Abstract
CD4+Foxp3+ regulatory T cells (T reg) are essential for maintaining self-tolerance, but their functional mechanisms and sites of action in vivo are poorly defined. We examined the homing receptor expression and tissue distribution of T reg cells in the steady state and determined whether altering their distribution by removal of a single chemokine receptor impairs their ability to maintain tissue-specific peripheral tolerance. We found that T reg cells are distributed throughout all nonlymphoid tissues tested, and are particularly prevalent in the skin, where they express a unique CCR4+CD103hi phenotype. T reg cell expression of CCR4 and CD103 is induced by antigen-driven activation within subcutaneous lymph nodes, and accumulation of T reg cells in the skin and lung airways is impaired in the absence of CCR4 expression. Mice with a complete loss of CCR4 in the T reg cell compartment develop lymphocytic infiltration and severe inflammatory disease in the skin and lungs, accompanied by peripheral lymphadenopathy and increased differentiation of skin-tropic CD4+Foxp3+ T cells. Thus, selectively altering T reg cell distribution in vivo leads to the development of tissue-specific inflammatory disease.
CD4+Foxp3+ regulatory T (T reg) cells are essential for the maintenance of self-tolerance, and T reg cell deficiency results in spontaneous autoimmunity in both mice and humans (1). T reg cells also control disease in several mouse models of organ-specific autoimmunity, including diabetes (2, 3), experimental autoimmune encephalomyelitis (4, 5), colitis (6), gastritis (7), and collagen-induced arthritis (8). Furthermore, CD25+ T reg cells ameliorate graft-versus-host disease (9, 10), and have been implicated in limiting immunopathology during responses to foreign antigens, including infectious agents such as Leishmania major (11) and influenza (12). Although extensively studied, the mechanisms used by T reg cells to control T cell responses remain controversial, with both cell contact– and cytokine-dependent mechanisms invoked in various in vitro and in vivo settings (13). This suggests that T reg cells may use multiple mechanisms to limit autoimmunity, and may reflect functional heterogeneity among T reg cell subsets that localize to distinct tissue environments.
Although the homing properties and in vivo distribution of T reg cells are still poorly characterized, their appropriate localization is likely to be essential for their ability to prevent autoimmunity. Both cell contact– and cytokine-based immunosuppressive mechanisms would require that T reg cells be in close proximity to their targets. It therefore follows that T reg cells must colocalize with these cells in vivo to effectively modulate their activities. In addition, T reg cell localization may be important for their access to survival and/or proliferative factors that define a unique homeostatic “T reg cell niche” (14).
In vivo, T reg cells express diverse patterns of homing receptors that are expected to target distinct subsets into a wide array of lymphoid and nonlymphoid sites (8, 15). Within the secondary lymphoid organs, T reg cells can block the initial priming and effector differentiation of autoreactive T cells. For instance, in a TCR transgenic mouse model of type 1 diabetes, transferred T reg cells inhibited the differentiation of effector T cells in the LN and blocked their subsequent migration to the pancreatic islets (16). Furthermore, in NOD mice, the ability of transferred T reg cells to prevent diabetes depended on their expression of CD62L and their migration to LNs (2). More recently, in vivo imaging studies have demonstrated that within LNs, T reg cells appear to function by interacting with DCs and limiting their ability to effectively prime naive T cells (17, 18).
Although the activity of T reg cells within nonlymphoid sites is still poorly understood, expression of CD103 (also known as the αE integrin) has been suggested as a marker for “effector memory” T reg cells that preferentially migrate to and function within these sites (8, 19). Indeed, several recent studies have implicated T reg cell activity in nonlymphoid tissues in limiting peripheral inflammation and autoimmunity. In the BDC2.5 adoptive transfer model of diabetes, cotransferred T reg cells neither inhibited the initial activation and expansion of the BDC2.5 effector T cells nor did they change the level of cellular infiltration of the pancreatic islets (20). Instead, their activity appeared to be restricted to controlling the progression and severity of islet destruction within the pancreas. Similarly, during L. major infection, CD103+ T reg cells migrated to the skin in a CCR5-dependent manner, where they served to dampen the immune response to the pathogen (21, 22). T reg cell migration to nonlymphoid tissues was also required for their ability to prevent acute graft-versus-host disease and cardiac graft rejection (23, 24). Finally, migration of T reg cells to the skin was required for the amelioration of OVA-induced, delayed-type hypersensitivity responses (15). Although these data indicate that T reg cell migration into nonlymphoid tissues is essential during inflammatory responses to foreign antigens or in acute models of autoimmunity, this requirement does not appear to be absolute. For example, in an adoptive transfer model of colitis, T reg cells can prevent disease even when their migration to the colon is severely impaired (25). In addition, the importance of T reg cells in maintaining tolerance in nonlymphoid tissues under steady state conditions has not been demonstrated.
A large fraction of T reg cells in human peripheral blood express the chemokine receptor CCR4 and display strong chemotactic responses to its ligands CCL17 and CCL22 (26, 27). CCR4−/− mice fail to develop allograft tolerance after administration of anti-CD154 and donor spleen cells, and this is associated with decreased accumulation of Foxp3-expressing cells in the graft (23). Nevertheless, the role of CCR4 in directing T reg cell migration and function in vivo is still poorly understood. Outside of the thymus, the CCR4 ligand CCL17 is constitutively expressed by endothelial cells in dermal postcapillary venules and by bronchial epithelial cells (28, 29). In addition, both CCL17 and a second CCR4 ligand, CCL22, are expressed by activated B cells, by macrophages, and by several DC subsets (30–32). Therefore, T reg cells may depend on the expression of CCR4 not only to localize in nonlymphoid tissues such as the skin and the lungs but also to direct their migration to different populations of APCs within the secondary lymphoid tissues.
In this study, we examined how the tissue distribution of T reg cells is related to their ability to maintain immune tolerance and prevent inflammatory disease. We demonstrate that the majority of Foxp3+ T reg cells in most murine nonlymphoid tissues are CCR4+ and that T reg cells in the skin have a unique CD103hiCCR4+ surface phenotype. Analysis of antigen-specific T reg cells demonstrates that these cells up-regulate CCR4, CD103, and other skin-homing receptors when they are stimulated by their cognate antigen within subcutaneous LNs under proinflammatory conditions. Furthermore, we show that CCR4 plays a role in the development or survival of skin-tropic CD103hi T reg cells, and in the accumulation of T reg cells in the skin and lung airways. To assess the impact of altering the tissue and microenvironmental distribution of T reg cells, we constructed mixed BM chimeras in which complete loss of CCR4 is restricted to the Foxp3+ T reg cell compartment. In these animals, the lack of CCR4 expression on T reg cells resulted in peripheral lymphadenopathy, and in an increased frequency of CD4+Foxp3− T cells expressing skin-homing receptors. Moreover, these mice developed severe lymphocytic infiltration and inflammation in the skin and the lungs, whereas all other tissues examined remained normal. Thus, selectively perturbing the migration of T reg cells through removal of CCR4 impaired their ability to effectively control CD4+ T cell activation and differentiation and to prevent tissue-specific inflammatory disease.
RESULTS
CCR4+ T reg cells are enriched in nonlymphoid tissues
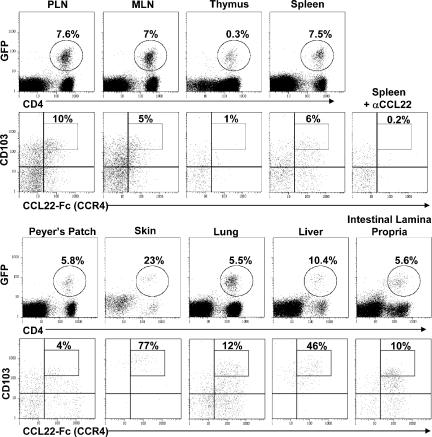
The activity of T reg cells isolated from lymphoid tissues has been extensively characterized, but the presence and activity of these cells within nonlymphoid tissues is not well understood, particularly under noninflammatory conditions. To determine if T reg cells are resident within nonlymphoid tissues, we analyzed CD4+ T cells isolated from Foxp3gfp mice (33). In these animals, GFP has been inserted in-frame upstream of the Foxp3 coding sequence, resulting in expression of a fully functional GFP-Foxp3 fusion protein. As a result, T reg cells can be easily identified on a single cell level in these animals via flow cytometry. In the Foxp3gfp mice, there was a substantial population of CD4+GFP+ cells not only in secondary lymphoid tissues such as the spleen, subcutaneous peripheral LNs (PLN), mesenteric LNs (MLN), and Peyer's patches, but also in all nonlymphoid tissues examined, including the skin, lung, liver, peritoneal cavity, intestinal epithelium, and intestinal lamina propria (Fig. 1 and not depicted). Thus, T reg cells are distributed throughout the body in a wide array of nonlymphoid tissues, even in the absence of any overt inflammatory responses.
Figure 1.
CD4+Foxp3+ T cells are resident in both lymphoid and nonlymphoid tissues. (first and third rows) Flow cytometry analysis of CD4 and GFP expression by lymphocytes isolated from the spleen, skin-draining PLNs, MLNs, thymus, Peyer's patches, skin, lung parenchyma, liver, and intestinal lamina propria of a 12-wk-old Foxp3gfp mouse. Percentage indicates the fraction of CD4+ cells expressing GFP. (second and fourth rows) Expression of CD103 and CCR4 by gated CD4+GFP+cells from each tissue. Percentage indicates the fraction of T reg cells that are CD103highCCR4+ as defined by the rectangular gate in the upper right quadrant. For the CCR4-negative control, spleen cells were stained with CCL22-Fc fusion protein that was preincubated for 5 min with a neutralizing anti-CCL22 monoclonal antibody. Results are representative of greater than six mice analyzed in this fashion
Two surface homing receptors previously associated with T reg cell migration into nonlymphoid tissues are the chemokine receptor CCR4 and the integrin CD103 (8, 23). We therefore examined expression of these molecules by T reg cells isolated from different organs. CCR4+ and CD103+ T reg cells were dramatically enriched in all nonlymphoid tissues examined, which is consistent with their proposed roles in directing T reg cells to these sites (Fig. 1). Interestingly, nearly all GFP+ T reg cells in the skin expressed a unique CD103hiCCR4+ phenotype that was uncommon among T reg cells from all other tissues examined (Fig. 1, boxed gate). CCR4 has been implicated in the migration of CD4+ T cells to the skin and in the development and/or survival of skin-tropic CD4+ T cells that express CD103 and functional E-selectin ligands (E-lig) (34). Likewise, CD103 has been associated with T cell accumulation in epithelial sites, where its ligand E-cadherin is expressed. Therefore, the CD103hiCCR4+ phenotype of cutaneous T reg cells suggests that the coordinated activities of these molecules may be particularly important for the proper localization and function of T reg cells within the skin.
Peripheral recognition of self-antigen alters T reg cell tissue tropism
Naive CD4+ T cells exit the thymus expressing homing receptors such as CD62L and CCR7, which mediate their continual recirculation through the various secondary lymphoid tissues. Upon stimulation with cognate antigen, T cells shift their tissue-tropism and acquire expression of homing receptors that direct their migration into specific nonlymphoid sites (35, 36). Similarly, T reg cells may up-regulate expression of CD103, CCR4, and other homing receptors that direct their entry into nonlymphoid tissues after recognition of self-antigens in the secondary lymphoid tissues. To directly test this hypothesis, we examined OVA-specific T reg cells from double transgenic DO11.10xRIP-mOVA mice. In these animals, OVA (driven by the rat insulin promoter) is expressed in both the thymus and in the pancreatic islets. As a result, ∼50% of the OVA-specific transgenic T cells (identified by staining with the TCR clonotypic antibody KJ1-26) differentiate into fully functional CD25+Foxp3+ T reg cells (37).
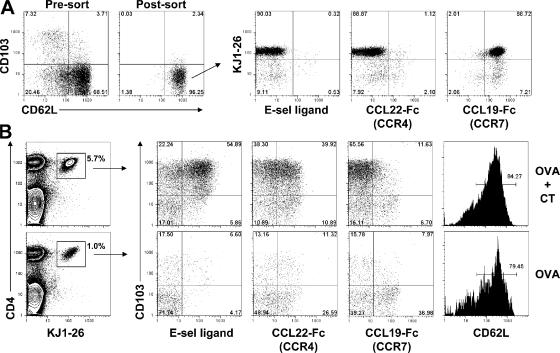
We transferred 105 FACS-sorted CD4+CD25+CD103−CD62L+ T reg cells from DO11.10xRIP-mOVA mice into WT BALB/c recipients. Approximately 90% of the transferred cells were KJ1-26+ and expressed the LN homing receptor CCR7, but were negative for expression of CCR4 and the skin-homing receptor E-lig (Fig. 2 A). To mimic recognition of cutaneous self-antigen in different conditions, the recipient animals were immunized with 200 μg of soluble OVA by subcutaneous injection in PBS, either alone or in combination with 1 μg of cholera toxin (CT) as a proinflammatory stimulus. Cells were harvested from the draining inguinal LNs 5 d after immunization, and homing receptor expression by the OVA-specific T reg cells was assessed by flow cytometry (Fig. 2 B). Upon stimulation with OVA+CT, the KJ1-26+ T reg cell underwent extensive expansion, and the vast majority became CD103hi. In addition, many of the CD103hi cells coexpressed CCR4 and E-lig, and nearly all cells down-regulated CCR7. Although many KJ1-26+ T reg cells also up-regulated CCR4 in response to soluble OVA alone, these cells did not expand as robustly without the proinflammatory stimulus, and few up-regulated CD103 or E-lig, whereas many retained expression of CCR7 and CD62L. Thus, after antigen recognition in the PLN under proinflammatory conditions, the OVA-specific T reg cells appear to have undergone a full program of differentiation that resulted in a dramatic shift in their tissue tropism. This involved robust expansion, accompanied by acquisition of homing receptors required for migration to the skin and loss of a chemokine receptor critical for T cell homing into secondary lymphoid organs. Indeed, 5 d after immunization with OVA+CT, CD4+KJ1-26+ T cells had migrated to the inflamed skin overlaying the injection site (unpublished data). In contrast, a large fraction of T reg cells activated by soluble antigen alone appeared to retain their preferential tropism for the secondary lymphoid organs. Importantly, after immunization with either OVA or OVA+CT, >95% of the KJ1-26+ cells remained Foxp3+, indicating that immunization under these conditions did not alter their functional status as T reg cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1).
Figure 2.
T reg cells alter their tissue tropism after antigen stimulation. (A) Flow cytometry analysis of CD62L and CD103 expression by gated CD4+CD25+ cells from the pooled spleen and PLN of a DO11. 10xRIP-mOVA mouse before and after sorting of CD62L+CD103− cells (left). (right) Expression of the DO11.10 clonotypic TCR (KJ1-26), E-selectin ligand, CCR4, and CCR7 by the sorted CD62L+CD103− T reg cells. (B) Flow cytometry analysis of lymphocytes from the draining inguinal LNs of recipient mice 5 d after immunization with OVA+CT (top) or OVA alone (bottom). (left) Gates used to define the OVA-specific T reg cells. Percentages indicate the frequency of KJ1-26+ cells among total CD4+ cells. (right) Expression of CD103, E-selectin ligand, CCR4, and CCR7 by gated CD4+KJ1-26+ cells.
T reg cell accumulation in the skin and lungs is impaired in the absence of CCR4
To determine if CCR4 has a role in T reg cell development or localization, we constructed mixed BM chimeras using congenically marked CCR4−/− (CD45.2+) and WT B6.SJL (CD45.1+) donors. We then determined if specific subsets of T reg cells were at a competitive disadvantage caused by their lack of CCR4, and whether CCR4−/− T reg cells accumulated in various tissues as efficiently as their WT counterparts.
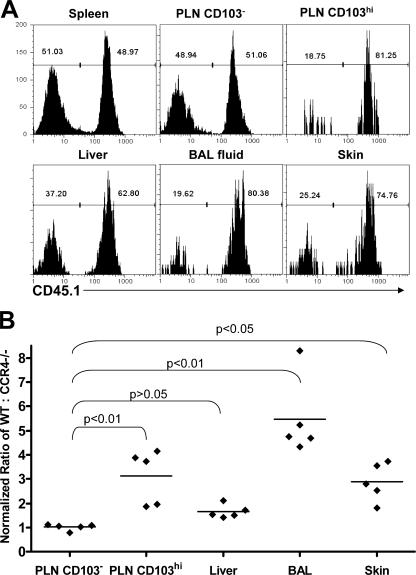
In the spleens of the mixed BM chimeras, the ratio of Foxp3+ T reg cells from the CCR4−/− and WT donors was similar to that observed for total CD4+ T cells, indicating that there was no gross developmental disadvantage for CCR4−/− T reg cells (unpublished data). However, within the subcutaneous PLN, T reg cells expressing high levels of CD103 were predominately derived from the WT donor (Fig. 3 A). For each animal analyzed, we normalized the ratio of WT/CCR4−/− cells among the CD103hi T reg cells in the PLN by the ratio found among total splenic T reg cells. On average, the ratio among the CD103hi cells was approximately threefold higher than the ratio in the spleen, indicating a clear developmental or survival advantage for WT cells in this compartment (Fig. 3 B). A similar threefold increase in the ratio of WT/CCR4−/− cells was observed among E-lig+ T reg cells in the PLN (unpublished data), which is consistent with the preferential expression of E-lig by CD103hi cells after antigen stimulation of OVA-specific T reg cells (Fig. 2 B). In contrast, the ratio of WT/CCR4−/− cells within the CD103low and CD103-negative populations of T reg cells in the PLN, or in any T reg cell populations examined in the MLN, was nearly identical to that found among T reg cells in the spleen (Fig. 3 B and not depicted).
Figure 3.
Impaired accumulation of CCR4−/− T reg cells in the skin and lung airways. (A) Representative flow cytometry analysis of CD45.1 expression by gated CD4+Foxp3+ cells from the indicated tissues of a WT+CCR4−/− mixed BM chimera. WT cells are CD45.1+. (B) The normalized ratio of WT to CCR4−/− T reg cells in the indicated tissues/compartments was derived by dividing the ratio of WT/CCR4−/− T reg cells in each by the ratio of WT/CCR4−/− T reg cells in the spleen. Each data point represents the normalized ratio from one individual chimera. Horizontal lines indicate the average normalized ratio (n = 5) in each tissue/compartment. Statistical analysis was performed using a one-way repeated measures ANOVA (P < 0.0001). P-values for the indicated pairwise comparisons were then computed using Dunnett's Multiple Comparison Test.
Because CCR4 is expressed by most T reg cells in nonlymphoid tissues, we also examined the ratio of WT/CCR4−/− T reg cells in the skin, liver, and lung airways of the chimeric mice. Similar to their CD103hi counterparts in the PLN, we found that cutaneous T reg cells were largely of WT origin, with a normalized ratio approximately threefold higher than that found among T reg cells in the spleen. Surprisingly, among all tissues and T reg cell subsets examined, the highest ratio of WT/CCR4−/− cells was found among T reg cells isolated from the lung airways, with a mean ratio approximately sixfold higher than splenic T reg cells. In contrast, there was only a slight (approximately twofold) preference for WT cells among T reg cells isolated from the livers of these mice that, when compared with the ratio of WT/CCR4−/− cells found in CD103− T reg cells from the PLN, did not reach statistical significance. From these data, we conclude that in the absence of CCR4, the development and/or survival of cutaneous CD103hi T reg cells is impaired, and there is a reduced accumulation of T reg cells in the skin and lung airways.
Lack of CCR4 expression on T reg cells results in cutaneous and pulmonary inflammation
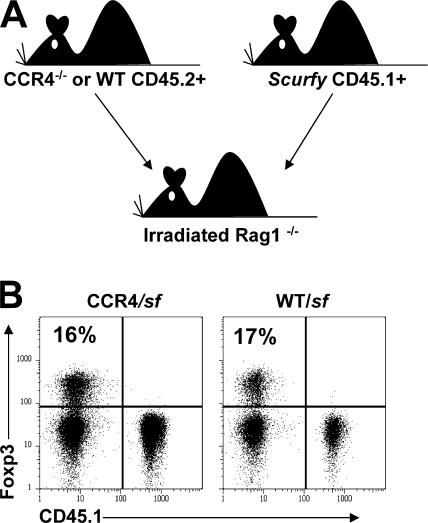
The impaired accumulation of CCR4−/− T reg cells in the skin and lung airways suggests that this receptor helps direct T reg cell migration to these sites in vivo. However, CCR4−/− mice do not develop any spontaneous inflammatory disease indicative of a defect in T reg cell function (38). This may be because migration of both T reg cells and Foxp3− effector memory CD4+ T cells are similarly impaired in these animals. Consistent with this notion, in the WT/CCR4−/− mixed BM chimeras, we found that the accumulation of both Foxp3+ and Foxp3− CD4+ T cells in the skin and lung airways was similarly impaired in the absence of CCR4 (Fig. 3 and Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1). Therefore, to assess the importance of CCR4 for T reg cell localization and function in the context of a largely WT Foxp3− T cell compartment, we constructed mixed BM chimeras by transferring BM from CD45.1+ Foxp3-deficient scurfy (sf) mice (39) and CD45.2+ CCR4−/− mice into irradiated RAG1−/− recipients (Fig. 4 A). In the resulting chimeras, only the CCR4−/− cells can give rise to Foxp3+ T reg cells, whereas all other T cell compartments are a mixture of CCR4+/+ and CCR4−/− cells. As controls, irradiated RAG−/− mice were reconstituted with BM from sf and WT CD45.2+ mice, or with sf BM alone. Hereafter, these mice will be referred to as CCR4/sf, WT/sf, and sf chimeras. In both CCR4/sf and WT/sf chimeras, normal numbers of lymphocytes develop and populate the periphery after BM reconstitution. Furthermore, as expected, all T reg cells developed from the CD45.2+CD45.1− CCR4−/− or WT donor cells (Fig. 4 B).
Figure 4.
Schematic of mixed BM chimera experimental setup and Foxp3+ T cell development. (A) Schematic representation of mixed BM chimera experimental set-up. (B) Representative flow cytometry analysis of CD45.1 and Foxp3 expression by gated CD4+ T cells from the PLN of one pair of CCR4/sf and WT/sf chimeras killed 153 d after BM transplant.
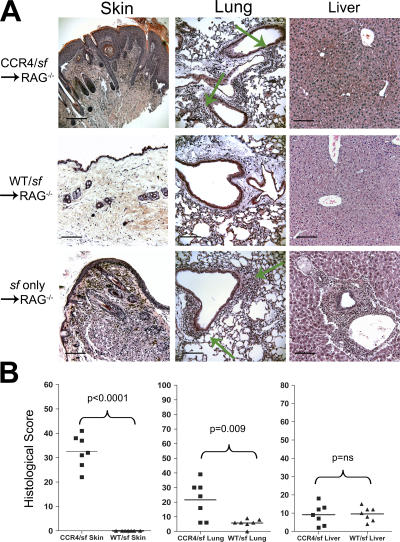
All chimeras that received only sf BM developed severe dermatitis and wasting, and were killed by 40 d after transplantation. Histological analysis of these animals revealed extensive inflammatory disease in the skin, lungs, and liver, which are the three major target tissues of autoimmunity in sf mice (Fig. 5 A, bottom). In contrast, the WT/sf chimeras remained phenotypically normal for up to 300 d after transfer, and showed little or no inflammatory disease in all tissues examined (Fig. 5 A, middle). Therefore, WT T reg cells were able to suppress the inflammatory disease caused by sf-derived CD4+ T cells. However, all CCR4/sf chimeras developed severe localized skin inflammation that was visible 50–150 d after transplantation. When cutaneous inflammatory disease became severe, with visible crusting, alopecia, and erythema in affected regions, each CCR4/sf chimeras was killed and analyzed along with a WT/sf counterpart (100–250 d after BM transplant). Within affected areas of the skin, there was extensive mixed leukocytic infiltration of the dermis accompanied by marked epidermal hyperplasia (Fig. 5 A, top). Inflammatory dermal infiltrates were composed largely of lymphocytes, neutrophils, eosinophils, and mast cells (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1). In addition, there was significant, albeit less severe, lymphocytic infiltration and inflammatory disease in the lungs, which was concentrated around the blood vessels and large airways (Fig. 5 A, arrows). To quantify disease severity in these mice, we developed a histological scoring system based on the severity and overall distribution of inflammatory infiltrates within each tissue section. Blinded analysis of sections from the skin, lungs, and livers from a panel of CCR4/sf- and WT/sf chimeras revealed that the ability of CCR4−/− T reg cells to prevent inflammatory disease in the skin and lungs is significantly impaired (Fig. 5 B). In contrast, WT and CCR4−/− T reg cells were both capable of preventing the extensive inflammatory hepatitis that developed in animals receiving only sf BM.
Figure 5.
Inflammatory disease in the skin and lungs of CCR4/sf chimeras. (A) Photomicrographs (20×) of hematoxylin and eosin–stained sections of the skin, lung, and liver from CCR4/sf, WT/sf (killed 114 d after BM transplant), or sf only chimeras (killed 38 d after BM transplant). Green arrows indicate the location of inflammatory infiltrates in the lungs of CCR4/sf and sf chimeras. (B) Blinded analysis of tissue sections from seven pairs of CCR4/sf and WT/sf chimeras (all killed in pairs 114–250 d after BM transplant). Each section was scored based on the severity and extent of inflammation. Statistical analysis was performed using two-tailed, paired Student's t test. ns, not significantly different (P > 0.05). Bars, 100 μm.
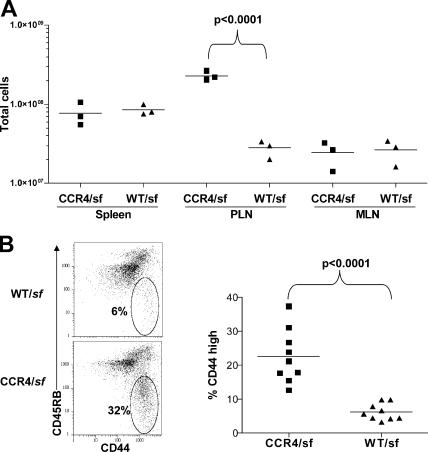
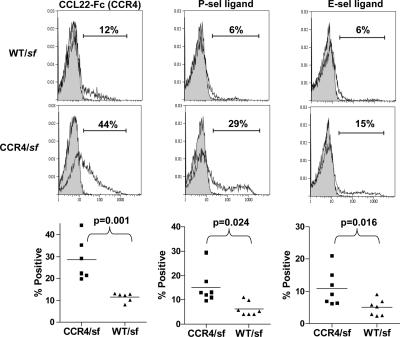
Consistent with the cutaneous inflammation observed in the CCR4/sf chimeras, these mice displayed severe lymphadenopathy selectively in the subcutaneous PLN (Fig. 6 B). This suggests that the CCR4−/− T reg cells failed to efficiently control the activation and differentiation of the sf-derived CD4+ T cells. Indeed, phenotypic analysis of the CD4+ CD45.1+ sf-derived T cells in the PLN showed a significantly increased frequency of CD44hiCD45RBlow effector memory cells when compared with cells from WT/sf chimeras (Fig. 6 B). T cells activated in cutaneous LNs up-regulate skin-homing receptors, such as P-selectin ligand (P-lig), E-lig, and CCR4 (35, 40). Accordingly, there was a significant increase in the fraction of sf-derived CD4+ T cells expressing these receptors in the cutaneous PLN of the CCR4/sf chimeras (Fig. 7). This accumulation of activated, skin-tropic CD4+ T cells in the PLN of CCR4/sf chimeras suggests that these cells migrated to the skin and mediated the cutaneous inflammation observed in these animals. Indeed, in both the inflamed and histologically normal skin of the CCR4/sf chimeras, there was a substantial accumulation of CD4+CD45.1+ sf-derived T cells that was not found in the phenotypically normal WT/sf chimeras (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1, and not depicted). Consistent with the pulmonary inflammation in the CCR4/sf chimeras, CD45.1+ T cells were also enriched in the both the lung airways and parenchyma of these mice. In addition, among CD4+ T cells in the skin and lung airways, there was a 3–4-fold reduction in the fraction of Foxp3+ T reg cells in the CCR4/sf chimeras compared with their WT/sf controls (unpublished data).
Figure 6.
Peripheral lymphadenopathy and enhanced CD4+ T cell activation in CCR4/sf chimeras. (a) Lymphocytes were isolated and counted from the spleen, subcutaneous PLNs, and MLNs of a matched group of three CCR4/sf chimeras (squares) and three WT/sf chimeras (triangles) killed 140 d after BM transplant. Data are representative of six experiments. Statistical analysis was performed using a two-tailed, unpaired Student's t test. (b) Representative flow cytometry analysis of CD44 and CD45RB expression by gated CD4+CD45.1+ sf-derived T cells from the PLN of one pair of WT/sf and CCR4/sf chimeras killed 140 d after BM transplant. (right) The frequency of CD44hi cells amongst gated CD4+CD45.1+ PLN cells from nine matched pairs of WT/sf- and CCR4/sf chimeras (killed 114–250 d after BM transplant). Statistical analysis was performed using a two-tailed, paired Student's t test.
Figure 7.
CCR4/sf chimeras have an elevated frequency of skin-tropic CD4+ T cells. (top) Representative flow cytometry analysis of CCR4, P-selectin ligand, and E-selectin ligand expression by gated CD4+CD45.1+ sf-derived T cells from the PLN of WT/sf and CCR4/sf chimeras (open area) killed 140 d after BM transplant. Shaded areas of the graph indicate background staining in the presence of anti-CCL22 (left) or 10 mM EDTA (middle and right). (bottom) Graphs indicating the frequency of gated CD4+CD45.1+ cells expressing the indicated receptor in the PLNs of five matched pairs of WT/sf- and CCR4/sf chimeras (all killed 114–250 d after BM transplant). Statistical analysis was performed using a two-tailed, paired Student's t test.
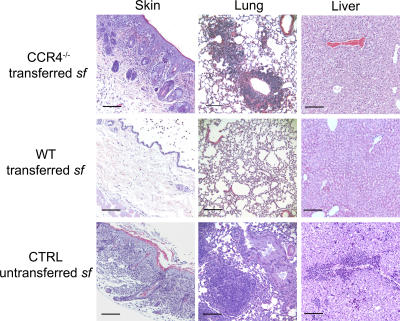
Adoptive transfer of purified CD4+CD25+ T reg cells into neonatal sf mice prevents the development of systemic autoimmune and inflammatory disease in these animals (14). Therefore, to further assess the importance of CCR4-dependent localization of T reg cells in vivo, we compared the ability of WT and CCR4−/− T reg cells to rescue animals from disease in this model. Whereas WT T reg cells effectively prevented inflammatory disease in all tissues examined, sf mice given CCR4−/− T reg cells developed a histological and cellular phenotype similar to the CCR4/sf chimeras, which was visibly apparent after 50 d of age, with severe inflammatory disease evident in the skin and lungs, but not the liver (Fig. 8 and not depicted). Thus, the cutaneous and pulmonary inflammation in the CCR4/sf chimeras is not simply a byproduct of the proinflammatory effects of the lethal irradiation used to condition the recipients, and it is not caused by the lymphopenia present in these animals. In addition, confirming previous results (23), we found that CCR4−/− T reg cells were as efficient as WT T reg cells at suppressing the proliferation of CD4+ T cells in vitro, indicating that there is not a gross defect in the function of CCR4−/− T reg cells (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1). Instead, our data demonstrate that altering the tissue and/or microenvironmental distribution of T reg cells by selective removal of CCR4 leads to excessive activation and differentiation of CD4+ T cells in the PLN and their subsequent accumulation in the skin and lungs, resulting in the development of tissue-specific inflammatory disease.
Figure 8.
CCR4−/− T reg cells fail to prevent cutaneous and pulmonary inflammation after transfer into neonatal sf mice. Photomicrographs of hematoxylin and eosin–stained sections of skin, lung, and liver from either an unmanipulated sf mouse (bottom) or from sf mice given purified WT or CCR4−/− CD4+CD25+ T reg cells (top and middle) shortly after birth. Data are representative of more than eight mice analyzed in each group. Bars, 100 μm
DISCUSSION
The regulation of immune responses by T reg cells in vivo is a complex process, involving multiple T reg cell subsets that appear to use unique functional mechanisms to suppress the activation, differentiation, and function of effector T cells within both lymphoid and nonlymphoid tissues. We examined the tissue distribution of T reg cells in the steady state, and determined the impact of altering this distribution by removal of the chemokine receptor CCR4. Our results have several important implications for current models of T reg cell migration and function.
Early studies of T reg cells suggested they predominantly functioned within secondary lymphoid tissues by inhibiting the initial priming of autoreactive T cells (1). However, data from several models have now convincingly demonstrated that T reg cells can also regulate effector T cell responses to both self- and foreign antigens during acute inflammation at nonlymphoid sites (41). Although T reg cells have been isolated from normal human skin and intestines (27, 42), their role in maintaining immune homeostasis in nonlymphoid sites under steady state conditions is not clear. Our analysis of T reg cell distribution in the Foxp3gfp mice further demonstrates that T reg cells are present within a wide variety of nonlymphoid organs, even in the absence of any overt inflammatory response. This suggests that T reg cells constitutively function to help maintain immune tolerance and prevent autoimmunity at these sites.
The integrin CD103 has been proposed as a marker of effector memory T reg cells with tropism for nonlymphoid tissues (8). Our analysis of T reg cells in Foxp3gfp animals demonstrated that CD103+ T reg cell are present at a higher frequency in nonlymphoid tissues than in the LNs, spleen, and Peyer's patches. However, within most nonlymphoid compartments we examined, including the lungs, liver, intestinal lamina propria, and intestinal epithelium, we found a mixture of both CD103+ and CD103− T reg cells. Thus, expression of CD103 alone cannot be used to define T reg cells with nonlymphoid tissue tropism. However, T reg cells isolated from the skin did uniformly express very high levels of CD103, the expression of which may be selectively induced after their migration into the skin. CD103 has previously been associated with cutaneous T cells (43), and the CD103 ligand E-cadherin is expressed by epidermal keratinocytes (44). Analogous to its proposed role in retaining T cells in the intestinal epithelium, CD103 may also facilitate T reg cell retention in the epidermis. This is consistent with the obligate function of CD103 in T reg cell–mediated immune regulation during cutaneous L. major infection (21), and suggests that the CD103hi subset of T reg cells is phenotypically specialized to localize to and function within the skin. Indeed, the phenotypic diversity of T reg cells resident in different tissues further supports the concept that the T reg cell population as a whole is made up of numerous subsets, each expressing different combinations of homing receptors that act to deliver specific immunoregulatory functions to distinct tissue sites in vivo.
Our analyses of OVA-specific T reg cells demonstrated that T reg cells acquire the ability to migrate to nonlymphoid sites after the recognition of cognate antigen in the secondary lymphoid tissues, and that addition of a proinflammatory stimulus greatly augmented this shift in T reg cell tissue tropism. Most subsets of APC undergo a low level of constitutive trafficking from nonlymphoid tissues into the corresponding draining LNs. Once there, they can present self-antigens they have acquired to the largely autoreactive T reg cell population (45). We propose that when T reg cells recognize self-antigen presented by immature APCs during these noninflammatory conditions (such as those found after administration of soluble antigen in our adoptive transfer model), they largely maintain their tropism for the secondary lymphoid tissues, where they function to suppress the initial priming and differentiation of self-reactive T cells. However, during a strong tissue inflammatory response (such as that induced by addition of CT in our model), T reg cells undergo extensive expansion, during which they acquire the ability to migrate to nonlymphoid tissues. This most likely occurs because of the increased migration of fully mature APCs to the draining LN. These APCs bear a wide range of T cell costimulatory molecules and can drive T reg cell expansion and differentiation. After migration to nonlymphoid sites, T reg cells can limit effector T cell responses, resulting in effective pathogen control without corresponding collateral tissue damage and immunopathology. This type of peripheral immune regulation may be especially important in tissues such as the skin, lungs, and intestines, which are frequently bombarded with foreign antigens and “pathogen-associated molecular patterns” that effectively activate APC. Consistent with this notion, we found that an unusually large fraction (20–40%) of CD4+ T cells in normal skin are Foxp3+ T reg cells (Fig. 1 and not depicted), suggesting that they have a critical function in preventing cutaneous inflammatory disease. Similarly, Foxp3+ T reg cells have been isolated from normal human skin, and a high proportion of T reg cells in human peripheral blood express cutaneous homing receptors (27). The skin and lungs (along with the liver) are primary targets of autoimmunity in Foxp3-deficient sf mice (39, 46), whereas humans deficient in FOXP3 expression and mice that lack T reg cells caused by mutations in various components of the IL-2 pathway generally develop severe enteropathy (47–49). Thus, Foxp3+ T reg cells appear to have a particularly important function in preventing autoimmune and inflammatory disease in these barrier tissues.
The chemokine receptor CCR4 has previously been associated with T reg cell activity in both humans and mice (23, 26). In humans, CCR4 is expressed by most E-selectin ligand+ skin-tropic CD4+ T cells found in peripheral blood, and by nearly all CD4+ T cells isolated from normal skin (28, 50). Accordingly, CCR4 helps direct CD4+ T cells to the skin during responses to protein antigens and haptens in mice (51, 52). In addition, by constructing mixed BM chimeras using WT and CCR4−/− mice as donors, Baekkevold et al. demonstrated that CCR4 plays a substantial role in the development and/or survival of cutaneous CD103+E-lig+ CD4+ T cells (34). In similar mixed chimeras, we confirmed these results (Fig. S2), and additionally demonstrated that the vast majority of CD103hi or E-lig+ T reg cells present in the skin-draining LNs were derived from the WT donor. Furthermore, both T reg cells and CD4+Foxp3− T cells in the skin of these animals were predominantly of WT origin (Fig. 3 and Fig. S2). Thus, our results confirm the role of CCR4 in the optimal accumulation of CD4+ T cells in the skin, and demonstrate that this requirement applies to both conventional CD4+Foxp3− T cells and to Foxp3+ T reg cells.
Surprisingly, loss of CCR4 also severely impaired the accumulation of both Foxp3+ and Foxp3− CD4+ T cells in the lung airways. The CCR4 ligand CCL17 is constitutively expressed in the lungs by bronchial epithelial cells and CD11c+ APCs (29, 32). Additionally, expression of both CCL17 and CCL22 is strongly up-regulated in the lungs during pulmonary inflammation (29, 53). Although CCR4 is expressed by nearly all CD4+ T cells recovered after bronchoalveolar lavage in humans, and at lower levels by most CD4+ cells isolated from human lung parenchyma (54), the function of this receptor in CD4+ T cell trafficking to the lungs remains poorly understood. Our results support a model in which CCR4 plays a significant role in the constitutive migration of CD4+ T cells to the lung airways. In addition, similar to the impaired development or survival of CD103hiE-lig+ skin-tropic T cells, loss of CCR4 may also impair the generation of “lung-tropic” CD4+ T cells, further contributing to their failure to accumulate in the lung airways.
Colocalization of T reg cells with their targets is thought to be important for their suppressive function in vivo. By constructing mixed BM chimeras using CCR4−/− and Foxp3-deficient sf mice as donors, we were able to determine how altering the distribution of T reg cells in vivo impacts their ability to prevent tissue-specific inflammatory disease. Indeed, the phenotype of the CCR4/sf chimeras is indicative of multiple roles for CCR4 in T reg cell migration and function. Within the secondary lymphoid tissues, T reg cells can modulate the ability of APCs to effectively prime naive T cells (17, 18). Upon maturation, many APC subsets produce both CCL17 and CCL22, and this may help attract recently activated CCR4+ T cells for further activation (30, 55, 56). Notably, compared with splenic DCs, both CCL17 and CCL22 are expressed at particularly high levels by epidermal Langerhans cells (LCs) upon their maturation and migration to the skin-draining LNs (57, 58). CCR4 may therefore play an important role in guiding T reg cells to LCs and other cutaneous APC subsets within the PLN. During T cell priming, LCs potently induce CD4+ T cell expression of skin homing receptors, such as P- and E-lig (40). Thus, if CCR4−/− T reg cells fail to effectively compete with WT T cells for access to LCs, we expect that this would result in uncontrolled activation and differentiation of skin-specific autoreactive effector T cells. This is consistent with the selective peripheral lymphadenopathy and increased frequency of sf-derived CD4+CD44hiCD45RBlo skin-tropic cells observed in the CCR4/sf chimeras. Thus, we propose that impaired T reg cell function within the PLN and the inefficient localization of CCR4−/− T reg cells to the skin both contributed to the severe cutaneous inflammation that developed in the CCR4/sf chimeras and in neonatal sf mice that received CCR4−/− T reg cells. Similarly, the pulmonary inflammation that developed in these animals was most likely caused by impaired T reg cell migration to the lung airways, and a corresponding failure to limit effector T cell function at this site.
Despite the fact that CCR4 is expressed by most T reg cells in all nonlymphoid tissues we examined from the Foxp3gfp mice, T reg cell-mediated protection of tissues other than the skin and lungs appeared to function normally, even in the absence of this receptor. However, among nonlymphoid tissues in unmanipulated mice, constitutive expression of CCL22 and/ or CCL17 is largely restricted to the skin and lungs (31, 32). Because we have shown that CCR4 is rapidly up-regulated by T reg cells upon antigen recognition, even in the absence of inflammation, the high frequency of CCR4+ T reg cells in other nonlymphoid tissues may simply reflect continual stimulation of T reg cells by self-antigen at these sites.
The critical function of T reg cells in maintaining immune tolerance in vivo has been firmly established. Indeed, acute depletion of T reg cells in adult mice leads to rapid development of systemic lymphadenopathy and splenomegaly accompanied by severe multiorgan inflammation, indicating that T reg cell–mediated immune suppression is critical throughout life (59). Our data demonstrate that T reg cells are found in a wide variety of lymphoid and nonlymphoid organs, and that simply altering the tissue and microenvironmental distribution of T reg cells results in tissue-specific inflammatory disease. This indicates that the balance of T reg cells and effector T cell activities in different tissues are precisely tuned to allow for effective immunosurveillance and pathogen control, while preventing the development of chronic inflammatory and autoimmune diseases. In addition, the possibility that subsets of T reg cells are phenotypically specialized to function in specific tissues is important to consider when designing therapies involving T reg cell manipulation or enrichment.
MATERIALS AND METHODS
Animals.
C57BL/6J, BALB/cJ, RAG−/− (B6.129S7-Rag1tm1Mom/J), and DO11.10 (C.Cg-Tg[DO11.10]10Dlo/J) mice were purchased from The Jackson Laboratory. CD45.1+ B6.SJL mice (B6.SJL-Ptprca/BoyAiTac) were purchased from Taconic Farms. Scurfy mice (B6.Cg-Foxp3sf/J) were obtained from The Jackson Laboratory and crossed to B6.SJL mice to generate CD45.1+ animals. CCR4−/− mice on the C57BL/6 genetic background were obtained from S. Ziegler (Benaroya Research Institute, Seattle, WA). Foxp3GFP mice have been described previously (33). BALB/c mice expressing RIP-mOVA were provided by A. Abbas (University of California, San Francisco, San Francisco, CA). All animals were housed and bred under specific pathogen-free conditions in the Benaroya Research Institute animal facility. All experiments were approved by the Benaroya Research Institute Institutional Animal Care and Use Committee
Cell isolation.
After whole-body perfusion with 50 ml of heparinized PBS, lymphocytes were isolated as follows. Single-cell suspensions were prepared from thymus, spleen, PLNs (pooled inguinal, axillary, brachial, and superficial cervical nodes), and MLNs by tissue disruption with glass slides, and filtered thru a 40-μM filter. Bronchoalveolar lavage fluid was collected by flushing the lungs with 10 ml cold sterile PBS. To isolate cells from the liver, lung, and skin (after removal of subcutaneous fat by scraping), tissues were finely minced with scissors and vigorously stirred in RPMI with 0.14 U/ml Blendzyme (Roche) and 100 μg/ml DNase I (Roche) for 20 min at 37°C. Supernatants were filtered through a 70-μM cell strainer, and the remaining tissue fragments were digested twice more, pooling all released cells. After dissection and removal of Peyer's patches, intestinal lamina propria lymphocytes (LPL) were isolated as follows. The intestinal epithelium was stripped, as previously described (60, 61), and the remaining intestinal pieces were washed three times in 40 ml of cold RPMI. Intestinal pieces were added to 50 ml of RPMI plus 100 μl 0.5 M MgCl2, 100 μl 0.5 M CaCl2, 500 μl 100× HGPG (111.9 mg/ml Hepes, 29.2 mg/ml L-glutamine, 1,000 U/ml penicillin, 1 mg/ml streptomycin, and 10 mg/ml gentamycin, all purchased from Invitrogen), and 150 U/ml collagenase (Roche). Samples were stirred at 37°C for 1 h, and the released cells were then filtered through nitex. Cells isolated from the skin, lung, liver, and lamina propria were pelleted, resuspended in 44% Percoll (GE Healthcare) in RPMI, layered over 67% Percoll, and spun at 2,800 rpm for 20 min. Lymphocytes were isolated from the interface and used for subsequent flow cytometry analyses.
Flow cytometry.
For cell-surface staining, 106 cells per sample were incubated with various antibodies in staining buffer (HBSS and 3% FCS) for 20 min on ice. Anti-murine antibodies included: anti-CD25 (PC61.5), anti-CD4 (RM4-5), anti-CD62L (MEL-14), anti-CD44 (IM7), anti-CD45RB (C363.16A), anti-CD45.1 (A20), anti-CD45.2 (104), anti-DO11.10 TCR (KJ1-26), and anti-CD103 (2E7); all antibodies were obtained from eBioscience. To assess the expression of CCR4 and CCR7, cells were incubated with CCL22- or CCL19-IgG3 fusion proteins, followed by anti–human IgG-APC (Jackson ImmunoResearch Laboratories). To assess expression of functional P- and E-selectin ligands, cells were sequentially incubated with either a P- or E-selectin–human IgM fusion protein (produced in COS-7 cells), followed by biotinylated goat anti–human IgM (Jackson Immuno Research) and streptavidin–PE (eBioscience). Foxp3 expression was assessed by staining with anti-Foxp3 (FJK-16s; eBioscience) according to the manufacturer's protocol. Data were acquired on a FACsCalibur (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Adoptive transfer and immunization.
CD4+CD25+CD62L+CD103− T reg cells were isolated from the spleen and PLNs of 5-wk-old double-transgenic DO11.10xRIP-OVA mice by FACS. BALB/c mice were given 105 sorted T reg cells by retroorbital injection in 100 μl of PBS. 1 d after transfer, the recipient mice were immunized by subcutaneous injection under the abdominal skin with 200 μg OVA, either alone or in conjunction with 1 μg CT in 100 μl PBS. 5 d after immunization, mice were killed, and lymphocytes were isolated from the draining inguinal LNs for analysis by flow cytometry.
BM chimeras.
BM cells were prepared by flushing the femurs and tibias with cold sterile PBS. The cells were filtered through a 40-μM filter and incubated in hemolytic buffer for 2 min at room temperature. CD4+ cells were depleted from sf-derived BM by magnetic depletion using anti-CD4 microbeads (Miltenyi Biotech), and they contained <1% contaminating CD4+ T cells after depletion. The cells were counted, washed, resuspended in sterile PBS, and injected retroorbitally into anesthetized RAG−/− mice (6–12 wk old) that had received 2 doses (separated by 4 h) of 450 Rad from a cesium irradiator. Recipients were given 2 × 106 CD4-depleted BM cells from sf donors, either alone or mixed with 106 CCR4−/− or WT BM cells. For the WT/CCR4−/− mixed chimeras, Rag−/− recipients received 3 × 106 of a 1:1 mixture of WT (CD45.1+) and CCR4−/− (CD45.2+) BM cells.
Tissue histology.
Tissues were immersion fixed in 10% neutral buffered formalin, paraffin embedded, and cut into 5 μm sections, which were stained with hematoxylin and eosin. All tissue sections were examined by a blinded observer for inflammatory infiltrates and scored for severity (normal, minimal, mild, moderate, and severe) and degree of distribution (focal, focally extensive, multifocal, coalescing, and diffuse) in different subregions of each tissue (see Table S1, available at http://www.jem.org/cgi/content/full/jem.20070081/DC1, for additional details). Each region was then assigned a subscore by multiplying the inflammation severity score by the distribution score. These subscores were then summed to generate a total histological score for each section.
In vitro suppression assay.
CD4+ T cells were isolated from the spleen and LNs of WT or CCR4−/− mice by negative selection with a Dynal CD4 T cell–negative isolation kit (Invitrogen). These cells were further separated into CD25+ and CD25− fractions by staining with CD25-PE, and by magnetic fractionation using anti-PE magnetic microbeads (Miltenyi Biotech). Final suspensions of CD4+CD25+ and CD4+CD25− cells were >90% pure. CD4+CD25− cells were incubated for 9 min at 37°C in 0.8 μM CFSE (Invitrogen) in PBS, washed with 100% FBS, and resuspended in complete DME. In each culture well, 106 CFSE-labeled WT cells were incubated with 106 irradiated (2,500 Rad) WT CD4-depleted spleen cells as APC, with or without addition of WT or CCR4−/− CD4+CD25+ T reg cells. All cultures (except unstimulated control) were stimulated with 3 μg/ml anti-CD3 and 1 μg/ml anti-CD28 for 110 h. Proliferation of CD4+CD25− T cells was measured by assessing relative CFSE dilution by flow cytometry.
Neonatal transfers.
CD4+CD25+ T reg cells (>90% purity in all experiments) were isolated from the spleen and LNs of 8–12-wk-old B6.SJL (CD45.1+) and CCR4−/− (CD45.2+) as described for the suppression assay. Neonatal sf mice (1–2 d old) were given 1–2 × 106 CD4+CD25+ T reg cells in 20 μl PBS by intraperitoneal injection. Mice were monitored for external signs of inflammatory disease and killed between 30 and 50 d after transfer for histological and phenotypic analyses.
Online supplemental material.
Fig. S1 demonstrates that transferred CD4+KJ1-26+ cells maintain Foxp3 expression after immunization in vivo. Fig. S2 illustrates that there is an impaired accumulation of CCR4-deficient CD4+Foxp3− T cells in the skin and lung airways in the WT + CCR4−/− mixed BM chimeras. Fig. S3 shows high-magnification pictures of the dermal infiltration by lymphocytes, neutrophils, eosinophils, and mast cells in CCR4/sf chimeras. Fig. S4 shows the accumulation of sf-derived CD4+ T cells in various tissues of two representative CCR4/sf chimeras compared with controls. Fig. S5 demonstrates that CCR4−/− T reg cells function normally in an in vitro suppression assay. Table S1 is a detailed description of the histological scoring system used to analyze tissue damage in our experimental model. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20070081/DC1.
Supplemental Material
Acknowledgments
We would like to thank Drs. Steve Ziegler and Jessica Hamerman for helpful comments on the manuscript; Dora Gyarmati for technical assistance; Matt Warren for administrative assistance; and Dr. Abul Abbas for providing the RIP-mOVA mice.
This work was supported by grants AI067750 and DK072295 from the National Institutes of Health to D.J. Campbell. B.D. Sather is the recipient of a departmental training grant from the University of Washington Department of Immunology (T32-CA09537). D.J. Campbell was the recipient of a career development award from the Crohn's and Colitis Foundation of America.
The authors have no conflicting financial interests.
Abbreviations used: APC, antigen-presenting cell; CT, cholera toxin; LC, Langerhans cell; PLN, peripheral LN; MLN, mesenteric LN.
References
- 1.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 2.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 3.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 4.Hori, S., M. Haury, A. Coutinho, and J. Demengeot. 2002. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 99:8213–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 6.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L.A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190–200. [DOI] [PubMed] [Google Scholar]
- 7.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huehn, J., K. Siegmund, J.C. Lehmann, C. Siewert, U. Haubold, M. Feuerer, G.F. Debes, J. Lauber, O. Frey, G.K. Przybylski, et al. 2004. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 199:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsley, C.I., M. Karim, A.R. Bushell, and K.J. Wood. 2002. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168:1080–1086. [DOI] [PubMed] [Google Scholar]
- 10.Trenado, A., F. Charlotte, S. Fisson, M. Yagello, D. Klatzmann, B.L. Salomon, and J.L. Cohen. 2003. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 112:1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 12.Suvas, S., U. Kumaraguru, C.D. Pack, S. Lee, and B.T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338–344. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 15.Siegmund, K., M. Feuerer, C. Siewert, S. Ghani, U. Haubold, A. Dankof, V. Krenn, M.P. Schon, A. Scheffold, J.B. Lowe, et al. 2005. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 106:3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarween, N., A. Chodos, C. Raykundalia, M. Khan, A.K. Abbas, and L.S. Walker. 2004. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J. Immunol. 173:2942–2951. [DOI] [PubMed] [Google Scholar]
- 17.Tadokoro, C.E., G. Shakhar, S. Shen, Y. Ding, A.C. Lino, A. Maraver, J.J. Lafaille, and M.L. Dustin. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 203:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang, Q., J.Y. Adams, A.J. Tooley, M. Bi, B.T. Fife, P. Serra, P. Santamaria, R.M. Locksley, M.F. Krummel, and J.A. Bluestone. 2006. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 7:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann, J., J. Huehn, M. de la Rosa, F. Maszyna, U. Kretschmer, V. Krenn, M. Brunner, A. Scheffold, and A. Hamann. 2002. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as. Proc. Natl. Acad. Sci. USA. 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, Z., A.E. Herman, M. Matos, D. Mathis, and C. Benoist. 2005. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suffia, I., S.K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444–5455. [DOI] [PubMed] [Google Scholar]
- 22.Yurchenko, E., M. Tritt, V. Hay, E.M. Shevach, Y. Belkaid, and C.A. Piccirillo. 2006. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 203:2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, I., L. Wang, A.D. Wells, M.E. Dorf, E. Ozkaynak, and W.W. Hancock. 2005. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wysocki, C.A., Q. Jiang, A. Panoskaltsis-Mortari, P.A. Taylor, K.P. McKinnon, L. Su, B.R. Blazar, and J.S. Serody. 2005. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 106:3300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denning, T.L., G. Kim, and M. Kronenberg. 2005. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. 174:7487–7491. [DOI] [PubMed] [Google Scholar]
- 26.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirahara, K., L. Liu, R.A. Clark, K. Yamanaka, R.C. Fuhlbrigge, and T.S. Kupper. 2006. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 177:4488–4494. [DOI] [PubMed] [Google Scholar]
- 28.Campbell, J.J., G. Haraldsen, J. Pan, J. Rottman, S. Qin, P. Ponath, D.P. Andrew, R. Warnke, N. Ruffing, N. Kassam, et al. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 400:776–780. [DOI] [PubMed] [Google Scholar]
- 29.Sekiya, T., M. Miyamasu, M. Imanishi, H. Yamada, T. Nakajima, M. Yamaguchi, T. Fujisawa, R. Pawankar, Y. Sano, K. Ohta, et al. 2000. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 165:2205–2213. [DOI] [PubMed] [Google Scholar]
- 30.Alferink, J., I. Lieberam, W. Reindl, A. Behrens, S. Weiss, N. Huser, K. Gerauer, R. Ross, A.B. Reske-Kunz, P. Ahmad-Nejad, et al. 2003. Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 197:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaniel, C., E. Pardali, F. Sallusto, M. Speletas, C. Ruedl, T. Shimizu, T. Seidl, J. Andersson, F. Melchers, A.G. Rolink, and P. Sideras. 1998. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J. Exp. Med. 188:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberam, I., and I. Forster. 1999. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur. J. Immunol. 29:2684–2694. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 34.Baekkevold, E.S., M.A. Wurbel, P. Kivisakk, C.M. Wain, C.A. Power, G. Haraldsen, and J.J. Campbell. 2005. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J. Exp. Med. 201:1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell, D.J., and E.C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson-Lindbom, B., M. Svensson, M.A. Wurbel, B. Malissen, G. Marquez, and W. Agace. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker, L.S.K., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chvatchko, Y., A.J. Hoogewerf, A. Meyer, S. Alouani, P. Juillard, R. Buser, F. Conquet, A.E. Proudfoot, T.N. Wells, and C.A. Power. 2000. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 191:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godfrey, V.L., J.E. Wilkinson, and L.B. Russell. 1991. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 40.Dudda, J.C., J.C. Simon, and S. Martin. 2004. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J. Immunol. 172:857–863. [DOI] [PubMed] [Google Scholar]
- 41.Huehn, J., K. Siegmund, and A. Hamann. 2005. Migration rules: functional properties of naive and effector/memory-like regulatory T cell subsets. Curr. Top. Microbiol. Immunol. 293:89–114. [DOI] [PubMed] [Google Scholar]
- 42.Uhlig, H.H., J. Coombes, C. Mottet, A. Izcue, C. Thompson, A. Fanger, A. Tannapfel, J.D. Fontenot, F. Ramsdell, and F. Powrie. 2006. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177:5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agace, W.W., J.M. Higgins, B. Sadasivan, M.B. Brenner, and C.M. Parker. 2000. T-lymphocyte-epithelial-cell interactions: integrin alpha(E) (CD103)beta(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 12:563–568. [DOI] [PubMed] [Google Scholar]
- 44.Jakob, T., M.J. Brown, and M.C. Udey. 1999. Characterization of E-cadherin-containing junctions involving skin-derived dendritic cells. J. Invest. Dermatol. 112:102–108. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh, C.S., Y. Liang, A.J. Tyznik, S.G. Self, D. Liggitt, and A.Y. Rudensky. 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 21:267–277. [DOI] [PubMed] [Google Scholar]
- 46.Chen, Z., C. Benoist, and D. Mathis. 2005. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc. Natl. Acad. Sci. USA. 102:14735–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A.C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261. [DOI] [PubMed] [Google Scholar]
- 48.Wildin, R.S., S. Smyk-Pearson, and A.H. Filipovich. 2002. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, and H. Griesser. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. [DOI] [PubMed] [Google Scholar]
- 50.Kunkel, E.J., J. Boisvert, K. Murphy, M.A. Vierra, M.C. Genovese, A.J. Wardlaw, H.B. Greenberg, M.R. Hodge, L. Wu, E.C. Butcher, and J.J. Campbell. 2002. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am. J. Pathol. 160:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiss, Y., A.E. Proudfoot, C.A. Power, J.J. Campbell, and E.C. Butcher. 2001. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell–attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell, J.J., D.J. O'Connell, and M.A. Wurbel. 2007. Cutting edge: chemokine receptor ccr4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 178:3358–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman, C.M., V.R. Stolberg, B.C. Chiu, N.W. Lukacs, S.L. Kunkel, and S.W. Chensue. 2006. CCR4 participation in Th type 1 (mycobacterial) and Th type 2 (schistosomal) anamnestic pulmonary granulomatous responses. J. Immunol. 177:4149–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell, J.J., C.E. Brightling, F.A. Symon, S. Qin, K.E. Murphy, M. Hodge, D.P. Andrew, L. Wu, E.C. Butcher, and A.J. Wardlaw. 2001. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J. Immunol. 166:2842–2848. [DOI] [PubMed] [Google Scholar]
- 55.Katou, F., H. Ohtani, T. Nakayama, K. Ono, K. Matsushima, A. Saaristo, H. Nagura, O. Yoshie, and K. Motegi. 2001. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am. J. Pathol. 158:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, H.L., and J.G. Cyster. 1999. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 284:819–822. [DOI] [PubMed] [Google Scholar]
- 57.Ross, R., X.L. Ross, H. Ghadially, T. Lahr, J. Schwing, J. Knop, and A.B. Reske-Kunz. 1999. Mouse Langerhans cells differentially express an activated T cell-attracting CC chemokine. J. Invest. Dermatol. 113:991–998. [DOI] [PubMed] [Google Scholar]
- 58.Fujita, H., A. Asahina, M. Sugaya, K. Nakamura, P. Gao, H. Fujiwara, and K. Tamaki. 2005. Differential production of Th1- and Th2-type chemokines by mouse Langerhans cells and splenic dendritic cells. J. Invest. Dermatol. 124:343–350. [DOI] [PubMed] [Google Scholar]
- 59.Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. [DOI] [PubMed] [Google Scholar]
- 60.Laky, K., L. Lefrancois, and L. Puddington. 1997. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J. Immunol. 158:1417–1427. [PubMed] [Google Scholar]
- 61.Goodman, T., and L. Lefrancois. 1989. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J. Exp. Med. 170:1569–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.