Abstract
Aire-expressing medullary thymic epithelial cells (mTECs) play a key role in preventing autoimmunity by expressing tissue-restricted antigens to help purge the emerging T cell receptor repertoire of self-reactive specificities. Here we demonstrate a novel role for a CD4+3− inducer cell population, previously linked to development of organized secondary lymphoid structures and maintenance of T cell memory in the functional regulation of Aire-mediated promiscuous gene expression in the thymus. CD4+3− cells are closely associated with mTECs in adult thymus, and in fetal thymus their appearance is temporally linked with the appearance of Aire+ mTECs. We show that RANKL signals from this cell promote the maturation of RANK-expressing CD80−Aire− mTEC progenitors into CD80+Aire+ mTECs, and that transplantation of RANK-deficient thymic stroma into immunodeficient hosts induces autoimmunity. Collectively, our data reveal cellular and molecular mechanisms leading to the generation of Aire+ mTECs and highlight a previously unrecognized role for CD4+3−RANKL+ inducer cells in intrathymic self-tolerance.
In the thymus, the α-β T cell receptor repertoire is subjected to selection events regulated by distinct thymic stromal cells (1). After positive selection by cortical epithelial cells (cTECs), thymocytes up-regulate CCR7, migrate to the medulla, and are subjected to negative selection (2). In the medulla, thymocytes interact with a specialized subset of medullary epithelium (medullary thymic epithelial cells [mTECs]) (3) expressing costimulatory molecules and self-tissue–restricted antigens (TRA), the latter regulated in part by Aire, a transcription factor defective in the autoimmune disease autoimmune polyendocrinopathy candidiasis extrodermal dystrophy (4). In Aire−/− mice, loss of TRAs correlates with the onset of multiorgan autoimmunity (4), and loss of a single TRA, interphotoreceptor-binding protein, triggers eye-specific autoimmunity (5). Importantly, this phenotype maps to a thymic epithelial cell (TEC) defect (4), underlining the importance of Aire+ mTECs in maintaining self-tolerance. Despite their key role and the recent identification of bipotent progenitors for cTECs and mTECs (6), the developmental pathways and mechanisms regulating development of Aire+ mTECs from this progenitor pool remain unclear. Here, we show that CD80+Aire+ mTECs derive from CD80−Aire− progenitors as a result of RANK-mediated signals from a previously unreported intrathymic CD4+3−RANKL+ lymphoid tissue inducer (LTi) population, and that RANK deficiency in TECs promotes the onset of autoimmunity. Collectively, our data define a novel role in thymus for CD4+3− inducer cells that to date have been associated with the development and function of secondary lymphoid tissue, and for the first time identify RANK as a key regulator of central tolerance.
RESULTS AND DISCUSSION
Haemopoietic cells regulate mTEC development
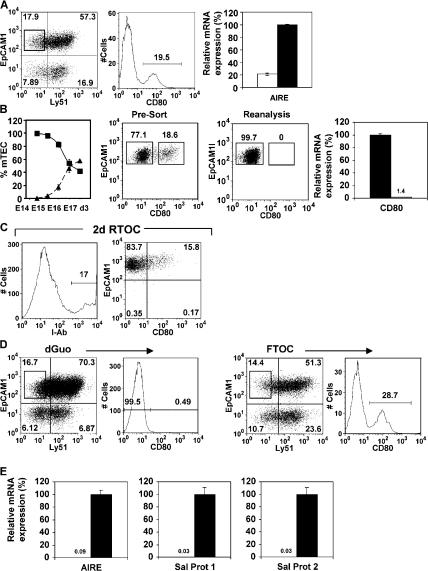
Ly51−EpCAM1+ mTECs (7) can be subdivided into CD80− and CD80+ subsets (Fig. 1 A), and quantitative PCR (qPCR) (8) analysis of purified CD80+ and CD80− mTECs shows Aire is associated with CD80+ mTECs (Fig. 1 A and reference 7), as are the Aire-dependent TRAs (4) salivary protein (SP)1 and SP2 (not depicted). Whether CD80−Aire− and CD80+Aire+ cells represent distinct mTEC lineages, or maturational states within a single mTEC lineage, is unclear (1, 9). To determine their developmental relationship, we examined their appearance in ontogeny. Early in development, mTECs are largely CD80−, with CD80+ mTECs appearing later (Fig. 1 B), consistent with a precursor–product relationship. To address this directly, purified Ly51−EpCAM1+CD80− mTECs (Fig. 1 B) from 7-d H-2b fetal thymus organ culture (FTOC) were mixed with disaggregated fetal thymus suspensions from MHC-mismatched H-2d embryos at a 1:5 ratio. Chimeric reaggregate thymus organ cultures (RTOCs) were cultured for 2 d, disaggregated, and analyzed by flow cytometry. Fig. 1 C shows the introduced IAb+ donor-derived cells persist over this period, and in contrast to the outset of culture when introduced mTECs were CD80− (Fig. 1 B), a proportion of IAb donor-derived mTECs are CD80+. These findings identify a precursor–product relationship within mTECs, consistent with the notion that Aire+CD80+ mTECs are generated from CD80− progenitors.
Figure 1.
Haemopoietic cells regulate mTEC development. EpCAM1+Ly51− mTECs in 7 d FTOCs (A) can be subdivided into CD80− and CD80+ subsets. qPCR anaysis shows mRNA for Aire is abundant in CD80+ mTECs (black bar) compared with CD80− mTECs (white bar). The left graph in B shows percentages of CD80− mTECs (▪) and CD80+ (▴) mTEC subsets within the total mTEC population, calculated after flow cytometric analysis of digested thymuses of the indicated ages. H-2b CD80− mTECs, shown by FACS (B) to lack surface CD80 expression and by PCR to lack CD80 mRNA (black bars, CD80+ mTEC; white bars, CD80− mTECs), were used to make RTOCs with H-2d thymus suspensions. RTOCs were analyzed for I-Ab (C, left), Ly51, EpCAM1, and CD80 expression after 2 d. Gating on I-Ab+ mTECs (C, right) shows CD80− mTECs have generated CD80+ mTECs. Analysis of mTECs in FTOCs or dGuo-treated FTOCs (D) shows absence of the CD80+ mTEC subset in dGuo FTOCs. qPCR analysis (E) shows Aire, SP1, and SP2 expression in mTECs from FTOCs (black bars) but not dGuo-treated FTOCs (white bars).
To study the mechanisms regulating maturation of CD80− mTEC progenitors into Aire+ mTECs, and as studies have implicated haemopoietic cell crosstalk in mTEC development (10), we compared mTECs in FTOC and 2-deoxyguanosine (dGuo) FTOCs, the latter to selectively eliminate haemopoietic cells (11). Although CD80− mTECs are detectable in both untreated and dGuo FTOC, the latter lack CD80+ mTECs (Fig. 1 D). Furthermore, dGuo FTOC lack expression of Aire and Aire-dependent TRAs SP1 and SP2 (Fig. 1 E), consistent with the absence of CD80+ mTECs. Together with lineage analysis (Fig. 1 C), this suggests that a dGuo-sensitive cell regulates Aire+CD80+ mTEC maturation.
CD4+3− LTi cells regulate mTEC development
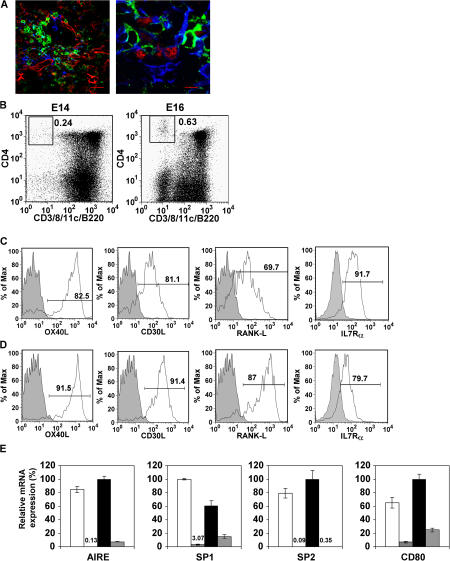
We next devised experiments to identify the haemopoietic cell regulating mTEC development. In agreement with earlier studies, mTECs from Rag1−/− mice express mRNA for Aire and Aire-dependent TRA (3, 12, 13, and unpublished data), suggesting that T cell development beyond the DN3 stage is not required for mTEC development. In secondary lymphoid tissues, CD4+3− LTi cells have been shown to be key regulators of stromal cells, and we recently identified their role in maintaining CCL21 expression (14) by interacting with podoplanin+ T-zone stroma (15). In line with a recent review by Derbinski and Kyewski (13), we wondered if a similar situation might operate in thymus, as CCR7 ligands regulate thymocyte migration to the medulla (2) where podoplanin+ stromal cells exist (16). Analysis of fetal and adult Rag1−/− mice revealed that CD4+3− LTi identical to those found in peripheral lymphoid organs were present in thymus, and confocal analysis demonstrated their close association with Aire+ mTECs (Fig. 2 A). Moreover, FTOCs initiated from E14 and E16 thymus contained CD4+3− LTi that lack CD8, B220, and CD11c (Fig. 2 B), showing that LTi cells are present in thymus at a time that correlates with the appearance of Aire+CD80+ mTECs (Fig. 1 B) and induction of Aire expression (12). Analysis of LTi cells in adult thymus (Fig. 2 C) showed expression of the TNF ligands OX40L and CD30L (17), RANKL, and IL7Rα, as seen by adult splenic LTi (Fig. 2 D). PCR analysis showed thymic LTi lack expression of Rag-1, but express RORγt (unpublished data), a gene expressed by LTi cells in developing secondary lymphoid tissue (18). Collectively, these observations identify LTi cells in fetal and adult thymus which possess key hallmarks of LTi cells in secondary lymphoid tissue (17–20).
Figure 2.
CD4+3− LTi cells regulate mTEC development. Thymic sections of 4-wk-old Rag1−/− mice (A, left) were stained with antibodies to mTECs (ERTR5, red), CD4 (green), CD3 (white), and CD11c (blue). In the right panel of A, sections were stained for Aire (red), CD4 (green), and keratin5+ mTECs (blue). In both cases, CD4+3− cells appear green. Bars, 5 μm. Flow cytometry of cells harvested from FTOCs from E14 and E16 WT embryos (B) shows CD4+3− cells that lack expression of CD8, CD11c, and B220, whereas CD4+3− cells from adult thymus (C) express OX40L, CD30L, IL-7Rα, and RANKL in a manner comparable to splenic CD4+3− cells (D). Shaded histograms are staining controls. E shows qPCR analysis of mTECs in RTOCs initiated from either dGuo-treated stroma alone (vertical bars), or with added CD4+3− LTi cells (E, black bars) or CD4+8+ thymocytes (E, hatched bars). Expression levels in unmanipulated FTOCs are shown for comparison (E, white bars.)
The close association of LTi cells with mTECs raised the possibility that they play a role in regulating maturation of Aire+ mTECs. Although previous analysis of Rag−/− mice suggested that CD4−8− thymocytes play a role in induction of Aire (12), the presence of LTi cells in the Rag−/− thymus (Fig. 2 A) highlights these cells as candidates in regulating mTEC development. To test this, LTi cells were isolated from E15 fetal spleen (14, 19) to avoid potential contamination by thymic stroma and were used to make RTOCs by mixing with dGuo-treated thymic stroma lacking Aire+CD80+ mTECs (Fig. 1, D and E). Importantly, apart from macrophages, dGuo treatment removes all haemopoietic cells, including LTi cells (11 and unpublished data). After 5 d, RTOCs were harvested and analyzed by qPCR. Fig. 2 E shows that unlike RTOCs made from dGuo-treated stroma alone, RTOCs of dGuo-treated stroma and LTi cells contained readily detectable levels of Aire, SP1, SP2, and CD80 mRNA (Fig. 2 E). In contrast, RTOCs initiated from dGuo stroma and CD4+8+ thymocytes (that give rise to mature thymocytes [11]) lacked Aire, SP1, and SP2 expression and showed little induction of CD80 expression (Fig. 2 E), indicating the specificity of the effect of LTi cells. Thus, CD4+3− LTi cells are found within the thymic medulla associated with Aire+ mTECs and are sufficient to induce Aire and CD80 expression and expression of Aire-dependent TRA.
RANK–RANKL signals from LTi cells regulate mTEC development
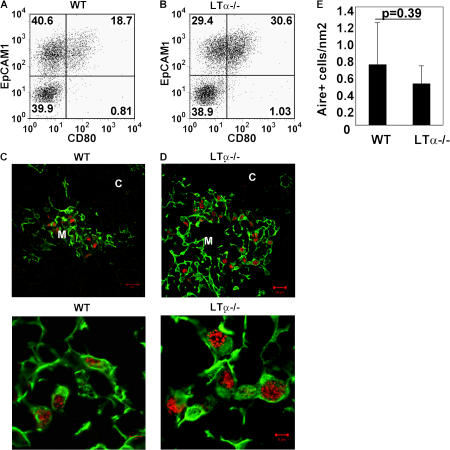
We next analyzed the molecular mechanism by which LTi cells induce development of Aire+ mTECs. As Aire is first expressed in fetal thymus (12) when LTi cells lack OX40L and CD30L (19), it seems unlikely these molecules would be required for initial development of Aire+ mTECs. Both fetal and adult LTi cells express lymphotoxin α (LTα) (18–20), and other studies have implicated LTα in Aire+ mTEC development (21). However, our analysis shows CD80+ mTECs are present in both LTα−/− (22) and WT mice (Fig. 3, A and B). Moreover, analysis using an anti-Aire antibody that does not stain Aire−/− tissue (23) shows that Aire+ mTECs are present at a similar frequency in WT and LTα−/− mice (Fig. 3, C–E). In addition, similar levels of Aire-dependent TRAs, SP1 and SP2 mRNA, were found in both WT and LTα−/− mice (unpublished data). Although the reason for this discrepancy is unclear, it is interesting that while Boehm et al. (24) showed a reduction in CD80+ mTEC frequency in LTβR−/− mice, they reported normal Aire expression, which correlates well with the normal frequency of Aire+ mTECs in LTα−/− mice shown here. Thus, although LTα-LTβR signaling may influence some aspects of mTEC development, such interactions are not essential for Aire+ mTEC development.
Figure 3.
Aire+ mTECs are present in LTα−/− mice. Stroma-enriched adult thymus digests from WT (A) and LTα−/− mice (B) were stained with antibodies to CD45, EpCAM1, Ly51, and CD80. Data are gated on CD45−Ly51− cells. Sections of WT (C) and LTα−/− (D) adult thymus were stained for keratin 5 (green) and Aire (red). The bottom two panels show higher power magnification. Bars: (C and D, top) 20 μm; (C and D, bottom) 5 μm. E shows quantitation of Aire+ mTECs in sections of WT and LTα−/− mice. Student's t test was run to determine the p-value, which was not significant.
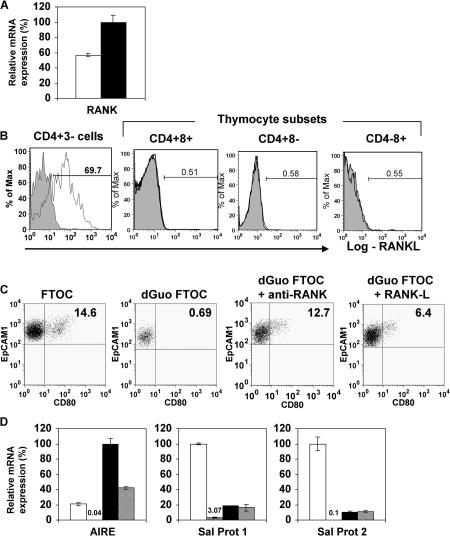
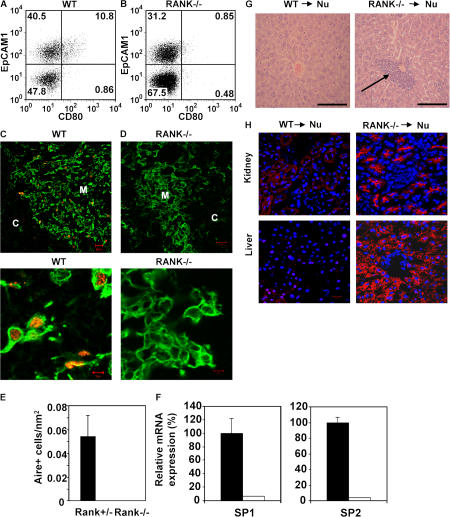
Studies on LTi cells in lymph node development show that interactions between the cell surface receptor RANK and its ligand RANKL (also known as TRANCE) are important (25, 26). RANKL is expressed by both thymic (Fig. 2) and splenic LTi cells (20), but not by thymocytes (Fig. 4 B). Mice lacking TRAF6, a downstream mediator of RANK signaling, lack Aire+ mTECs (27), yet the TNF-R responsible for their absence was not identified. Analysis of cTECs and mTECs shows that the latter express higher levels of RANK mRNA (Fig. 4 A), indicating mTECs may be responsive to RANKL. To obtain direct evidence that RANK–RANKL interactions regulate development of Aire+CD80+ mTECs, dGuo FTOCs that lack this population (Fig. 1, D and E) were cultured for 2 d with either RANKL or an agonistic antibody to RANK, disaggregated, and analyzed for the presence of CD80+ mTECs and Aire expression. Importantly, treatment of dGuo FTOC with either anti-RANK or RANKL induced the appearance of CD80+ mTECs (Fig. 4 C), mRNA for Aire, and to a lesser extent, SP1 and SP2 (Fig. 4 D), the latter perhaps reflecting the short-term (2 d) period of stimulation and the requirement for initial Aire-dependent transcription of these TRAs. Collectively, these findings provide direct evidence that RANK signaling can induce the appearance of Aire+CD80+ mTECs and Aire-dependent TRAs. To provide definitive evidence for a role for RANK in development of Aire+ mTECs in vivo, we analyzed the thymic microenvironment of RANK−/− mice, previously reported to support normal T cell development (26). In contrast to littermate controls, CD80+ mTECs were absent from RANK−/− mice (Fig. 5, A and B). Moreover, confocal analysis demonstrated that although keratin5+ medullary areas were present in RANK−/− mice, there was a marked absence of Aire+ mTECs (Fig. 5, C–E), correlating with an absence of Aire-dependent TRAs (Fig. 5 F).
Figure 4.
RANK–RANKL interactions promote Aire+ mTEC development. qPCR analysis of RANK expression (A) in EpCAM1+Ly51− mTECs (black bar) and EpCAM1+Ly51+ cTEC (white bar). B shows analysis of RANKL expression in thymic CD4+3− LTi cells and thymocyte subsets. Shaded histograms are staining controls. C shows analysis of EpCAM1+Ly51−CD80+ mTEC in FTOCs and dGuo-treated FTOCs cultured in the absence or presence of anti-RANK or recombinant RANKL for 2 d. Quadrant gates are set using staining levels obtained using isotype-matched control antibodies. (D) qPCR analysis of Aire, SP1, and SP2 expression was performed in untreated FTOCs (white bars) dGuo FTOC (vertical bars), and in dGuo FTOCs treated with either anti-RANK (black bars) or recombinant RANKL (hatched bars).
Figure 5.
Lack of Aire+CD80+ mTECs in RANK−/− mice promotes autoimmunity. Stroma-enriched adult thymus digests from WT (A) and RANK−/− mice (B) were stained for CD45, EpCAM1, Ly51, and CD80. Data are gated on CD45−Ly51− cells. In C and D, tissue sections of WT and RANK−/− adult thymus were stained for keratin 5 (green) and Aire (red). Higher power magnification is shown in the bottom panels. Bars: (C and D, top) 20 μm; (C and D, bottom) 5 μm. E shows quantitation of Aire+ mTECs in sections of RANK+/− and RANK−/− thymus. F is qPCR highlighting absence of TRA expression in RANK−/− thymus (white bars) compared with WT thymus (black bars). Histological analysis of leukocytic infiltrates in the liver of nude mice (arrow) transplanted with RANK−/− dGuo-treated, but not WT thymus, is in G. (H) Immunohistochemical analysis of Rag−/− tissues using serum obtained from RANK−/− and WT transplanted nude mice. Red, autoantibody staining; blue, DAPI staining. Bars: G, 100 μm; H, 20 μm.
Transplantation of RANK−/− thymic stroma leads to autoimmunity
As the RANK−/− thymus lacks Aire expression and transplantation of Aire−/− thymus into nude mice promotes T cell–mediated autoimmunity (4), we predicted that similar symptoms would be seen after transplant of RANK−/− thymic tissue. Relevant to this, the absence of a reported autoimmune phenotype in RANK−/− mice could be caused by the severity of the RANK−/− phenotype outside the immune system, which leads to abnormal tooth and skeletal development and premature death (26). To address the importance of RANK in relation to thymic tolerance, dGuo-treated E15 RANK−/− and littermate thymuses were transplanted under the kidney capsule of nude mice (4). After 4 wk, in contrast to mice receiving control thymus lobes, mice receiving RANK−/− thymus lobes showed signs of a wasting disease, including weight loss and diarrhea. After being killed, recovery of control and RANK−/− thymus grafts showed the presence of CD4+8+, CD4+8−, and CD4−8+ thymocytes in both cases (unpublished data). However, unlike control-grafted mice, mice grafted with RANK−/− thymus displayed inflammatory infiltrates in liver (Fig. 5 G), and immunostaining of Rag−/− tissue sections (4) with serum obtained from RANK−/− thymus transplanted mice revealed autoantibodies to several tissues (Fig. 5 H). Thus, RANK deficiency in thymic stroma is sufficient to induce symptoms of autoimmunity similar to those observed after transplantation of Aire−/− thymic stroma (4).
In conclusion, we have identified RANK signals from LTi cells as being key in the regulation of central tolerance by promoting Aire+ mTEC development. As mentioned earlier, these events have also been linked with LTα–LTβR interactions (13, 21, 24), which suggests that although induction of Aire+ mTEC development can occur in the absence of LTα, some aspects of mTEC development and organization may involve both RANK and LTβR signaling. Of significance is that LTi cells express both LTα and RANKL (20), underlining their importance in regulating both signaling pathways. We have also provided evidence that CD80+Aire+ mTECs are derived from CD80−Aire− mTECs, and this finding together with a recent study (28) helps to clarify previously poorly defined stages of mTEC development. Two further crucial points emerge from our studies. First, the role of RANK in regulating mTEC maturation parallels its role in mammary epithelial development (29), highlighting this pathway in epithelial cell differentiation in two distinct settings. Second, the fact that LTi cells regulate Aire+ mTEC development suggests they also play a role in determining central tolerance to self. As the TNF ligands linked with T cell survival are missing from LTi cells in the neonate (17, 19), these cells may also aid to purge T cells activated on peripheral self-antigens by failing to provide the signals for T cell survival in secondary lymphoid tissues (17), a process that leads to tolerance rather than immunity. Thus, manipulation of LTi cells and the TNF ligands they express may be beneficial in therapeutic strategies such as transplantation, where manipulating the balance of tolerance and immunity is desirable.
MATERIALS AND METHODS
Mice.
Mice were bred at the University of Birmingham, and all experiments were performed in accordance with UK Home Office regulations. Mice with a floxed RANK allele were generated at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences, Institute of Molecular Biology. These mice were crossed onto actin-Cre mice to generate RANK-deficient mice. Day of vaginal plug detection was designated day zero, and adult mice were used at 4–6 wk.
Antibodies.
The following antibodies were used for flow cytometry (11): anti-CD80 (16-10A1) FITC, anti-Ly51 (BP-1) PE, anti-CD45 biotin (clone 30F11), streptavidin PECy7 (all eBioscience), anti-EpCAM1 APC, (G8.8), anti–I-Ab PE (AF6120.1; BD Biosciences), anti-CD3 (145-2C11), anti-CD8 (53–6.7), anti-CD11c (HL3), and anti-B220 (RA36B2) conjugated with FITC or PE (BD Biosciences), anti-CD4 APC (GK1.5; eBioscience), and biotinylated anti-OX40L (RM134L), anti-CD30L (RM153) (BD Biosciences), TRANCE (R&D Systems), streptavidin cychrome, and anti–IL-7Rα PE (A7R34; eBioscience).
Cell isolation.
CD4+3− cells for use in RTOCs were prepared as described (14). CD4+8+ thymocytes, CD45−EpCAM1+Ly51+ cTECs, and CD45−EpCAM1+Ly51− mTECs (3, 7) were prepared by MoFlo (Dako Cytomation) to a purity >99% (unpublished data). CD45−EpCAM1+Ly51− mTECs were also subdivided into CD80− and CD80+ subsets by MoFlo. TECs from adult mice were prepared as described (3).
FTOCs and RTOCs.
FTOCs were prepared as described (11). RTOCs were made from mixtures of dGuo thymic stroma and either CD4+8+ thymocytes or CD4+3− LTi cells at a 1:1 ratio (11). In some experiments, dGuo-treated FTOCs were cultured with 10 μg/ml anti-RANK or 2.5 μg/ml RANK ligand (R&D Systems) for 2 d.
Precursor–product relationships in mTECs.
7-d H-2b FTOCs were digested, and EpCAM1+Ly51−CD80− cells were sorted by MoFlo. Preparations were mixed with freshly disaggregated H-2d thymus lobes (E15) at a ratio of 1:5, cultured as RTOCs for 2 d (11), and analyzed for EpCAM1, Ly51, CD80, and I-Ab expression.
Confocal microscopy.
Sections were analyzed (17) and stained with the following: rat anti-Aire (B1/02-5H12-2), ERTR5, goat anti–rat IgG Alexa594 or Alexa350 (Molecular Probes), anti–keratin 5 (Covance), anti-CD4 (GK1.5, purified or FITC; eBioscience), anti-CD3, anti-CD11c, anti–hamster Cy5 (Jackson ImmunoResearch Laboratories), and Streptavidin Alexa488 or Alexa594 (Molecular Probes). To calculate the number of Aire+ cells, five different K5+ medullary areas were studied, the area being automatically calculated by the LSM10 Carl Zeiss MicroImaging, Inc. confocal software. Aire+ cells were counted and divided by the number of areas to give the mean and SD.
qPCR.
mRNA was isolated using the μMacs One-step cDNA kit (Miltenyi Biotec). qPCR was performed using SYBR green with primers for β-actin, Aire, SP1, and SP2. PCR reactions were performed in reaction buffer containing ABsolute QPCR SYBR Green mix (ABgene) and 200–300 nM primers. The fluorescent signal produced from the amplicon was acquired at the end of each polymerization step, and a melt curve profile was obtained. Reaction amplification efficiency and the Ct values were obtained from Rotor Gene 6.0 software (Corbett Research) using standard curves generated from FTOC cDNA. Relative expression values for samples normalized to β-actin were obtained (8).
Primer sequences are as follows, and GenBank accession numbers are given: β-actin (X03672) forward, 5′-ATCTACGAGGGCTATGCTCTCC-3′ and reverse, 5′-CTTTGATGTCACGCACGATTTCC-3′ (148 bp); AIRE (NM_009646) forward, 5′-TGCATAGCATCCTGGACGGCTTCC-3′ and reverse, 5′-CCTGGGCTGGAGACGCTCTTTGAG-3′ (187 bp); SP1 (NM_009267) forward, 5′-CTGGTGAAAATACTGGCTCTGAA-3′ and reverse, 5′-AGCAGTGTTGGTATCATCAGTG-3′ (116 bp); SP2 (NM_009268) forward, 5′- TCAGACCAAAGTGGGTGACA-3′ and reverse, 5′-CCTCTTGTTTCTCATTGGAGGT-3′ (122 bp); and CD80 (AY278186) forward, 5′-GCTGCTGATTCGTCTTTCACAA-3′ and reverse, 5′-GGGCCACACACTTTTAGTTTCCC-3′ (190 bp).
Thymus grafting and analysis of autoimmunity.
E15 RANK−/− and littermate control embryos were used as thymus tissue for transplantation. Thymus lobes were cultured for 5 d in the presence of dGuo before transplantation under the kidney capsule of adult nude recipients (4). Analysis of lymphocyte infiltrates was performed on paraffin-embedded haemotoxylin and eosin–stained sections, and autoantibodies were detected by incubating frozen sections from Rag−/− mice with serum from grafted mice (4).
Acknowledgments
We thank M. Kim and W. van Ewijk for antibodies.
This work was supported by grants from Medical Research Council, Wellcome Trust, and the EU FP6 Thymaide Project.
The authors have no conflicting financial interests.
References
- 1.Kyewski, B., and L. Klein. 2006. A central role for central tolerance. Annu. Rev. Immunol. 24:571–606. [DOI] [PubMed] [Google Scholar]
- 2.Ueno, T., F. Saito, D.H. Gray, S. Kuse, K. Hieshima, H. Nakano, T. Kakiuchi, M. Lipp, R.L. Boyd, and Y. Takahama. 2004. CCR7 signals are essential for cortex-to-medulla migration of developing thymocytes. J. Exp. Med. 200:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derbinski, J., A. Schulte, B. Kyewski, and L. Klein. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2:1032–1039. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projections of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 5.Devoss, J., Y. Hou, K. Jahannes, W. Lu, G.I. Liou, J. Rinn, H. Chang, R. Caspi, L. Fong, and M.S. Anderson. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi, S., W.E. Jenkinson, G. Anderson, and E.J. Jenkinson. 2006. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 441:988–991. [DOI] [PubMed] [Google Scholar]
- 7.Derbinski, J., J. Gabler, B. Brors, S. Tierling, S. Jonnakuty, M. Hergenmhahn, L. Peltonen, J. Walter, and B. Kyewski. 2005. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vegiopolous, A., P. Garcia, N. Emambokus, and J. Frampton. 2006. Coordination of erythropoiesis by the transcription factor c-Myb. Blood. 107:4703–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray, D.H., N. Seach, T. Ueno, M.K. Milton, A. Liston, A.M. Lew, C.C. Goodnow, and R.L. Boyd. 2006. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 108:3777–3785. [DOI] [PubMed] [Google Scholar]
- 10.Shores, E.W., W. van Ewijk, and A. Singer. 1991. Disorganisation and restoration of thymic medullary epithelial cells in T-cell receptor-negative SCID mice: evidence that receptor-bearing lymphocytes influence the maturation of the thymic microenvironment. Eur. J. Immunol. 21:1657–1661. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson, E.J., G. Anderson, and J.J. Owen. 1992. Studies on T-cell maturation on defined thymic stromal cell populations in vitro. 1992. J. Exp. Med. 176:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuklys, S., G. Balciunaite, A. Agarwal, E. Fasler-Kan, E. Palmer, and G.A. Hollander. Normal thymic architecture and negative selection are associate with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). 2000. J. Immunol. 165:1976–1983. [DOI] [PubMed] [Google Scholar]
- 13.Derbinksi, J, and B. Kyewski. Linking signalling pathways, thymic stroma integrity and autoimmunity. 2005. Trends Immunol. 26:503–506. [DOI] [PubMed] [Google Scholar]
- 14.Kim, M.Y., F.M. McConnell, F.M. Gapsal, A. White, S.H. Glanville, V. Bekiaris, L.S. Walker, J. Caamano, E.J. Jenkinson, G. Anderson, and P.J. Lane. 2007. Function of CD4+CD3− cells in relation to B and T zone stroma in the spleen. Blood. 109:1602–1610. [DOI] [PubMed] [Google Scholar]
- 15.Farr, A.G., M.L. Berry, A. Kim, A.J. Nelson, M.P. Welch, and A. Aruffo. 1992. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med. 176:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farr, A., A. Nelson, and S. Hosier. 1992. Characterization of an antigenic determinant preferentially expressed by type-1 epithelial cells in the murine thymus. J. Histochem. Cytochem. 40:651–654. [DOI] [PubMed] [Google Scholar]
- 17.Kim, M.Y., F.M. Gaspal, H.E. Wiggett, F.M. McConnell, A. Gulbranson-Judge, C. Raykundalia, L.S. Walker, M.D. Goodall, and P.J. Lane. 2003. CD4+CD3− accessory cells costimulate primed CD4 T-cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 18:643–654. [DOI] [PubMed] [Google Scholar]
- 18.Eberl, G., S. Marmon, M.J. Sunshine, P.D. Rennert, Y. Choi, and D.R. Littman. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid inducer cells. Nat. Immunol. 5:64–73. [DOI] [PubMed] [Google Scholar]
- 19.Kim, M.Y., K.M. Toellner, A.J. White, F.M. McConnell, F.M. Gaspal, S.M. Parnell, E.J. Jenkinson, G. Anderson, and P.J. Lane. 2006. Neonatal and adult CD4+3− cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15). J. Immunol. 177:3074–3081. [DOI] [PubMed] [Google Scholar]
- 20.Cupedo, T., G. Kraal, and R.E. Mebius. 2002. The role of CD45+CD4+CD3− cells in lymphoid organ development. Immunol. Rev. 189:41–50. [DOI] [PubMed] [Google Scholar]
- 21.Chin, R.K., J.C. Lo, O. Kim, S.E. Blink, P.A. Christiansen, P. Peterson, Y. Wang, C. Ware, and Y.X. Fu. 2003. Lymphotoxin pathway directs thymic Aire expression. Nat. Immunol. 4:1121–1127. [DOI] [PubMed] [Google Scholar]
- 22.Sean Riminton, D., H. Korner, D.H. Strickland, F.A. Lemckert, J.D. Pollard, and J.D. Sedgwick. 1998. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumor necrosis factor-deficient, mice. J. Exp. Med. 187:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liston, A., D.H. Gray, S. Lesage, A.L. Fletcher, J. Wilson, K.E. Webster, H.S. Scott, R.L. Boyd, L. Peltonen, and C.C. Goodnow. 2004. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J. Exp. Med. 200:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehm, T., S. Scheu, K. Pfeffer, and C.C. Bleul. 2003. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial crosstalk via LtßR. J. Exp. Med. 198:757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong, Y.Y.H., I. Yoshida, H. Sarosi, L. Tan, E. Timms, C. Capparelli, S. Morony, A.J. Oliveria-dos-Santos, G. Van, A. Itie, et al. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 397:315–323. [DOI] [PubMed] [Google Scholar]
- 26.Dougall, W.C., M. Glaccum, K. Charrier, K. Rohrbach, K. Brasel, T. DeSmedt, E. Daro, J. Smith, M.E. Tometsko, C.R. Maliszewski, et al. 1999. RANK is essential for osteoclast and lymph node development. Genes Dev. 13:2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama, T., S. Maeda, S. Yamane, K. Ogino, M. Kasai, F. Fujiwara, M. Matsumoto, and J. Inoue. 2005. Dependence of self-tolerance in TRAF6-directed development of thymic stroma. Science. 308:248–251. [DOI] [PubMed] [Google Scholar]
- 28.Hamazaki, Y., H. Fujita, T. Kobayashi, Y. Choi, H.S. Scott, M. Matsumoto, and N. Minato. 2007. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat. Immunol. 8:304–311. [DOI] [PubMed] [Google Scholar]
- 29.Kim, N.S., H.J. Kim, B.K. Koo, M.C. Kwon, Y.W. Kim, Y. Cho, Y. Yokota, J.M. Penninger, and Y.Y. Kong. 2006. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 26:1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]