Abstract
Antigen-induced immune suppression, like T cell activation, requires antigen-presenting cells (APCs); however, the role of APCs in mediating these opposing effects is not well understood, especially in vivo. We report that genetic inactivation of CD11b, which is a CD18 subfamily of integrin receptors that is highly expressed on APCs, abolishes orally induced peripheral immune tolerance (oral tolerance) without compromising APC maturation or antigen-specific immune activation. The defective oral tolerance in CD11b−/− mice can be restored by adoptive transfer of wild-type APCs. CD11b deficiency leads to enhanced interleukin (IL) 6 production by APCs, which subsequently promotes preferential differentiation of naive T cells to T helper 17 (Th17) cells, which are a T cell lineage characterized by their production of IL-17. Consequently, antigen feeding and immunization of CD11b−/− mice results in significant production of IL-17 within the draining lymph nodes that interferes with the establishment of oral tolerance. Together, we conclude that CD11b facilitates oral tolerance by suppressing Th17 immune differentiation.
The induction, execution, and maintenance of immune tolerance requires direct cell–cell contact mediated by specific surface molecules on APCs, notably MHC-II, B7.1, B7.2, and ICOSL, and their corresponding partners on T lymphocytes (1–5). As these molecules are also involved in T cell activation, it is unclear how APCs function to facilitate both immune tolerance and activation. One potential mechanism that controls such opposite effects is the current dogma on APC maturation status, which is that immature DCs and nonprofessional APCs that lack sufficient surface expression of costimulatory molecules induce T cell apoptosis, anergy, or differentiation of suppressor T cells, whereas mature DCs that express high levels of costimulatory molecules support immune activation (2).
Another mechanism by which APCs control immune activation versus suppression involves their ability to modulate the production of pro- (IL-6, GM-CSF, and IFN-γ) and antiinflammatory cytokines (IL-10 and TGF-β) (1, 6–8). In particular, it was recently reported that IL-6, together with TGF-β, promotes the generation of Th17 cells, which are a distinct T cell lineage characterized by their ability to produce large quantities of IL-17 (9, 10). Interestingly, despite the abundant expression of costimulatory molecules such as CD80 and CD40, certain differentiated DCs are fully capable of suppressing T cell activation (7, 11). Thus, the mechanism that dictates the ability of APCs to induce immune activation versus immune tolerance cannot be explained only by the levels of costimulatory molecules.
One of the highly expressed molecules on APCs is integrin CD11b/CD18 (αM, Mac-1, and CR3). Based on the observation that C3bi, which is a specific ligand of CD11b/CD18 (12), functions critically in the development of specific forms of immune suppression (13, 14), we hypothesize that the development of peripheral immune tolerance is dependent on CD11b/CD18. In this study, we tested the role of CD11b in orally induced peripheral immune tolerance (oral tolerance) using CD11b−/− mice. Our data show that genetic inactivation of CD11b does not significantly affect the maturation of APCs or the cellular compositions of the draining LNs. Rather, CD11b deficiency leads to increased expression of IL-6, preferential immune deviation toward the Th17 pathway, and enhanced production of IL-17, which interferes with the establishment of oral tolerance. Together, this study identifies CD11b/CD18 as an important player in the development of antigen-induced immune tolerance, at least in part because of its ability to suppress Th17 differentiation.
RESULTS AND DISCUSSION
CD11b−/− mice exhibit defective antigen-induced oral tolerance
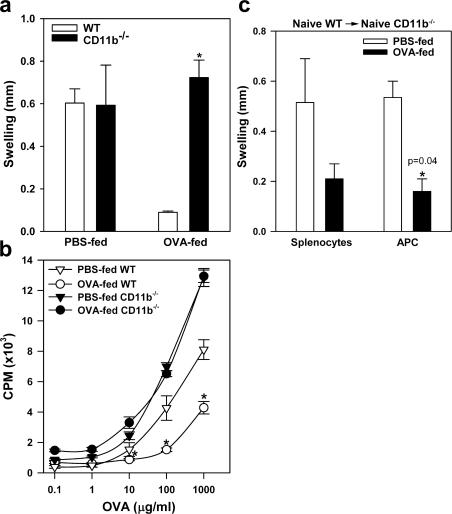
Recent studies demonstrate that unlike its homologue receptor CD11a, genetic inactivation of CD11b does not protect mice from the development of autoimmune diseases. On the contrary, CD11b deficiency worsens the inflammation and disease progression in several autoimmune disease models, including systemic lupus erythematosus, asthma, and arthritis (15–17), suggesting that CD11b/CD18 is potentially involved in immune suppression rather than immune activation. To test our hypothesis that CD11b is required for peripheral immune tolerance, we studied the role of CD11b in low-dose antigen-induced oral tolerance, based on a commonly used feeding regimen (18). Thus, wild-type (WT) and CD11b−/− mice were fed with 1 mg OVA in PBS or PBS alone daily for 7 d, and then immunized with OVA emulsified in complete Freund's adjuvant (CFA). 7 d later, OVA-specific delayed-type hypersensitivity (DTH) was determined. In both WT and CD11b−/− mice that received PBS only, immunization with OVA induced strong immune responses, as indicated by increment in footpad thickness upon challenge with particulate OVA (Fig. 1 a). Thus, CD11b deficiency does not affect immune activation. On the other hand, feeding WT mice with low-dose OVA strongly suppressed the subsequent immune response, as no significant footpad swelling developed in the fed WT mice. In contrast, similarly fed CD11b−/− mice still developed strong DTH responses (Fig. 1 a), demonstrating that CD11b−/− mice are defective in developing immune suppression upon low-dose antigen feeding.
Figure 1.
CD11b is required for induction of oral tolerance. (a) CD11b deficiency prevents orally induced peripheral tolerance as determined by DTH. WT C57BL/6 or CD11b−/− mice were fed daily with PBS or 1 mg OVA for 7 d, and then immunized with OVA/CFA. After 7 d, these mice were challenged with OVA in the left footpad and saline in the right footpad. Footpad thickness was measured after 24 h, and the increase in thickness (swelling) between the left and right footpads is given. *, P = 0.0015, WT versus CD11b−/−. n = 3–6. (b) CD11b deficiency prevents tolerance in OVA-stimulated lymphocyte proliferation. Total LN cells from the OVA-fed and immunized WT and CD11b−/− mice were stimulated with graded concentrations of OVA, and proliferation was measured after 72 h by [3H]thymidine incorporation. P = 0.00003, WT versus CD11b−/−. n = 4. (c) Restoration of oral tolerance in CD11b−/− mice by passive transfer of WT APCs. CD11b−/− mice were injected i.v. with splenocytes or adhesion-enriched APCs from naive WT mice, and then fed with PBS or 1 mg OVA per day for 7 d. Establishment of oral tolerance in recipient CD11b−/− mice is determined by DTH response to OVA. *, P = 0.04, PBS versus OVA. n = 3. Data shown are means ± the SEM and are representative of three to four independent experiments.
The lack of immune suppression in CD11b−/− mice was directly verified by in vitro antigen-induced lymphocyte proliferation assays. Single-cell suspensions prepared from the draining LNs of immunized mice with or without low-dose antigen feeding were restimulated in vitro with graded concentrations of OVA in 96-well plates for 72 h, and cell proliferation was measured on day 3 by [3H]thymidine incorporation. Lymphocytes from immunized WT and CD11b−/− mice showed similarly strong antigen-dependent proliferation (Fig. 1 b), again demonstrating that CD11b deficiency does not adversely affect antigen-specific immune responses. Low-dose oral OVA administration significantly suppressed the resultant proliferation of lymphocytes from WT mice, but did not affect the proliferation of lymphocytes from similarly fed CD11b−/− mice (Fig. 1 b). Together, these results revealed that unlike other known costimulatory molecules present on APCs, such as B7.1, B7.2, and ICOSL, which support both activation and suppression (4, 5, 19), CD11b/CD18 is uniquely involved in oral tolerance, but not in immune activation.
The defective immune tolerance in CD11b−/− mice can be restored by passive transfer of WT APCs
Professional APCs, which express high levels of CD11b, may depend on this molecule to support oral tolerance. In support of this hypothesis, adoptive transfer of total splenocytes or adhesion-enriched APCs from naive WT mice into CD11b−/− mice, followed by antigen feeding, restored oral tolerance in CD11b−/− mice, as indicated by the substantial reduction in OVA-induced footpad swelling (Fig. 1 c), strongly arguing that CD11b expressed on APCs is likely involved in the process of oral tolerance in OVA-fed mice.
Given a potential role of CD11b/CD18 in leukocyte trafficking, CD11b deficiency may change the cellular composition of the lymphoid organs in response to antigen feeding, thus abolishing oral tolerance. However, no significant differences in either the percentage or the total number of CD19+ (B cells), CD3+ (T cells), and MHC-II+ (APC) cells within the draining mesenteric LNs (mLNs) were observed between OVA-fed WT and CD11b−/− mice (Table S1, available at http://www.jem.org/cgi/content/full/jem.20062292/DC1). CD11b deficiency may also interfere with DC maturation within the draining LNs in response to antigen feeding, which is very unlikely given the normal immune response observed in Fig. 1 a. Indeed, two-color flow cytometric analysis of the DC population within the draining mLN cells, using CD11c as a marker, showed similar surface expression of MHC-II, CD40, and CD86 between OVA-fed WT or CD11b−/− mice (Fig. S1).
CD11b deficiency promotes Th17 immune deviation under the oral tolerance condition
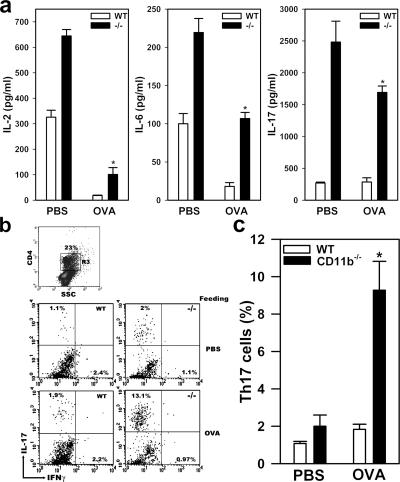
To understand the cellular mechanism by which CD11b deficiency abolished the establishment of oral tolerance, we analyzed cytokine production by draining LN cells in response to antigen restimulation. Single-cell suspension was prepared from the inguinal LNs (iLNs) of PBS- or OVA-fed and immunized WT and CD11b−/− mice and restimulated with OVA for 3 d, and their supernatants were analyzed for cytokine production using multiplex microbead-based cytokine assay kit. Compared with naive mice (not depicted), immunization increased the production of IL-2 and -6 similarly in WT and CD11b−/− mice (Fig. 2 a). Under antigen-feeding conditions, CD11b−/− mice exhibited approximately sixfold increased cytokine production when compared with similarly fed WT mice (P < 0.001). Production of IL-17 was only observed in CD11b−/− mice.
Figure 2.
CD11b deficiency leads to Th17 immune deviation under the condition of oral tolerance induction. (a) Cytokine production in response to antigen restimulation. Singe-cell suspension, obtained from the iLNs of PBS- or OVA-fed and immunized WT (open bar) or CD11b−/− (shaded bar) mice, were restimulated in vitro with 100 μg/ml OVA for 72 h, and the cytokine concentrations in the supernatants were determined by microbead multiplex assay. *, P < 0.001, WT versus CD11b−/−. n = 4. (b) Identification of Th17 cells by intracellular staining. Total CD4+ T cells obtained from PBS- or OVA-fed and immunized mice were stimulated with PMA and ionomycin in the presence of GolgiPlug for 5 h, and then analyzed for intracellular expression of IL-17 and IFNγ by three-color flow cytometry. Plots were gated on CD4+ cells (top, gate R3) and the percentages of cells staining positive for IL-17 or IFNγ were shown. (c) Existence of a Th17 cell population in CD11b−/− mice under conditions of oral tolerance induction. The percentages of Th17 cells, identified by their production of IL-17, but not IFNγ, within CD4+ subpopulation (gate R3), were determined by flow cytometry. Data shown are the means ± the SEM and are representative of three independent experiments. *, P = 0.0001, WT versus CD11b−/−. n = 12.
A major source of IL-17 expression has been attributed to a newly described Th17 cell population (20, 21). Thus, the presence of large quantities of IL-17 suggested that CD11b deficiency may result in Th17 immune deviation in vivo. In support of this hypothesis, IL-6, which is another cytokine that is associated with the development of Th17 cells (9, 10), was also detected in large quantities in the supernatants from antigen-fed and immunized CD11b−/− mice (Fig. 2 a). To directly test our hypothesis, we examined whether CD11b−/− mice contained a Th17 cell population under the condition of oral tolerance induction. Thus, single-cell suspension was obtained from the draining iLNs of PBS- or OVA-fed and immunized WT or CD11b−/− mice, and the presence of Th 17 cells within CD4+ population, as characterized by expression of IL-17, but not IFNγ, was determined by intracellular staining with their corresponding antibodies according to an established protocol (20, 21). Indeed, flow cytometric analyses revealed fivefold more IL-17+IFNγ− cells within the gated CD4+ cells (i.e., Th17 cells) obtained from OVA-fed CD11b−/− mice than that from OVA-fed WT mice (Fig. 2 b). Statistical analysis showed a significant increase in the percentage of Th17 cells within the draining LNs of CD11b−/− mice compared with WT mice (P = 0.0001; n = 12; Fig. 2 c). No detectable Th17 population was observed in either naive WT or CD11b−/− mice (unpublished data).
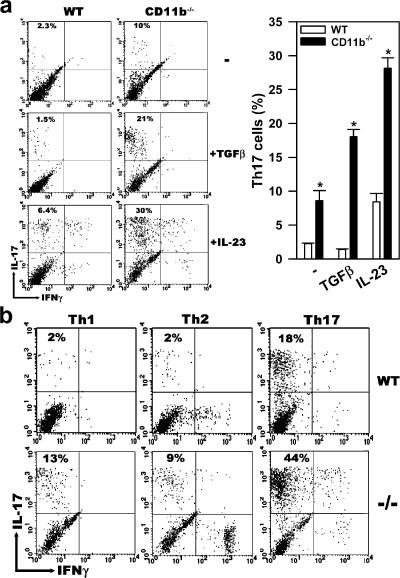
To verify if the IL-17–expressing T cells represented a mature and stable Th17 population, we tested whether they could respond to restimulation by TGFβ, which is a cytokine that promotes the differentiation of Th17 cells (9, 10), and by IL-23, which is a cytokine that promotes their proliferation (9, 22). Thus, total CD4+ T cells were isolated from OVA-fed and immunized WT and CD11b−/− mice, and then restimulated with TGFβ or IL-23 for 3 d in the presence of their corresponding irradiated APCs and anti-CD3. Only CD4+ T cells derived from CD11b−/− mice, but not from WT mice, responded vigorously to either TGFβ or IL-23 treatment (Fig. 3 a). When subjected to different T cell–polarizing conditions, CD4+ T cells isolated from CD11b−/− mice responded vigorously to restimulation by TGFβ plus IL-6 (Th17 polarizing), such that the Th17 population expanded from 13% (basal level; Fig. 2 b) to 44%. In contrast, no significant expansion of Th17 cells was observed when they were restimulated with IFNγ plus anti–IL-4 (Th1 polarizing) or with IL-4 plus anti-IFNγ (Th2 polarizing). In agreement with recent reports (9, 22), a small population of CD4+ T cells (most likely the naive population) from WT mice also responded to the Th17 polarizing condition, and differentiated to Th17 cells (18% for WT vs. 44% for CD11b−/−). Interestingly, although Th17 cells did not respond to the Th2 polarizing condition, restimulation with IL-4 plus anti-IFNγ led to a small population (7–14%) of IFNγ-expressing cells for both WT and CD11b−/− cells (Fig. 3 b).
Figure 3.
Existence of a mature Th17 population in antigen-fed and immunized CD11b−/− mice. (a) The IL-17–expressing cells represent a mature and stable Th17 population. Total CD4+ T cells, which were purified by MACS from the iLNs of OVA-fed and immunized WT or CD11b−/− mice, were cocultured with their corresponding irradiated APCs in the presence of anti-CD3 mAbs with or without 2 ng/ml TGFβ or 20 ng/ml IL-23 plus anti–IL-4 and anti-IFNγ for 3 d. They were then restimulated with PMA and ionomycin in the presence of GolgiPlug for 5 h to retain the cytokines intracellularly. The restimulated cells were analyzed by intracellular staining with anti–IL-17 and anti-IFNγ, and the percentages of CD17+IFNγ− cells within CD4+ population were determined by three-color flow cytometry and shown on the right. *, P < 0.0001, WT versus CD11b−/−. n = 4. (b) Th17 cells from CD11b−/− mice are capable of maintaining a stable Th17 phenotype under different polarizing conditions. Purified CD4+ T cells from OVA-fed and immunized WT or CD11b−/− mice were cocultured with their irradiated APCs in the presence of anti-CD3 under either Th1 (IFNγ plus anti-IL-4), Th2 (IL-4 plus anti-IFNγ), or Th17 (TGFβ plus IL-6) polarizing conditions for 3 d. The percentage of CD17+IFNγ− CD4+ T cells was determined by intracellular cytokine staining. Data shown are representative of three mice per group; these experiments were repeated three times, with similar results.
Suppression of IL-17 production by CD11b is critical to the development of oral tolerance
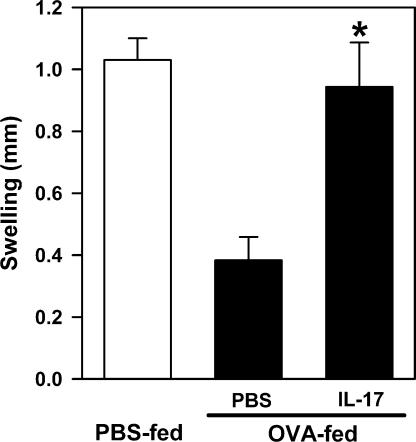
To test our hypothesis, we evaluated whether administration of exogenous IL-17 could block the establishment of oral tolerance in WT mice. Thus, WT mice were injected i.v. with IL-17 (60 pg/mouse/d) and simultaneously fed with low-dose OVA for 7 d. The development of OVA-specific immune tolerance was assessed by the DTH response, as described in Fig. 1 a. Low-dose IL-17 injections did not significantly affect the total blood leukocyte counts (unpublished data), but completely abrogated OVA-specific immune tolerance, leading to the development of OVA-specific DTH response in WT mice, when compared with the PBS-injected and antigen-fed WT control mice (Fig. 4). Based on the above results, we propose that genetic inactivation of CD11b leads to enhanced production of IL-6 by APCs, which, in the presence of TGF-β, promote Th17 immune deviation (9, 10). The presence of Th17 cells and their production of IL-17 within the draining LNs interfere with oral tolerance in CD11b−/− mice.
Figure 4.
IL-17 administration in WT mice interferes with the establishment of oral tolerance. WT mice were injected i.v. with PBS or IL-17 (60 pg/mouse) during OVA feeding, immunized with OVA/CFA, and challenged. Development of immune tolerance was determined by inhibition of footpad swelling in the DTH response, as in Fig. 1 a. *, P = 0.003, PBS versus IL-17. n = 5–6 mice per group. Data shown are means ± the SEM and are representative of two independent experiments.
Th17 cells and the cytokine IL-17 they produce are associated with a variety of allergic and autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and asthma (23–26). Our observation that skewed differentiation toward Th17 pathway in CD11b−/− mice blocks the establishment of oral tolerance is consistent with these clinical observations. Thus, the information provided from this study not only helps us better understand the function of APCs in oral tolerance but also may have clinical implications for patients with autoimmune diseases.
MATERIALS AND METHODS
Mice.
WT and CD11b−/− strains were all in the C57BL/6J background, used at 8–13 wk old. WT mice were purchased from the National Cancer Institute; CD11b−/− mice were provided by C.M. Ballantyne (Baylor College of Medicine, Houston, TX) (27) and have been backcrossed to the C57BL/6J background for more than seven generations. Animals were housed in a pathogen-free facility, and all procedures were performed in accordance with the Institutional Animal Care and Use Committee approval.
Antibodies and reagents.
FITC–anti–mouse CD4 (clone GK1.5) was purchased from Miltenyi Biotec Inc. FITC–anti–mouse CD25 (clone 7D4), PE–anti-CD3 (clone17A2), PerCP–anti-CD4 (clone L3T4), FITC–anti-CD86 (clone 24F), FITC–anti-CD19 (clone 1D3), and FITC–anti-IFNγ (clone XMG1.2) were obtained from BD Biosciences. R-PE–anti–mouse IL-17 (clone TC11-18H10.1) was purchased from BioLegend. Rat anti-CD11b (clone M1/70), FITC–anti-MHC-II (clone M5/114.15.2), and their respective control IgGs were purchased from eBioscience. Hamster anti-CD3 (clone 1452C11) was obtained from the American Type Culture Collection. Recombinant IL-17 was purchased from Biosource. IL-4 was obtained from Pierce Chemical Co., IL-6 and -23 were purchased from R&D Systems, and IFNγ was purchased from CHEMICON International, Inc.
Tolerance induction and determination of DTH.
The method of Miller et al. was used for tolerance induction, with minor modifications (18). In brief, low-dose oral tolerance was induced by feeding 1 mg OVA per day via drinking water for 7 d. In certain experiments, wild-type mice (5–6 mice per group) were injected i.v. daily with either IL-17 (60 pg/mouse) or PBS, while being fed with 1 mg OVA for 7 d. The extent of tolerance induced by these methods was measured by DTH in response to OVA immunization. Accordingly, mice were primed by injecting s.c. 200 μl OVA/CFA emulsion (100 μl of 250 μg OVA plus 100 μl CFA) at the tail base, and then challenged after 7 d with 50 μl of aggregated OVA (10 mg/ml) injected into the left footpad. The right footpad received 50 μl of saline serving as control. The thickness of both hind footpads was measured 24 and 48 h later with a caliper, and the thickness increment was calculated as the difference between the left and right footpad measurements.
Adoptive cell transfer.
Single-cell suspensions were prepared from spleens harvested from naive WT mice and allowed to adhere to tissue culture dishes in complete media (RPMI-1640, 10% FBS, 10 mM Hepes, 1 mM sodium pyruvate, 1× nonessential amino acids, 50 μM 2-mercaptoethanol, and penicillin (100 U/ml streptomycin [100 μg/ml]) at 37°C for 30 min, and the nonadherent cells were removed by washing. The adherent cells (i.e., adhesion-enriched APCs) were detached mechanically without trypsin, which contained <5% contaminating T cells based on flow cytometry. The WT splenocytes or APCs (2 × 106) were injected i.v. into the recipient CD11b−/− mice, which were then fed with 1 mg OVA daily for 7 d. Immune responses to OVA were assessed by DTH as indicated by OVA-induced footpad swelling, as described in the pervious section.
Cell proliferation and cytokine measurement.
Single-cell suspensions were prepared from the iLNs of WT or CD11b−/− mice that were fed with PBS or OVA and primed with OVA. Cells (5 × 105 cells/well) were cultured in 200 μl/well of RPMI-1640 plus 5% FBS at 37°C and 5% CO2 in 96-well flat-bottom culture plates in the presence of graded concentrations of OVA (0–1,000 μg/ml). Cell proliferation was measured after 3 d by [3H]thymidine incorporation. In brief, [3H]thymidine (1 μCi/well; PerkinElmer) was added during the last 16 h of incubation, cells were harvested onto glass-fiber filters (Millipore), and [3H]thymidine incorporation was measured by liquid scintillation. Results are reported as the mean ± the SEM of triplicate or quadruplicate wells per group, and they are representative of two to four independent experiments. For cytokine determination, cultured supernatants were collected after 72 h, and cytokine concentrations were measured by Luminex fluorescent bead–based multiplex cytokine assay system using the BioPlex program (Bio-Rad Laboratories) according to the manufacturer's instructions.
In vitro differentiation/expansion of Th17 cells.
Total CD4+ T cells were prepared using CD4+ T cell isolation kit (Miltenyi Biotec) from the iLNs of WT or CD11b−/− mice that were fed with OVA for 7 d and immunized with OVA/CFA at the tail base for 7 d. Isolated CD4+ T cells (105 cells/well) were cocultured with their corresponding irradiated APCs (3 × 105 cells/well; adhesion-enriched) in 200 μl/well of complete media plus 10 μg/ml anti-CD3 (mAb 1452C11) and different sets of cytokines and antibodies, as follows: 20 ng/ml IFNγ plus 10 μg/ml anti–IL-4 (for Th1 polarization); 20 ng/ml IL-4 plus 10 μg/ml anti-IFNγ (for Th2 polarization); or 2 ng/ml TGFβ plus 8 ng/ml IL-6 (for Th17 polarization) at 37°C for 3 d. Differentiation/expansion of Th17 cells was determined by intracellular staining using anti–IL-17 and anti-IFNγ, and the Th17 cells were identified as IL-17+IFNγ− CD4+ cells by three-color flow cytometry.
Intracellular cytokine staining.
To retain the cytokines intracellularly, the cell mixtures obtained from the draining LNs of different mice or from the in intro cell cultures were restimulated with 50 ng/ml PMA and 2 μM ionophore in the presence of 1 μl GolgiPlug (BD Biosciences) in complete media at 37°C for 5 h. These cells were then incubated with Fc Blocker (clone 2.4G2; BD Biosciences) at 4°C, stained with a first set of fluorescence-labeled (FITC, PE, or PerCP) antibodies for 25 min, and then washed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) for 20 min. These cells were then incubated with a second set of antibodies at 4°C for 30 min, washed, and analyzed by two- or three-color flow cytometry (FACScan; BD Biosciences) using CellQuest software (BD Biosciences).
Statistical analysis.
Statistical analyses were performed using Student's t test (Systat Software, Inc.). P values <0.05 were considered significant.
Online supplemental materials.
Fig. S1 shows that CD11b deficiency did not compromise DC maturation in response to antigen feeding. Table S1 shows that CD11b deficiency did not affect leukocyte trafficking into the draining LNs. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062292/DC1.
Supplemental Material
Acknowledgments
We thank Drs. David Scott and Mark Williams for critical reading of this manuscript.
This work was supported, in part, by grants from the National Institutes of Health (NHLBI R01 HL61589 and NHLBI 2P01 HL54710), the United States Public Health Services (AI43384 and AI50222), and the National Space Biomedical Research Institute (IIH00208), and by the Intramural Research Program of the National Institute for Dental and Craniofacial Research.
The authors declare that they have no competing financial interests.
References
- 1.Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. [DOI] [PubMed] [Google Scholar]
- 3.Weiner, H.L. 2000. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J. Clin. Invest. 106:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbari, O., G.J. Freeman, E.H. Meyer, E.A. Greenfield, T.T. Chang, A.H. Sharpe, G. Berry, R.H. DeKruyff, and D.T. Umetsu. 2002. Antigen- specific regulatory T cells develop via the ICOS-ICOS- ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 5.Vigouroux, S., E. Yvon, E. Biagi, and M.K. Brenner. 2004. Antigen-induced regulatory T cells. Blood. 104:26–33. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., J. Inobe, R. Marks, P. Gonnella, V.K. Kuchroo, and H.L. Weiner. 1995. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 376:177–180. [DOI] [PubMed] [Google Scholar]
- 7.Akbari, O., R.H. DeKruyff, and D.T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., M.E. Frank, W. Jin, and S.M. Wahl. 2001. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 14:715–725. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, M., H. Tang, Z. Guo, H. An, X. Zhu, W. Song, J. Guo, X. Huang, T. Chen, J. Wang, and X. Cao. 2004. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat. Immunol. 5:1124–1133. [DOI] [PubMed] [Google Scholar]
- 12.Ross, G.D., and J.D. Lambris. 1982. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J. Exp. Med. 155:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerberg, C., S.K. Katiyar, M.C. Carroll, and K.D. Cooper. 1998. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J. Exp. Med. 187:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn, J.H., P.S. Bora, H.J. Suk, H. Molina, H.J. Kaplan, and N.S. Bora. 2003. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat. Med. 9:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts, G.M., F.J. Beurskens, I. Martin-Padura, C.M. Ballantyne, L.B. Klickstein, M.B. Brenner, and D.M. Lee. 2005. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J. Immunol. 174:3668–3675. [DOI] [PubMed] [Google Scholar]
- 16.Kevil, C.G., M.J. Hicks, X. He, J. Zhang, C.M. Ballantyne, C. Raman, T.R. Schoeb, and D.C. Bullard. 2004. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am. J. Pathol. 165:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanwar, S., C.W. Smith, F.R. Shardonofsky, and A.R. Burns. 2001. The role of Mac-1 (CD11b/CD18) in antigen-induced airway eosinophilia in mice. Am. J. Respir. Cell Mol. Biol. 25:170–177. [DOI] [PubMed] [Google Scholar]
- 18.Miller, A., O. Lider, A.B. Roberts, M.B. Sporn, and H.L. Weiner. 1992. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl. Acad. Sci. USA. 89:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmits, R., T.M. Kundig, D.M. Baker, G. Shumaker, J.J. Simard, G. Duncan, A. Wakeham, A. Shahinian, A. van der Heiden, M.F. Bachmann, et al. 1996. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J. Exp. Med. 183:1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 21.Weaver, C.T., L.E. Harrington, P.R. Mangan, M. Gavrieli, and K.M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688. [DOI] [PubMed] [Google Scholar]
- 22.Mangan, P.R., L.E. Harrington, D.B. O'Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, and C.T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234. [DOI] [PubMed] [Google Scholar]
- 23.Fujino, S., A. Andoh, S. Bamba, A. Ogawa, K. Hata, Y. Araki, T. Bamba, and Y. Fujiyama. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie, B.S., R.A. Kastelein, and D.J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17–23. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, H., C.W. Smith, J. Perrard, D.C. Bullard, L. Tang, M.L. Entman, A.L. Beaudet, and C.M. Ballantyne. 1997. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1 deficient mice. J. Clin. Invest. 99:1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.