Abstract
It has been suggested that T cell immunoglobulin mucin (Tim)-1 expressed on T cells serves to positively costimulate T cell responses. However, crosslinking of Tim-1 by its ligand Tim-4 resulted in either activation or inhibition of T cell responses, thus raising the issue of whether Tim-1 can have a dual function as a costimulator. To resolve this issue, we tested a series of monoclonal antibodies specific for Tim-1 and identified two antibodies that showed opposite functional effects. One anti–Tim-1 antibody increased the frequency of antigen-specific T cells, the production of the proinflammatory cytokines IFN-γ and IL-17, and the severity of experimental autoimmune encephalomyelitis. In contrast, another anti–Tim-1 antibody inhibited the generation of antigen-specific T cells, production of IFN-γ and IL-17, and development of autoimmunity, and it caused a strong Th2 response. Both antibodies bound to closely related epitopes in the IgV domain of the Tim-1 molecule, but the activating antibody had an avidity for Tim-1 that was 17 times higher than the inhibitory antibody. Although both anti–Tim-1 antibodies induced CD3 capping, only the activating antibody caused strong cytoskeletal reorganization and motility. These data indicate that Tim-1 regulates T cell responses and that Tim-1 engagement can alter T cell function depending on the affinity/avidity with which it is engaged.
We recently identified a new gene family encoding the T cell immunoglobulin mucin (Tim) proteins (1, 2). The Tim family consists of eight genes in mouse (Tim-1–8) and three genes in human (TIM-1, -3, and -4). Tim family members are cell surface glycoproteins that share a common motif, including an IgV domain, a mucin-like domain, a transmembrane domain, and an intracellular tail (1, 2). Tim family members are differentially expressed on Th1 cells, Th2 cells, or DCs, and they are implicated in the regulation of asthma and autoimmunity (1).
TIM-1 was first identified as hepatitis A virus cellular receptor 1 (3, 4), and later as a kidney injury molecule 1 (5, 6). Interestingly, hepatitis A virus infection is associated with a reduced risk of developing asthma (7), and in mouse models, Tim-1 has been genetically linked to murine airway hypersensitivity (8). Furthermore, polymorphic forms of TIM-1 in humans have been associated with susceptibility to asthma, eczema, and rheumatoid arthritis (9–13), suggesting that Tim-1 may play a role in regulating immune responses. In addition to its expression on kidney cells, Tim-1 is also expressed on activated T cells. Upon CD4+ T cell polarization, it is expressed at a higher level on Th2 cells than on Th1 cells (14, 15). Initial studies suggested that Tim-1 expressed on T cells is a positive costimulatory molecule that results in enhancement of T cell proliferation, cytokine production, and abrogation of tolerance (14). Our laboratory has recently reported that Tim-4, which is expressed on APCs, is a natural ligand for Tim-1 (15). Interestingly, dependent on the dose, Tim-4 binding to Tim-1 has different effects on T cell proliferation. A higher dose of Tim-4-Ig consistently led to an increase in T cell proliferation upon TCR ligation, whereas a lower concentration of Tim-4-Ig inhibited T cell proliferation (15). Therefore, it was not clear whether Tim-1 is a positive or a negative T cell costimulatory molecule. It is also possible, however, that Tim-4 could induce these opposite effects by engaging different receptors on the T cell surface. One possibility that would account for this apparent discrepancy is that the Tim-1 molecule itself may be a positive regulator of T cell responses, but that it may also act as an inhibitory molecule depending on how and when the molecule is engaged during T cell activation. To date, however, all known costimulatory molecules have been categorized as positive costimulators (e.g., CD28 and ICOS) or negative costimulatory molecules (e.g., CTLA-4 and PD-1), although some reports suggested that CTLA4, which is an inhibitory molecule, can up-regulate LFA-1 (16).
In this paper, we tested a series of anti–Tim-1 mAbs and identified one antibody that positively costimulated T cell responses and another that inhibited T cell responses. The two antibodies also differentially regulated the expansion of antigen-specific T cells, cytokine production, and development of autoimmunity in vivo. The two antibodies differed 17-fold in their binding avidity for Tim-1 and in the regulation of T cell cytoskeletal movement and TCR–CD3 capping, suggesting that Tim-1 might be intimately involved in regulating TCR-driven activation.
RESULTS
Identification of anti–Tim-1 antibodies that either increase or inhibit T cell proliferation
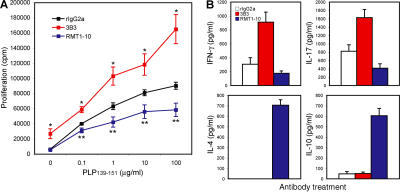
Tim-4 is a natural ligand for Tim-1 (15). Depending on the dose, Tim-4-Ig can either increase or inhibit T cell proliferation upon TCR ligation, suggesting that Tim-1 may deliver both stimulatory and inhibitory costimulatory signals into T cells (15). However, the inhibitory effects of Tim-4-Ig might be caused by binding to a receptor other than Tim-1 on T cells. To understand the mechanism by which Tim-1 regulates T cell expansion and effector functions, we tested a series of antibodies with binding specificity for Tim-1. It was previously reported that Tim-1 engagement by an agonistic anti–Tim-1 antibody (clone 3B3) could costimulate T cells along with TCR ligation (14). Indeed, when spleen cells isolated from SJL mice immunized with the encephalitogenic peptide proteolipid protein (PLP)139-151 in CFA were treated with antigen in vitro, addition of 3B3 anti–Tim-1 antibody to the cultures significantly increased T cell proliferation at all doses of antigens, compared with the treatment with control rat IgG2a (rIgG2a; Fig. 1 A). Interestingly, in contrast to 3B3, addition of another anti–Tim-1 antibody (RMT1-10) reduced T cell proliferation in the cultures (Fig. 1 A). The data suggest that Tim-1 cross-linking with different antibodies can deliver either a positive or negative costimulatory signal in T cells, similar to what has been observed with Tim-4-Ig (15).
Figure 1.
Opposite effects of the anti–Tim-1 antibodies 3B3 and RMT1-10 on T cell responses. (A) Anti–Tim-1 antibody 3B3 enhances, whereas RMT1-10 inhibits, antigen-specific T cell proliferation. Spleen cells from PLP139-151-immunized SJL mice were cultured in vitro with different concentrations of PLP139-151 plus 10 μg/ml RMT1-10, 3B3, or rIgG2a. Proliferation was measured after 48 h by 3[H]thymidine incorporation. The mean ± SEM of three independent experiments are shown. *, P < 0.0001; **, P < 0.01, relative to isotype control. (B) Treatment with RMT1-10 skews the Th1/Th17 responses toward a Th2 response. Spleen cells from PLP139-151-immunized SJL mice were cultured in vitro with 1 μg/ml PLP139-151 plus 10 μg/ml RMT1-10, 3B3, or rIgG2a. Supernatants were taken from the culture at 48 h and assessed by cytokine ELISA for IFN-γ, IL-17, -4, and -10. Data are representative of three independent experiments. Error bars represent the SEM of triplicate measurements in the same experiment.
We next determined the cytokine production in these cultures after antigen-specific activation and addition of anti–Tim-1 antibodies. As observed previously (17, 18), activation of PLP139-151-primed lymph node cells with the specific antigen in the presence of control rIgG2a resulted in the production of the proinflammatory cytokines IFN-γ and IL-17, with no detectable IL-4 or -10 in the cultures (Fig. 1 B). The addition of 3B3 increased the production of both IFN-γ and IL-17 with no detectable production of IL-4 or -10 (Fig. 1 B). However, the addition of RMT1-10 to the cultures resulted in inhibition of IFN-γ and IL-17, but induced the production of the Th2 cytokines IL-4 and -10 (Fig. 1 B). Thus, the two anti–Tim-1 antibodies not only differed in their ability to regulate T cell proliferation but also differentially induced cytokine production from the responding T cells.
Anti–Tim-1 antibodies 3B3 and RMT1-10 bind to the same or closely related epitopes in the IgV domain of the Tim-1 molecule
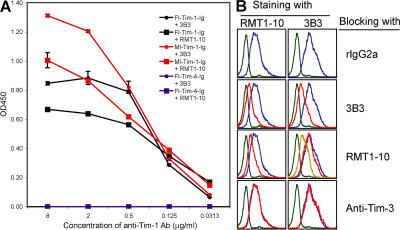
Because Tim-1 molecules contain both IgV and mucin domains in the extracellular region, it was possible that opposite costimulatory functional effects on T cell responses by Tim-1 might be caused by engagement of different domains of Tim-1 by the two antibodies. Therefore, 3B3 and RMT1-10 were tested in an ELISA assay for binding to full-length and mucinless forms of Tim-1-Ig. Both 3B3, which was generated by immunizing rats with Tim-1 IgV-only fusion protein, (14) and RMT1-10 bound to both full-length and mucinless forms of Tim-1 (Fig. 2 A), suggesting that both the antibodies are specific for the IgV domain of Tim-1. The binding of both antibodies to the mucinless form was stronger than the binding to full-length Tim-1. Neither antibody bound to the negative control Tim-4-Ig fusion proteins.
Figure 2.
Characterization of 3B3 and RMT1-10 anti–Tim-1 antibodies. (A) ELISA plates were coated with goat anti–mouse IgG. Full-length (Fl) or mucinless (Ml) Tim-1-Ig fusion proteins were used to coat ELISA plates, and the binding of 3B3 and RMT1-10 to the fusion proteins was determined. Tim-4-Ig was included as a negative control. Data are representative of three independent experiments. Error bars represent the SEM of triplicate measurements in the same experiment. (B) EL-4–Tim-1 transfectants were incubated with 0.5 μg/106 cells of unlabeled rIgG2a, 3B3, RMT1-10, or anti–Tim-3 antibody (clone 2C12) for 1 h on ice, and 0.5 μg/106 cells of either biotin-labeled RMT1-10 or PE-labeled 3B3 was added into the reaction for another 30 min. The binding of 3B3 or RMT1-10 (followed by PE-conjugated streptavidin) was detected by flow cytometric analysis. The green lines show isotype control staining; the blue lines show Tim-1 staining blocked with rIgG2a; the red lines show the Tim-1 staining blocked with unlabeled 3B3, RMT1-10, or anti–Tim-3; the yellow line shows the Tim-1 staining blocked with 5 μg/106 cells of unlabeled RMT1-10.
To further determine whether 3B3 and RMT1-10 bind to the same epitope in the Tim-1 IgV domain, we used one antibody to stain Tim-1 transfectants and added in the other antibody as a competitor to block this binding. As shown in Fig. 2 B, when we used RMT1-10 to stain EL-4 cells transfected with Tim-1 (EL-4–Tim-1), addition of rIgG2a did not block the binding, whereas the addition of unlabeled RMT1-10 strongly blocked the binding. The binding of RMT1-10 to EL-4–Tim-1 cells was also strongly blocked by 3B3 anti–Tim-1 antibody but not by anti–Tim-3 antibody. When we used 3B3 to stain the EL-4–Tim-1 cells, addition of unlabeled 3B3 strongly inhibited the labeled 3B3 binding to the Tim-1 transfectants, whereas addition of RMT1-10 in a 1:1 ratio weakly blocked the 3B3 binding (Fig. 2 B). However, when higher amounts of RMT1-10 were used, we observed an obvious inhibition of 3B3 binding to the EL-4–Tim-1 cells (e.g., 10:1 ratio, as shown in Fig. 2 B). These data suggest that both antibodies bind to the same or closely related epitopes in the Tim-1 IgV domain.
Increase in early T cell dynamics induced by anti–Tim-1 antibody 3B3, but not by RMT1-10
Given that the two antibodies bind to the same or closely related epitopes, but differed in their functional outcomes, we hypothesize that the two antibodies might differ in the dynamics of early activation of T cells. Cytoskeletal changes, T cell motility, and CD3 capping have been used as markers for the early stages of T cell activation (19). Using live imaging of T cells, we determined how the two antibodies affect the kinetics of early CD4+ T cell responses.
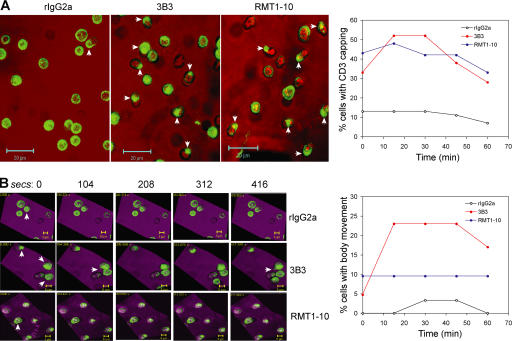
Changes in cytoskeletal arrangements and motility offer a readout for early stages in T cell activation, in addition to membrane ruffling, and formation of lamellopodia resulting from reorganization of TCR–CD3 and other costimulatory/adhesion molecules (19). After addition of the anti–Tim-1 antibodies, there was no observable difference in the CD3 capping induced by the two antibodies. As shown in Fig. 3 A, when treated with control rIgG2a, only a few cells showed CD3 capping, and no more CD3 capping was observed 60 min after the rIgG2a treatment, whereas addition of either 3B3 or RMT1-10 within minutes induced rapid CD3 capping, as demonstrated by three-dimensional reconstruction of z-stack scanning of individual CD4+ T cells over time. Both 3B3 and RMT1-10 treatment induced a similar percentage of CD4+ T cells with CD3 capping during a 60-min observation (Fig. 3 A).
Figure 3.
Effects of treatment with 3B3 or RMT1-10 on early T cell responses. CD4+ T cells were stained with Alexa Fluor 488–conjugated anti-CD3 on ice and treated with rIgG2a, 3B3, or RMT1-10 in a Live Imaging Microincubator at 37°C and 5% CO2. The responses of labeled CD4+ T cells were recorded for 1 h in 15-min intervals. CD3 capping at the 15-min time point and time courses have been shown in A. The arrows represent cells with CD3 capping. Pictures of the cell mobility were taken from 15 to 22 min, with 104-s intervals, and are shown in B. The arrows represent the cells with active body movement. The time course of the cell mobility has been shown in B. The video of T cell mobility from 15 to 22 min is shown Video 1. Green, CD3; red (A) and purple (B), differential interface contrast. Video 1 is available at http://www.jem.org/cgi/content/full/jem.20062498/DC1.
However, 3B3 treatment showed an increased percentage of motile T cells with persistent changes in morphology and behavior. These effects peaked after 15 min, lasted for 30 min, and then began to drop. In contrast, the percentage of cells with body movement in the RMT1-10–treated culture remained unchanged over 60 min (Fig. 3 A). Treatment with RMT1-10 antibody and control rIgG2a resulted in only a very small percentage of motile CD4+ cells, and the majority of the cells have a stationary behavior during the recorded time points. Most strikingly, treatment with 3B3 caused persistent changes in T cell morphology and motility, whereas both rIgG2a and RMT1-10 treatments induced very little motility, and no major change in morphology (Video 1, available at http://www.jem.org/cgi/content/full/jem.20062498/DC1).
This suggested that the Tim-1 molecule induced important changes in T cell activation. Both appear to engage receptors in the membrane that induced a robust CD3 capping; strikingly, only 3B3 antibody resulted in a subsequent robust motility and cytoskeletal rearrangements (20).
3B3 has a 17-fold higher binding avidity than RMT1-10 anti–Tim-1 antibody
Because the two antibodies bound to the same or closely related epitopes in the Tim-1 IgV domain, the divergent effects of 3B3 and RMT1-10 on early T cell activation events and proliferation could not be explained by their binding specificity of the Tim-1 molecule. However, because 3B3 strongly blocked the binding of RMT1-10 to EL-4–Tim-1 cells, whereas RMT1-10 only inhibited 3B3 binding to the cells only at a very high concentration (Fig. 2 B), 3B3 might have a higher Tim-1 binding avidity than RMT1-10. Therefore, biacore analysis of 3B3 and RMT1-10 binding to Tim-1 molecules was undertaken to determine their binding kinetics and avidities.
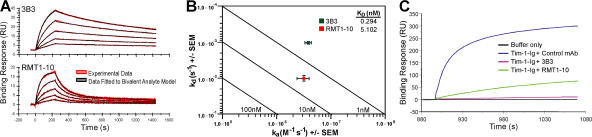
After capture of 3B3 or RMT1-10 on the surface of a goat anti–rat IgG Fc biosensor chip, a concentration series of Tim-1-Ig was injected over the antibodies and used to generate high-resolution kinetic data (Fig. 4 A). Association (K a) and dissociation (K d) rate constants for each antibody were derived from a global fit of Tim-1-Ig binding data using a bivalent analyte model. Both 3B3 and RMT1-10 had rapid association rates with Tim-1, mean K a = 3.8 × 106 and 3.2 × 106 M−1s−1, respectively, but significantly different rates of antibody–antigen complex dissociation, mean K d = 1.05 × 10−3 and 1.02 × 10−2 s−1, respectively (P = 0.007). The resulting difference in binding avidities (K D) was primarily dependent on the significantly faster off-rate of Tim-1 from RMT1-10 (Fig. 4 B). The mean avidity of 3B3 for Tim-1 is 17-fold higher than that of RMT1-10 (mean K D = 0.294 vs. 5.102 nM, respectively). Thus, the stronger T cell activation is likely a consequence of a higher-avidity interaction of 3B3 and Tim-1 molecules. This might result in a more stable Tim-1 complex, which associates with the TCR–CD3 complex to form large supramolecular activation clusters (19). Furthermore, the biacore analysis confirms that 3B3 and RMT1-10 bind closely related epitopes on Tim-1 (Fig. 4 C). Compared with control rIgG2a, preincubation of Tim-1 with 3B3 almost completely eliminated the ability of 3B3 that was captured on the surface of the biosensor chip to bind Tim-1. Interestingly, preincubation of Tim-1 with RMT1-10 partially inhibited the binding of Tim-1 by captured 3B3, further corroborating that the opposite effects of 3B3 and RMT1-10 antibodies do not have different binding specificity, but differential binding avidity for the Tim-1 molecule.
Figure 4.
Biacore analysis of 3B3 and RMT1-10 interactions with Tim-1 molecules. (A) 3B3 and RMT1-10 were captured on the surface of a biosensor chip, and a concentration series of Tim-1-Ig was used to generate high-resolution kinetic data. Tim-1 association and dissociation data were acquired and rate constants (K a and K d) were generated from a global fit of the experimental binding data using a bivalent analyte model (mean χ2 = 0.329 for 3B3 and 0.471 for RMT1-10). Sensogram data are representative of five independent experiments. (B) Association/dissociation rate plot showing resolution of binding affinities for each antibody. Isoaffinity diagonals indicate constant affinity values that increase from bottom left to top right. Mean affinities (K D = K d/K a) for 3B3 and RMT1-10 are indicated within the plot. Data are the mean ± the SEM of five independent experiments. (C) Antibody blocking was used to examine the 3B3 and RMT1-10 epitopes on Tim-1. Tim-1-Ig was preincubated with anti–Tim-1 antibodies and observed for binding to 3B3 captured on the surface of a biosensor chip. RMT1-10 significantly reduced, but did not eliminate, Tim-1 binding to 3B3. Data are representative of three independent experiments. The RUs of immobilized anti–Tim-1 for the experiment in A were 137–141 for 3B3 and 108–113 for RMT1-10. In the experiment shown in C, the RUs were 360–365. In all cases, the “DeltaRUs” in the Tim-1 antibody immobilized on the chips was <5%.
Administration of activating anti–Tim-1 antibody 3B3 expands autopathogenic Th1 and Th17 cells
Tim-1 regulates the early activation of T cells. Depending on the avidity of its cross-linking, it can either enhance or inhibit T cell proliferation and regulate production of cytokines (Fig. 1). These traits are similar to those of agonistic/antagonistic TCR peptide ligands (also known as altered peptide ligands) (21). Because the two antibodies have divergent effects on T cell responses in vitro, we next examined their effects in vivo on T cell responses and the development of experimental autoimmune encephalomyelitis (EAE), which is an animal model of autoimmune inflammatory disease in the central nervous system (CNS) that serves as an animal model for multiple sclerosis (22). EAE is mediated by myelin- reactive CD4+ T cells, and many studies suggest that both Th1 and Th17 cells are crucial for its development (18, 23–25), whereas Th2 cells that produce IL-4 and -10 have been shown to inhibit and reverse EAE (26, 27). Our in vitro results predict that the activating anti–Tim-1 antibody 3B3 would enhance pathogenic T cell responses and autoimmunity, whereas RMT1-10 would inhibit them.
To determine the effect of 3B3 on the development of antigen-specific T cell responses, spleen cells from PLP139-151-immunized SJL mice treated with 3B3 or control reagents were tested for T cell proliferation and cytokine production ex vivo. As shown in Fig. 5 A, spleen cells from 3B3-treated mice had 3–5 times the basal proliferation in the absence of any exogenous antigen. These cells also showed increased proliferation upon addition of low doses of antigen, compared with those from control mice. These data suggest that administration of activating anti–Tim-1 antibody results in a hyperproliferation of T cells in vivo, such that they continue to proliferate ex vivo, even in the absence of further activation. Cytokine ELISA showed that even without antigenic restimulation, spleen cells from 3B3-treated mice secreted large quantities of IFN-γ, IL-17, -2, -6, and TNF-α (Fig. 5 A and not depicted), which correlated with the higher level of basal proliferation observed in these cultures. Spleen cells from mice treated with rIgG or PBS produced only little of these cytokines in the absence of antigen. However, spleen cells from control-treated mice showed a dose-dependent increase in the production of these cytokines upon in vitro activation with PLP139-151. Spleen cells from 3B3-treated mice showed a similar dose-dependent increase in cytokine production, but the amounts of cytokines produced were two to four times higher than those from control-treated mice. The production of IL-4 and -10 was very low and comparable between 3B3-treated and control groups (Fig. 5 A and not depicted). These data indicate that treatment with activating high-avidity anti–Tim-1 antibody enhances proinflammatory Th1 and Th17 responses.
Figure 5.
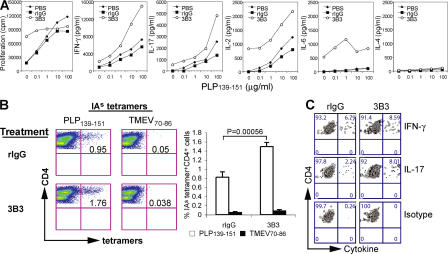
Treatment with 3B3 enhances PLP139-151-specific Th1 and Th17 responses. (A) Spleen cells from immunized SJL mice treated in vivo with 3B3, or with rIgG or PBS as control, were cultured in vitro for 48 h with PLP139-151 restimulation. Proliferation was measured in triplicate wells after 48 h by 3[H]thymidine incorporation. Supernatants were taken at 48 h from the culture and assessed by cytokine ELISA for IL-2, IFN-γ, IL-17, -6, -4, and -10. Splenocytes from individual mice (n = 4) were analyzed separately, and mean data for all mice are shown. (B) Lymphocytes from spleen and lymph nodes of immunized SJL mice treated with 3B3 or rIgG were cultured in vitro for 5 d with PLP139-151 restimulation. Live cells were obtained by Ficoll-Hypaque density gradient centrifugation and used for triple color staining with PE-conjugated IAs tetramer, APC–anti-CD4, and 7-AAD. The PLP139-151/IAs tetramer-positive cells were determined in the live CD4+ cell population (CD4+7-AAD−). Cells were also reactivated with PMA and ionomycin for 4 h and used for four-color staining with APC-conjugated IAs tetramer, FITC–anti-CD4, 7-AAD, and PE-cytokine. (C) The number of cytokine-producing cells was determined in the PLP139-151/IAs tetramer-positive CD4+ cell population. Data are representative of three independent experiments.
We next analyzed the frequency of PLP139-151-specific CD4+ T cells using MHC class II/IAs tetramers specific for PLP139-151 peptide (17, 28). In cultures derived from 3B3-treated mice immunized with PLP139-151, there were approximately twofold more PLP139-151/IAs tetramer-positive CD4+ T cells than in cultures from control mice (Fig. 5 B; 1.76 vs. 0.95%, P = 0.00056). Furthermore, the frequency of IL-17–producing PLP139-151/IAs-reactive CD4+ T cells was almost fourfold higher in 3B3-treated mice than that in control mice (Fig. 5 C; 8.01 vs. 2.24%, P = 0.0016). 3B3 treatment also increased the frequency of IFN-γ–producing PLP139-151-specific CD4+ T cells, although the increase in IFN-γ–producing cells was not as high as observed for IL-17–producing cells. These data demonstrate that administration of high-avidity anti–Tim-1 antibody promotes the expansion of antigen-specific proinflammatory Th1/Th17 cells, as well as production of proinflammatory cytokines from these cells. These results would predict that increased proinflammatory cells and cytokines that were induced by activating anti–Tim-1 3B3 antibody would promote autoimmunity.
Treatment with activating anti–Tim-1 antibody 3B3 enhances the severity of EAE
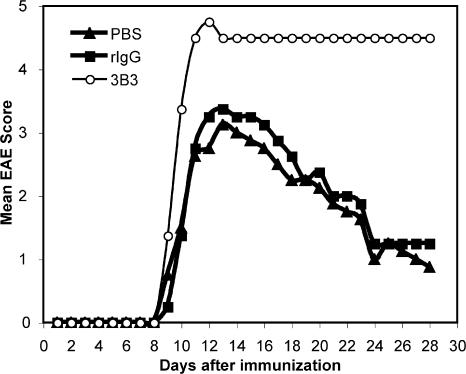
To test the potential effect of the activating anti–Tim-1 antibody on the regulation of EAE, SJL mice were immunized with the encephalitogenic PLP139-151 peptide, and treated with 3B3 or control reagents. Whereas the control groups (rIgG and PBS) showed a typical EAE course, treatment with 3B3 dramatically altered the course of EAE (Fig. 6). 80% of 3B3-treated mice died 3–4 d after the onset of disease (Table I). Histological examination of brains and spinal cords did not show striking differences in the numbers of inflammatory/demyelinating lesions in 3B3 versus control-treated mice (Table I). However, inflammatory lesions were observed as early as day 8 in the 3B3 antibody-treated mice, but not in the rIgG-treated group. Furthermore, large numbers of neutrophils were regularly identified in the lesions of 3B3-treated mice, but not in control mice (unpublished data). There was also a trend toward larger areas of demyelination in the 3B3-treated mice, suggesting that treatment with 3B3 resulted in more leukocyte infiltration and more tissue damage in the CNS that correlated with severe, unremitting disease in the treated mice. Collectively, these results suggest that cross-linking of Tim-1 by an activating anti–Tim-1 antibody in vivo during T cell activation not only enhances proinflammatory Th1 and Th17 responses, but also has functional consequences in that it dramatically enhanced the progression and severity of EAE.
Figure 6.
Administration of 3B3 anti–Tim-1 antibody enhances EAE severity. 8–12-wk-old female SJL mice were actively immunized with PLP139-151 emulsified in CFA, and treated with 3B3, rIgG, or PBS every other day from day 0 to 8. Mice were evaluated daily for the signs of EAE.
Table I.
Clinical and histological EAE in anti–Tim-1 antibody 3B3-treated SJL mice
| Clinical disease | Histopathology (Number of inflammatory lesions) |
||||||
|---|---|---|---|---|---|---|---|
| Treatment | Incidence (sick/total) |
Mean day of onset (mean ± SEM) |
Mean maximum score (mean ± SEM) |
Mortality (death/total) |
Meninges | Parenchyma (mean ± SEM) |
Total |
| PBS | 8/8 | 9.75 ± 0.46 | 3.25 ± 0.60 | 0/8 | 78 ± 29 | 110 ± 17 | 188 ± 18 |
| rIgG | 8/8 | 10.25 ± 0.89 | 3.50 ± 0.89 | 1/8 | 72 ± 30 | 110 ± 40 | 182 ± 56 |
| 3B3 | 10/10 | 9.25 ± 0.46 | 4.75 ± 0.46a | 8/10b | 68 ± 42 | 120 ± 33 | 188 ± 61 |
P < 0.05, 3B3-treated group compared with rIgG- or PBS-treated group.
Inhibitory anti–Tim-1 antibody RMT1-10 decreases antigen-specific CD4+ T cell expansion and production of IFN-γ and IL-17 by these cells
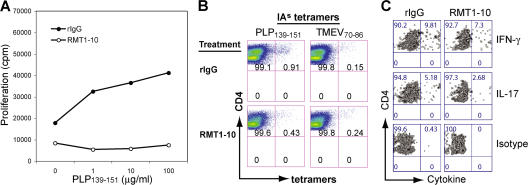
When SJL mice were immunized with PLP139-151 and treated with the anti–Tim-1 antibody RMT1-10, their spleen cells had a lower basal proliferation in the absence of antigen and decreased proliferation in the presence of antigen, compared with the control (Fig. 7 A). PLP139-151/IAs tetramer staining demonstrated that the frequency of PLP139-151-specific CD4+ T cells decreased nearly twofold in cells derived from RMT1-10–treated mice compared with those from the control mice (Fig. 7 B; 0.43 vs. 0.91%, P = 0.0018). Furthermore, the frequency of IL-17–producing PLP139-151/IAs-reactive CD4+ T cells was about twofold lower in RMT1-10–treated mice than in control mice (Fig. 7 C; 2.68 vs. 5.18%, P = 0.0019). The frequency of IFN-γ–producing PLP139-151/IAs-reactive CD4+ T cells was slightly decreased in RMT1-10–treated mice. These data suggest that administration of the low-avidity anti–Tim-1 antibody RMT1-10 inhibits expansion and cytokine production of proinflammatory T cells, especially Th17 cells.
Figure 7.
Treatment with RMT1-10 anti–Tim-1 antibody inhibits PLP139-151-specific T cell responses. (A) Spleen cells from immunized SJL mice treated in vivo with RMT1-10 or rIgG were cultured in vitro for 48 h with PLP139-151 restimulation. Proliferation was measured in triplicate wells after 48 h by 3[H]thymidine incorporation. (B and C) Lymphocytes from spleen and lymph nodes of immunized SJL mice were cultured for 5 d with PLP139-151 restimulation. Live cells were then obtained by Ficoll-Hypaque density gradient centrifugation and used for determining the frequency of PLP139-151/IAs tetramer-positive CD4+ cells and cytokine production from these cells as described in Fig. 5 (B and C). Data are representative of three independent experiments.
Administration of RMT1-10 inhibitory anti–Tim-1 antibody inhibits the development of EAE
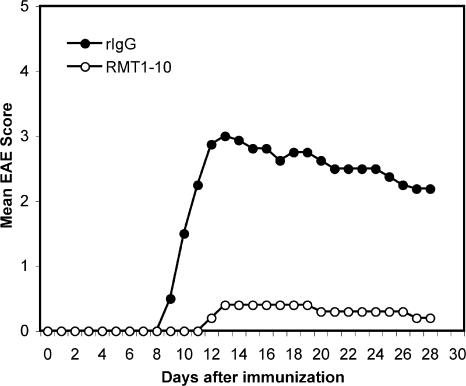
Next, we determined whether the reduced frequency of Th1 and Th17 cells upon RMT1-10 treatment also translated into an attenuation of EAE. Indeed, in contrast to treatment with 3B3, administration of RMT1-10 inhibited the development of EAE (Fig. 8). Only 30% of RMT1-10–treated mice developed EAE, whereas all of the rIgG-treated mice developed severe EAE. Furthermore, RMT1-10 treatment not only dramatically decreased the severity of EAE but also delayed the disease onset (Table II). Histological examination of brain and spinal cords demonstrated a significant decrease in the number of inflammatory lesions in the meninges and parenchyma in RMT1-10–treated mice compared with control animals (Table II). These data suggest that the low-avidity anti–Tim-1 antibody RMT1-10 inhibits EAE when administrated in vivo during the induction phase, which is opposite to the effect of the activating high-avidity anti–Tim-1 antibody 3B3.
Figure 8.
Administration of RMT1-10 anti–Tim-1 antibody inhibits EAE. Female SJL mice were actively immunized with PLP139-151 emulsified in CFA and treated with RMT1-10 or rIgG every other day from day 0 to 10. Mice were evaluated daily for the signs of EAE.
Table II.
Clinical and histological EAE in anti–Tim-1 antibody RMT1-10-treated SJL mice
| Clinical disease | Histopathology (Number of inflammatory lesions) |
||||||
|---|---|---|---|---|---|---|---|
| Treatment | Incidence (sick/total) |
Mean day of onset (mean ± SEM) |
Mean maximum score (mean ± SEM) |
Mortality (death/total) |
Meninges | Parenchyma (mean ± SEM) |
Total |
| rIgG | 6/6 | 10.75 ± 1.16 | 3.38 ± 0.92 | 1/6 | 97 ± 33 | 87 ± 18 | 185 ± 33 |
| RMT1-10 | 3/10 | 14.00 ± 3.46a | 2.00 ± 1.00b | 0/10 | 35 ± 26c | 27 ± 19d | 63 ± 35e |
Representing the mice that showed clinical signs of EAE (3 out of 10 mice).
P < 0.05, RMT1-10–treated group compared with rIgG- or PBS-treated group.
DISCUSSION
T cell activation is a tightly regulated event involving complex receptor–ligand interaction, ultimately leading to downstream signaling events. Optimal T cell activation requires at least two signals, antigen recognition and costimulation (29–31). The TCR–CD3 complex, which recognizes antigens presented by MHC molecules, is critical in maintaining the specificity of T cell responses. Signal two, or costimulation, is an antigen-independent signal required for sustained T cell activation, proliferation, and survival. Although several costimulatory molecules have been identified that can either enhance (e.g., CD28 and ICOS) or inhibit (e.g., CTLA4 and PD-1) T cell responses in terms of T cell activation, none of these molecules has been found to both activate and inhibit T cell proliferation/cytokine responses. With the specific anti–Tim-1 antibodies described here, we provide evidence that engagement of Tim-1 can both activate and inhibit T cell responses. We have shown that two mAbs that are both specific for the IgV domain of Tim-1 have different effects on T cell activation and subsequent responses mainly because of the difference of their binding avidity, but not binding specificity. Because both anti–Tim-1 antibodies were specific for Tim-1 and did not cross react with other Tim family members, this places Tim-1 in a unique position in the category of T cell costimulatory molecules. Tim-1 may represent a third category of costimulatory molecules that can deliver both positive and negative costimulatory signals depending on how it is engaged during T cell activation.
The anti–Tim-1 antibodies 3B3 and RMT1-10 had opposite effects on T cell responses and the development of an autoimmune disease. The opposite effects of the two antibodies apparently are not caused by their binding specificity because both antibodies bound to closely related epitopes in the Tim-1 IgV domain. However, based on the biacore analysis, it was evident that the activating anti–Tim-1 antibody had an avidity that was 17 times higher than the inhibitory antibody RMT1-10. Because Tim-1 appears to be a costimulatory signaling molecule (14, 32), higher-avidity antibodies like 3B3 could enhance T cell activation by forming a stable Tim-1 complex and bringing Tim-1 into the TCR–CD3 complex, and they also help form large supramolecular activation clusters for full T cell activation (19). On the other hand, low-avidity antibodies like RMT1-10 have a significantly faster off-rate and may not support the formation of stable Tim-1–TCR–CD3 complexes. Akin to partial agonist/antagonist TCR ligands, low-avidity engagement of Tim-1 by RMT1-10 might only lead to partial T cell activation. Many of the partial agonist/antagonist TCR ligands have been shown to induce a partial signal into T cells, induce Th2 responses in vivo, and prevent development of autoimmune diseases (21, 33, 34). Strikingly, in agreement with this, treatment with RMT1-10, indeed, induced a Th2 response, even under proinflammatory immunization conditions. Our data clearly show that the 3B3 antibody induced a stronger cytoskeletal activation and motility, although the percentage of CD4+ T cells forming CD3 caps is not different between the two antibody treatments (Fig. 3 and Video 1). Furthermore, we found that monovalent Fab′ fragments of 3B3, like bivalent 3B3, enhanced Th1 and Th17 responses, whereas Fab′ fragments of RMT1-10, such as bivalent RMT1-10, decreased Th1 and Th17 responses and increased Th2 responses. However, the Fab′ fragments of both anti–Tim-1 antibodies did not change Th cell proliferation significantly upon antigen restimulation (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062498/DC1). These data suggest that the mere affinity (binding strength of Tim-1 and antibody) of Tim-1 engagement might contribute to the quality of the resulting T cell response. Also, because, similar to the intact antibodies, the Fab′ fragments of 3B3 and RMT1-10 induced skewing of the T cell response, it is unlikely that the results observed with the intact antibodies were caused by differences in the binding of Tim-1 (monovalent vs. bivalent) (35), but instead the stability of the Tim-1– antibody complex and the number of Tim-1 molecules engaged at a given time point might account for the differential T cell responses. However, the differences in the effect of the two antibodies on proliferation appears to be significantly affected when intact rather than when the Fab′ fragment was used in T cell proliferation assays, suggesting that the two T cell responses (proliferation vs. cytokine production) can be separated, depending on whether monovalent or bivalent antibodies are used.
Our data cannot exclude the possibility that interaction of Tim-1 with its ligand is an inhibitory interaction and that antibodies like 3B3 and RMT1-10 are differentially blocking this inhibitory interaction. Tim-1 has been shown to bind to itself by a homophilic interaction (36), and also to Tim-4, which is preferentially expressed on the antigen- presenting cells (15). Indeed, the Tim-1–Tim-4 interaction can be strongly blocked by high-avidity 3B3 antibody, whereas low-avidity antibody RMT1-10 showed a weak inhibition only at a very high dose (unpublished data). Whether the blockade of Tim-1–Tim-4 or Tim-1–Tim-1 interaction by anti–Tim-1 antibodies (3B3 or RMT1-10) contributes to the differential T cell responses observed here need to be further investigated. Furthermore, biochemical differences in the signaling mediated by the affinity/avidity of the two antibodies will further help discern the mechanism by which the two anti–Tim-1 antibodies mediate such opposite effects.
It has been reported that administration of 3B3 prevented development of respiratory tolerance and led to the development of airway hyperreactivity (14). Using this activating high-avidity anti–Tim-1 antibody, we showed that 3B3 in vivo strongly enhanced pathogenic Th1/Th17 responses, leukocyte infiltration, and tissue injury in the CNS, and increased disease severity of EAE. Both Th1 and Th17 T cell subsets have been shown to be critical for the tissue inflammation and pathogenesis of EAE (18, 23–25, 37). Using PLP139-151/IAs tetramers, we further analyzed the expression of cytokines in tetramer-positive cells. In 3B3-treated mice, the frequency of IFN-γ–producing PLP139-151-reactive Th cells was only slightly increased, indicating that the effect of 3B3 treatment on increase of IFN-γ production might mainly be caused by expansion of the responding T cells. There was an even bigger expansion in Th17 cells (nearly fourfold increase) in 3B3-treated mice. Besides increasing IFN-γ and IL-17, 3B3 enhanced IL-6 production from the responding T cells as well. Because we and others have recently shown that IL-6 is a key cytokine that, together with TGF-β, promotes differentiation of Th17 cells (18, 38), increased generation of Th17 cells by activating anti–Tim-1 antibody may be partly caused by the induction of IL-6 by 3B3 in T cells. Together with TGF-β, this induction of IL-6 might be responsible for the generation of Th17 cells in 3B3-treated mice. A previous report suggested that 3B3 antibody could enhance both production of IFN-γ and Th2 cytokines (14); however, we could not detect IL-4 or -10 in our study. This is likely caused by strain differences (SJL vs. BALB/c) in that in the previous study the authors used a Th2-prone strain, BALB/c, whereas most of our studies are undertaken with the SJL mice. The low-avidity anti–Tim-1 antibody RMT1-10 inhibited T cell expansion and proinflammatory cytokine production and promoted the generation of Th2 responses. This reduction in T cell proliferation and change in cytokine profile was paralleled by the inhibition of EAE in the RMT1-10–treated mice. The mechanism by which this anti–Tim-1 antibody inhibited EAE could be caused by both reduction of encephalitogenic Th1/Th17 responses and induction of Th2 responses. Both Th2 cytokines IL-4 and -10 have previously been shown to suppress EAE, and an increase in Th2 cytokines has been shown to accumulate in the CNS during recovery and to precede remission (25). Of the two cytokines, IL-10 has been shown to have more profound effect in regulating EAE (27). IL-4 has also recently been shown to potently inhibit the generation of Th17 cells (24, 37).
A subset of CD4+ cells called CD4+CD25+ regulatory T cells (T reg cells) that express Forkhead box P3 (Foxp3) can control autoimmune responses (39, 40). T reg cells have recently been shown to confer significant protection from the development of EAE (41), but also traffic to the target tissue and impact on the local milieu (42). We reported that there is a dichotomy in Th17 and FoxP3+ T reg cells (18). Whether increased severity of EAE in 3B3-treated mice or inhibition of EAE in RMT1-10–treated mice is also caused by alteration in the number and/or function of FoxP3+ T reg cells is not clear. Our preliminary data suggest that there is no decrease in the number of T reg cells in 3B3-treated mice or increase of T reg cells in RMT1-10–treated mice. However, whether treatment with 3B3 or RMT1-10 antibody alters the generation or function of antigen-specific T reg cells will need to be further investigated.
In conclusion, we found that Tim-1 plays an important role in the regulation of T cell responses and the development of autoimmune disease. The high-avidity anti–Tim-1 antibody enhances the severity of EAE because of increasing autopathogenic Th1 and Th17 responses, whereas the low-avidity antibody inhibits autopathogenic Th1 and Th17 responses and EAE and induces a strong Th2 response. The data suggest that Tim-1 may represent a new category of T cell costimulators that can positively and negatively costimulate T cell responses depending on how they are engaged during T cell activation, similar to the TCR ligands that can change functional outcomes depending on avidity with which TCR is engaged. Manipulating the Tim-1 pathway may have a therapeutic potential for many different diseases. Although antibodies with characteristics of 3B3 would be useful as vaccine adjuvants and in the treatment of cancers and infectious diseases, antibodies like RMT1-10 would be useful in treating autoimmune diseases and transplant rejections.
MATERIALS AND METHODS
Mice and antigen.
SJL mice were purchased from The Jackson Laboratory. The mice were maintained and all animal experiments were done according to the animal protocol guidelines of Harvard Medical School. PLP139-151 (HSLGKWLGHPDKF) was synthesized by Quality Controlled Biochemicals and was >90% pure, as determined by HPLC.
Generation of Tim transfectants and anti–Tim-1 mAbs.
Mouse Tim-1 cDNA was cloned into the pDisplay vector (Invitrogen) and transfected into Chinese hamster ovary (CHO) and EL-4 cells using a previously described method (43). Anti–Tim-1 mAb 3B3 (rat IgG2a, κ) was generated using mucinless Tim-1-Ig, which contains the IgV domain only, as immunogen (14). Anti–mouse Tim-1 mAb RMT1-10 (rat IgG2a, κ) was generated by immunizing SD rats with full-length Tim-1-Ig that contained both IgV and mucin domains of Tim-1. Lymph node cells were then fused with P3U1 myeloma cells and cloned. The hybridomas were screened for binding to mouse Tim-1–transfected CHO cells, but not the parental CHO cells. The specificity of the anti–Tim-1 antibodies were further determined by staining EL-4 and CHO cells transfected with different Tim family members.
Induction and clinical evaluation of EAE.
8–12-wk-old female SJL mice were immunized subcutaneously in the flanks with an emulsion containing PLP139-151 (80 μg/mouse) and 4 mg/ml Mycobacterium tuberculosis H37Ra extract (DIFCO) in CFA.
Pertussis toxin (100 ng/mouse; List Biological Laboratories) was administered intravenously on days 0 and 2. Mice were intraperitoneally injected with 100 μg 3B3, RMT1-10, rIgG, or PBS every other day from day 0 to 8. Mice were monitored and assigned grades for clinical signs of EAE using the following scoring system: 0, healthy; 1, limp tail; 2, impaired righting reflex or waddled gait; 3, hind limb paralysis; 4, total limb paralysis; 5, moribund or death. At different time points, brains and spinal cords were removed and fixed in 10% phosphate-buffered formalin and examined histologically for numbers of inflammatory foci and demyelination.
Proliferation assays and ELISA.
Female SJL mice were immunized with PLP139-151/CFA and treated with anti–Tim-1 or control antibodies as described in the previous section. Mice were killed at the time of disease onset (on day 10 for 3B3 treatment and on day 14 for RMT1-10 treatment) and spleens were removed. Spleen cells were isolated and plated in round-bottomed 96-well plates (BD Biosciences) in culture medium with various concentrations of PLP139-151. After 48 h, culture supernatants were removed for cytokine ELISA and cytokine production was measured by quantitative capture ELISA, as previously described (43). Plates were pulsed for 16 h with 1 μCi [3H]thymidine per well. Proliferation was measured as counts per minute by using a Wallac Liquid Scintillation Counter (Perkin Elmer).
IAs Tetramer staining and intracellular staining.
IAs tetramers for PLP139-151 and TMEV70-86 were generated as previously described (17, 28). TMEV tetramers were used as negative controls. Lymphocytes from spleen and lymph nodes of PLP139-151-immunized SJL mice treated with different antibody were cultured with 20 μg/ml of antigen for 5 d. Cells were purified by Ficoll-Hypaque density gradient centrifugation. After washing, cells were incubated with the tetramers (30 μg/ml) for 3 h at 37°C, followed by staining with anti–CD4-APC (clone RM4.5) and 7-amino-actinomycin D (7-AAD; BD Biosciences). Cells were acquired by using the FACSort flow cytometer (Becton Dickinson), and tetramer-positive cells were determined within the CD4+ population after gating the viable cells (7-AAD−). FACS data were analyzed with the CELLQUEST (Becton Dickinson) and FlowJo (Tree Star) programs. To determine the frequency of cytokine-producing cells, Ficoll-purified cells were reactivated with 20 ng/ml PMA (Sigma- Aldrich) and ionomycin (300 ng/ml; Sigma-Aldrich) and 2 mM monensin (GolgiStop; BD Biosciences) for 4 h at 37°C. After staining with tetramer, anti–CD4, and 7-AAD, the cells were fixed, permeabilized, and stained with cytokine antibody as recommended by the manufacturer (BD Biosciences). The frequencies of cytokine-producing cells were analyzed by gating on tetramer+CD4+7-AAD− populations.
Anti–Tim-1 binding ELISA.
ELISA plates were coated with 10 μg/ml of AffiniPure goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, Inc.). After overnight incubation at 4°C and washing, the plates were then coated with 50 nM of full-length or mucinless mouse Tim-1-Ig or Tim-4-Ig fusion protein. After blocking, different concentrations of anti–Tim-1 were added, followed by peroxidase-conjugated AffiniPure goat anti–rat IgG secondary antibody (Jackson ImmunoResearch Laboratories). Assays were developed with TMB Microwell Peroxidase Substrate (Kierkegaard and Perry Laboratories) and read at 450 nm using a Benchmark microplate reader (Bio-Rad Laboratories).
Confocal microscopy and live imaging.
CD4+ T cells were purified from SJL mouse using CD4 T cell enrichment column (R&D Systems) and stained with Alexa Fluor 488–conjugated anti–mouse CD3 mAb (BioLegend). Labeled cells were placed in a Live Imaging Microincubator (Carl Zeiss MicroImaging, Inc.) at 37°C and 5% CO2 for monitoring cell responses to the treatment with rIgG2a, 3B3, or RMT1-10 antibodies. Data were recorded for 1 h with a LSM510 laser-scanning confocal microscope (Carl Zeiss MicroImaging, Inc.) by using z-stack of the cultures to obtain the behavior of the entire body of cells and not just a single optical plane over time; these allow us to firmly establish the activity of both antibodies in CD3 capping and cell movement. Three-dimensional reconstructions were performed for every experiment and followed for the duration of the experiments, and the percentage of cells demonstrating capping and extended short-range movement, increased motility were annotated. Laser scanning parameters were maintained to a minimum to avoid any potential photodamage, and results were analyzed with LSM 510 software (Carl Zeiss MicroImaging, Inc.) and processed using LSM 510 confocal software, as previously described (24, 44).
Biacore analysis.
A Biacore T100 system (Biacore, Inc.) was used for kinetic analysis of anti–Tim-1 antibodies 3B3 and RMT1-10 with a reaction temperature of 37°C. Goat anti–rat IgG Fc was immobilized to the CM5 carboxymethylated dextran sensor chip. 5 nM of 3B3 or RMT1-10 was injected over the chip surface for 3 min at 10 μl/min. The reference flow cell was injected with rIgG2a. Tim-1-Ig, at concentrations of 5, 2.5, 1.25, 0.625, 0.3125, 0.156, and 0.078 nM, and buffer only was introduced to the chip surface using a high-performance injection for 4 min at 30 μl/min. For each concentration, dissociation of bound antigen in the buffer flow was recorded for an additional 20 min. The sensor chip surface was regenerated with a 30-s injection of 20 mM HCl at 60 μl/min, followed by a 30-s stabilization period.
For anti–Tim-1 epitope mapping, a Biacore 3000 system was used with a reaction temperature of 25°C. 10 μg/ml of anti–Tim-1 antibody 3B3 were injected over the goat anti–rat IgG Fc-immobilized chip surface for 1 min at 10 μl/min. The chip surface was then saturated with rIgG. A mixture of 10 μg/ml Tim-1-Ig and 100 μg/ml anti–Tim-1 antibody (3B3, RMT1-10, or control antibody) was injected over the chip surface for 3 min at 10 μl/min. The sensor chip surface was regenerated with a 40-s injection of 20 mM HCl at 60 μl/min.
Data transformation and sensogram plot overlays were prepared using BIAevaluation software for Biacore 3000 data, and Biacore T100 evaluation software was used for kinetic analysis of association and dissociation rates. All sensogram data were subtracted from the reference flow cell and double referenced using buffer injection data from each condition tested. Association and dissociation kinetics were determined using a bivalent analyte algorithm with global fit analysis.
Statistics.
Comparisons of the differences in biacore data or immune responses, such as proliferation and cytokine production and numbers of IAs tetramer-positive CD4+ T cells, were made using Student's t tests. The clinical score and incidence of PLP139-151-induced EAE was analyzed by Fisher's exact test. P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 shows the effects of Fab′ fragments of 3B3 and RMT1-10 on T cell proliferation and cytokine production. Video 1 shows that treatment with 3B3, but not RMT1-10, anti–Tim-1 antibody causes highly motile T cells with changes in morphology. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062498/DC1.
Supplemental Material
Acknowledgments
We thank Deneen Kozoriz for cell sorting and members in the laboratory for their helpful discussions and comments on the manuscript.
This work is supported by research grants from the National Multiple Sclerosis Society (RG3666 and RG2571D9) and the National Institutes of Health (NS045973, NS046414, NS35685, NS30843, AI44880, AI058680, P01AI139671, PO1AI41521, and P01NS38037). S. Xiao is a recipient of the National Multiple Sclerosis Society Postdoctoral Fellowship. N. Najafian is the recipient of the American Heart Association Scientist Development Grant. V.K. Kuchroo is a recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
The authors have no conflicting financial interests.
Abbreviations used: AAD, amino-actinomycin D; CHO, Chinese hamster ovary; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; PLP, proteolipid protein; Tim, T cell immunoglobulin mucin.
S. Xiao and N. Najafian, and S.J. Khoury and V.K. Kuchroo contributed equally to this work.
References
- 1.Meyers, J.H., C.A. Sabatos, S. Chakravarti, and V.K. Kuchroo. 2005. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 11:362–369. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo, V.K., D.T. Umetsu, R.H. DeKruyff, and G.J. Freeman. 2003. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 3:454–462. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan, G., A. Totsuka, P. Thompson, T. Akatsuka, Y. Moritsugu, and S.M. Feinstone. 1996. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 4.Feigelstock, D., P. Thompson, P. Mattoo, Y. Zhang, and G.G. Kaplan. 1998. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 72:6621–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichimura, T., J.V. Bonventre, V. Bailly, H. Wei, C.A. Hession, R.L. Cate, and M. Sanicola. 1998. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 273:4135–4142. [DOI] [PubMed] [Google Scholar]
- 6.Han, W.K., V. Bailly, R. Abichandani, R. Thadhani, and J.V. Bonventre. 2002. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 62:237–244. [DOI] [PubMed] [Google Scholar]
- 7.McIntire, J.J., S.E. Umetsu, C. Macaubas, E.G. Hoyte, C. Cinnioglu, L.L. Cavalli-Sforza, G.S. Barsh, J.F. Hallmayer, P.A. Underhill, N.J. Risch, et al. 2003. Immunology: hepatitis A virus link to atopic disease. Nature. 425:576. [DOI] [PubMed] [Google Scholar]
- 8.McIntire, J.J., S.E. Umetsu, O. Akbari, M. Potter, V.K. Kuchroo, G.S. Barsh, G.J. Freeman, D.T. Umetsu, and R.H. DeKruyff. 2001. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2:1109–1116. [DOI] [PubMed] [Google Scholar]
- 9.Graves, P.E., V. Siroux, S. Guerra, W.T. Klimecki, and F.D. Martinez. 2005. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J. Allergy Clin. Immunol. 116:650–656. [DOI] [PubMed] [Google Scholar]
- 10.Chae, S.C., J.H. Song, Y.C. Lee, J.W. Kim, and H.T. Chung. 2003. The association of the exon 4 variations of Tim-1 gene with allergic diseases in a Korean population. Biochem. Biophys. Res. Commun. 312:346–350. [DOI] [PubMed] [Google Scholar]
- 11.Chae, S.C., J.H. Song, J.C. Heo, Y.C. Lee, J.W. Kim, and H.T. Chung. 2003. Molecular variations in the promoter and coding regions of human Tim-1 gene and their association in Koreans with asthma. Hum. Immunol. 64:1177–1182. [DOI] [PubMed] [Google Scholar]
- 12.Chae, S.C., J.H. Song, S.C. Shim, K.S. Yoon, and H.T. Chung. 2004. The exon 4 variations of Tim-1 gene are associated with rheumatoid arthritis in a Korean population. Biochem. Biophys. Res. Commun. 315:971–975. [DOI] [PubMed] [Google Scholar]
- 13.Gao, P.S., R.A. Mathias, B. Plunkett, A. Togias, K.C. Barnes, T.H. Beaty, and S.K. Huang. 2005. Genetic variants of the T-cell immunoglobulin mucin 1 but not the T-cell immunoglobulin mucin 3 gene are associated with asthma in an African American population. J. Allergy Clin. Immunol. 115:982–988. [DOI] [PubMed] [Google Scholar]
- 14.Umetsu, S.E., W.L. Lee, J.J. McIntire, L. Downey, B. Sanjanwala, O. Akbari, G.J. Berry, H. Nagumo, G.J. Freeman, D.T. Umetsu, and R.H. DeKruyff. 2005. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 6:447–454. [DOI] [PubMed] [Google Scholar]
- 15.Meyers, J.H., S. Chakravarti, D. Schlesinger, Z. Illes, H. Waldner, S.E. Umetsu, J. Kenny, X.X. Zheng, D.T. Umetsu, R.H. DeKruyff, et al. 2005. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 6:455–464. [DOI] [PubMed] [Google Scholar]
- 16.Schneider, H., E. Valk, S. da Rocha Dias, B. Wei, and C.E. Rudd. 2005. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proc. Natl. Acad. Sci. USA. 102:12861–12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy, J., H. Waldner, X. Zhang, Z. Illes, K.W. Wucherpfennig, R.A. Sobel, and V.K. Kuchroo. 2005. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 175:5591–5595. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 19.Dustin, M.L. 2005. A dynamic view of the immunological synapse. Semin. Immunol. 17:400–410. [DOI] [PubMed] [Google Scholar]
- 20.Villalba, M., N. Coudronniere, M. Deckert, E. Teixeiro, P. Mas, and A. Altman. 2000. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 12:151–160. [DOI] [PubMed] [Google Scholar]
- 21.Stefanova, I., J.R. Dorfman, M. Tsukamoto, and R.N. Germain. 2003. On the role of self-recognition in T cell responses to foreign antigen. Immunol. Rev. 191:97–106. [DOI] [PubMed] [Google Scholar]
- 22.Swanborg, R.H. 1995. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin. Immunol. Immunopathol. 77:4–13. [DOI] [PubMed] [Google Scholar]
- 23.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchroo, V.K., A.C. Anderson, H. Waldner, M. Munder, E. Bettelli, and L.B. Nicholson. 2002. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 20:101–123. [DOI] [PubMed] [Google Scholar]
- 26.Kuchroo, V.K., M.P. Das, J.A. Brown, A.M. Ranger, S.S. Zamvil, R.A. Sobel, H.L. Weiner, N. Nabavi, and L.H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 80:707–718. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli, E., M.P. Das, E.D. Howard, H.L. Weiner, R.A. Sobel, and V.K. Kuchroo. 1998. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4- deficient and transgenic mice. J. Immunol. 161:3299–3306. [PubMed] [Google Scholar]
- 28.Reddy, J., Z. Illes, X. Zhang, J. Encinas, J. Pyrdol, L. Nicholson, R.A. Sobel, K.W. Wucherpfennig, and V.K. Kuchroo. 2004. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 101:15434–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretscher, P.A. 1999. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc. Natl. Acad. Sci. USA. 96:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe, A.H., and G.J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116–126. [DOI] [PubMed] [Google Scholar]
- 31.Chitnis, T., and S.J. Khoury. 2003. Role of costimulatory pathways in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J. Allergy Clin. Immunol. 112:837–849. [DOI] [PubMed] [Google Scholar]
- 32.de Souza, A.J., T.B. Oriss, K.J. O'Malley, A. Ray, and L.P. Kane. 2005. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc. Natl. Acad. Sci. USA. 102:17113–17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson, L.B., and V.K. Kuchroo. 1996. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr. Opin. Immunol. 8:837–842. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson, L.B., and V.K. Kuchroo. 1997. T cell recognition of self and altered self antigens. Crit. Rev. Immunol. 17:449–462. [PubMed] [Google Scholar]
- 35.Koch, C., G. Staffler, R. Huttinger, I. Hilgert, E. Prager, J. Cerny, P. Steinlein, O. Majdic, V. Horejsi, and H. Stockinger. 1999. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 11:777–786. [DOI] [PubMed] [Google Scholar]
- 36.Santiago, C., A. Ballesteros, C. Tami, L. Martinez-Munoz, G.G. Kaplan, and J.M. Casasnovas. 2007. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 26:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17- producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 38.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 39.Shevach, E.M., R.S. McHugh, C.A. Piccirillo, and A.M. Thornton. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182:58–67. [DOI] [PubMed] [Google Scholar]
- 40.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, X., D.N. Koldzic, L. Izikson, J. Reddy, R.F. Nazareno, S. Sakaguchi, V.K. Kuchroo, and H.L. Weiner. 2004. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int. Immunol. 16:249–256. [DOI] [PubMed] [Google Scholar]
- 42.Korn, T., J. Reddy, W. Gao, E. Bettelli, A. Awasthi, T.R. Petersen, B.T. Backstrom, R.A. Sobel, K.W. Wucherpfennig, T.B. Strom, et al. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakravarti, S., C.A. Sabatos, S. Xiao, Z. Illes, E.K. Cha, R.A. Sobel, X.X. Zheng, T.B. Strom, and V.K. Kuchroo. 2005. Tim-2 regulates T helper type 2 responses and autoimmunity. J. Exp. Med. 202:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monsonego, A., J. Imitola, S. Petrovic, V. Zota, A. Nemirovsky, R. Baron, Y. Fisher, T. Owens, and H.L. Weiner. 2006. Abeta-induced meningoencephalitis is IFN-gamma-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 103:5048–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.