Equils, P.A. Sieling, L. Thomas, F.X. Zhang, C.J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058–11063. [DOI] [PubMed] [Google Scholar]
- 26.Lin, Y., H. Lee, A.H. Berg, M.P. Lisanti, L. Shapiro, and P.E. Scherer. 2000. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J. Biol. Chem. 275:24255–24263. [DOI] [PubMed] [Google Scholar]
- 27.Pivarcsi, A., L. Bodai, B. Rethi, A. Kenderessy-Szabo, A. Koreck, M. Szell, Z. Beer, Z. Bata-Csorgoo, M. Magocsi, E. Rajnavolgyi, et al. 2003. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 15:721–730. [DOI] [PubMed] [Google Scholar]
- 28.Stoll, L.L., G.M. Denning, W.G. Li, J.B. Rice, A.L. Harrelson, S.A. Romig, S.T. Gunnlaugsson, F.J. Miller Jr., and N.L. Weintraub. 2004. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J. Immunol. 173:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagnoff, M.F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 100:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philpott, D.J., S.E. Girardin, and P.J. Sansonetti. 2001. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr. Opin. Immunol. 13:410–416. [DOI] [PubMed] [Google Scholar]
- 31.Schilling, J.D., S.M. Martin, C.S. Hung, R.G. Lorenz, and S.J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA. 100:4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noulin, N., V.F. Quesniaux, S. Schnyder-Candrian, B. Schnyder, I. Maillet, T. Robert, B.B. Vargaftig, B. Ryffel, and I. Couillin. 2005. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J. Immunol. 175:6861–6869. [DOI] [PubMed] [Google Scholar]
- 33.Sato, A., and A. Iwasaki. 2004. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc. Natl. Acad. Sci. USA. 101:16274–16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinzel, F.P. 1990. The role of IFN-gamma in the pathology of experimental endotoxemia. J. Immunol. 145:2920–2924. [PubMed] [Google Scholar]
- 36.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, H. Charest, D. Jankovic, R.N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dieu, M.C., B. Vanbervliet, A. Vicari, J.M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto, F., P. Schaerli, P. Loetscher, C. Schaniel, D. Lenig, C.R. Mackay, S. Qin, and A. Lanzavecchia. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28:2760–2769. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kontoyiannis, D., M. Pasparakis, T.T. Pizarro, F. Cominelli, and G. Kollias. 1999. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 10:387–398. [DOI] [PubMed] [Google Scholar]
- 42.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubo, M., T. Hanada, and A. Yoshimura. 2003. Suppressors of cytokine signaling and immunity. Nat. Immunol. 4:1169–1176. [DOI] [PubMed] [Google Scholar]
- 44.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 45.Li, Y., N. Chu, A. Rostami, and G.X. Zhang. 2006. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J. Immunol. 177:1679–1688. [DOI] [PubMed] [Google Scholar]
- 46.Pape, K.A., A. Khoruts, A. Mondino, and M.K. Jenkins. 1997. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 159:591–598. [PubMed] [Google Scholar]
- 47.Belardelli, F., and M. Ferrantini. 2002. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 23:201–208. [DOI] [PubMed] [Google Scholar]
- 48.Eaton, S.M., E.M. Burns, K. Kusser, T.D. Randall, and L. Haynes. 2004. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J. Exp. Med. 200:1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heufler, C., F. Koch, and G. Schuler. 1988. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J. Exp. Med. 167:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beutler, B., I.W. Milsark, and A.C. Cerami. 1985. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 229:869–871. [DOI] [PubMed] [Google Scholar]
- 51.Tracey, K.J., Y. Fong, D.G. Hesse, K.R. Manogue, A.T. Lee, G.C. Kuo, S.F. Lowry, and A. Cerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 330:662–664. [DOI] [PubMed] [Google Scholar]
- 52.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D.F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 99:3263–3271. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto, F., and A. Lanzavecchia. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177:134–140. [DOI] [PubMed] [Google Scholar]
- 54.De Trez, C., B. Pajak, M. Brait, N. Glaichenhaus, J. Urbain, M. Moser, G. Lauvau, and E. Muraille. 2005. TLR4 and Toll-IL-1 receptor domain-containing adapter-inducing IFN-beta, but not MyD88, regulate Escherichia coli-induced dendritic cell maturation and apoptosis in vivo. J. Immunol. 175:839–846. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Fontecha, A., S. Sebastiani, U.E. Hopken, M. Uguccioni, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spörri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163–170. [DOI] [PubMed] [Google Scholar]
- 57.Starr, R., T.A. Willson, E.M. Viney, L.J. Murray, J.R. Rayner, B.J. Jenkins, T.J. Gonda, W.S. Alexander, D. Metcalf, N.A. Nicola, and D.J. Hilton. 1997. A family of cytokine-inducible inhibitors of signalling. Nature. 387:917–921. [DOI] [PubMed] [Google Scholar]
- 58.Bode, J.G., A. Nimmesgern, J. Schmitz, F. Schaper, M. Schmitt, W. Frisch, D. Haussinger, P.C. Heinrich, and L. Graeve. 1999. LPS and TNFalpha induce SOCS3 mRNA and inhibit IL-6-induced activation of STAT3 in macrophages. FEBS Lett. 463:365–370. [DOI] [PubMed] [Google Scholar]
- 59.Tsuda, Y., H. Takahashi, M. Kobayashi, T. Hanafusa, D.N. Herndon, and F. Suzuki. 2004. CCL2, a product of mice early after systemic inflammatory response syndrome (SIRS), induces alternatively activated macrophages capable of impairing antibacterial resistance of SIRS mice. J. Leukoc. Biol. 76:368–373. [DOI] [PubMed] [Google Scholar]
- 60.Xiao, T., H. Fujita, H. Saeki, H. Mitsui, M. Sugaya, Y. Tada, T. Kakinuma, H. Torii, K. Nakamura, A. Asahina, and K. Tamaki. 2003. Thymus and activation-regulated chemokine (TARC/CCL17) produced by mouse epidermal Langerhans cells is upregulated by TNF-alpha and IL-4 and downregulated by IFN-gamma. Cytokine. 23:126–132. [DOI] [PubMed] [Google Scholar]
- 61.Vulcano, M., C. Albanesi, A. Stoppacciaro, R. Bagnati, G. D'Amico, S. Struyf, P. Transidico, R. Bonecchi, A. Del Prete, P. Allavena, et al. 2001. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur. J. Immunol. 31:812–822. [DOI] [PubMed] [Google Scholar]
- 62.Menges, M., S. Rossner, C. Voigtlander, H. Schindler, N.A. Kukutsch, C. Bogdan, K. Erb, G. Schuler, and M.B. Lutz. 2002. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 195:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svensson, M., A. Maroof, M. Ato, and P.M. Kaye. 2004. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 21:805–816. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, M., H. Tang, Z. Guo, H. An, X. Zhu, W. Song, J. Guo, X. Huang, T. Chen, J. Wang, and X. Cao. 2004. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5:1124–1133. [DOI] [PubMed] [Google Scholar]
- 65.Reis e Sousa, C., G. Yap, O. Schulz, N. Rogers, M. Schito, J. Aliberti, S. Hieny, and A. Sher. 1999. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 11:637–647. [DOI] [PubMed] [Google Scholar]
- 66.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311–316. [DOI] [PubMed] [Google Scholar]
- 67.Rimoldi, M., M. Chieppa, P. Larghi, M. Vulcano, P. Allavena, and M. Rescigno. 2005. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 106:2818–2826. [DOI] [PubMed] [Google Scholar]
- 68.Rimoldi, M., M. Chieppa, V. Salucci, F. Avogadri, A. Sonzogni, G.M. Sampietro, A. Nespoli, G. Viale, P. Allavena, and M. Rescigno. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6:507–514. [DOI] [PubMed] [Google Scholar]
- 69.Bogunovic, M., F. Ginhoux, A. Wagers, M. Loubeau, L. Isola, L. Lubrano, V. Najfeld, R. Phelps, C. Grosskreutz, E. Scigliano, et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 203:2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGonagle, D., and M.F. McDermott. 2006. A proposed classification of the immunological diseases. PLoS Med. 3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rehberg, E., B. Kelder, E.G. Hoal, and S. Pestka. 1982. Specific molecular activities of recombinant and hybrid leukocyte interferons. J. Biol. Chem. 257:11497–11502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.