Abstract
There is growing evidence for an interplay between inflammatory and coagulation pathways in acute and chronic inflammatory diseases. However, it remains unclear whether components of the coagulation pathway, such as tissue factor (TF), contribute to intestinal inflammation, and whether targeting TF will blunt the inflammatory cell recruitment, tissue injury, and enhanced thrombus formation that occur in experimental colitis. Mice were fed 3% dextran sodium sulfate (DSS) to induce colonic inflammation, with some mice receiving a mouse TF-blocking antibody (muTF-Ab). The adhesion of leukocytes and platelets in colonic venules, light/dye-induced thrombus formation in cremaster muscle microvessels, as well as disease activity index, thrombin–antithrombin (TAT) complexes in plasma, and histopathologic changes in the colonic mucosa were monitored in untreated and muTF-Ab–treated colitic mice. In untreated mice, DSS elicited the recruitment of adherent leukocytes and platelets in colonic venules, caused gross and histologic injury, increased plasma TAT complexes, and enhanced thrombus formation in muscle arterioles. muTF-Ab prevented elevation in TAT complexes, reduced blood cell recruitment and tissue injury, and blunted thrombus formation in DSS colitic mice. These findings implicate TF in intestinal inflammation and support an interaction between inflammation and coagulation in experimental colitis.
Inflammatory bowel disease (IBD) is associated with a hypercoagulable state and an increased risk for thromboembolism (1–3). The IBD-associated hypercoagulable state is manifested in systemic blood and in both intestinal and extraintestinal vascular beds. Although no single consistent coagulation abnormality has been identified, blood samples from patients with active IBD have revealed thrombocytosis, accelerated thrombin generation, and increased circulating levels of thrombin–antithrombin (TAT) complexes (2–4). Clinical studies indicate that there is a substantially increased incidence of extraintestinal thromboembolism in IBD patients (1, 2, 4), with the thromboembolism frequently manifested as deep vein thrombosis or a pulmonary embolism. Although thrombosis is known to contribute to morbidity and mortality in IBD, the mechanisms that underlie the hypercoagulable state during intestinal inflammation remain poorly defined.
Efforts to better understand the processes of hemostasis and coagulation in different pathologic conditions have revealed an intimate link between coagulation and the inflammatory response (5, 6). Inflammation appears to shift the hemostatic mechanisms in favor of thrombosis by altering the three dominant anticoagulant pathways, i.e., the heparin–antithrombin system, the tissue factor (TF) pathway inhibitor system, and the protein C anticoagulant pathway (5, 6). Although clinical evidence suggests that these pathways are altered in IBD (7, 8), there is no direct evidence supporting the participation of the natural anticoagulant pathways in the induction of a prothrombotic state in either human or experimental IBD.
There is also emerging evidence that the coagulation pathways exert an influence on the inflammatory response. Different components of the coagulation pathways, including thrombin and TF, appear to promote inflammation, whereas activated protein C exerts an antiinflammatory effect. For example, TF itself activates protease-activated receptor (PAR) 2 and, to a lesser extent, PAR1 (9), whereas thrombin is known to activate PAR1, 3, and 4 (10–12). The activation of PARs elicits the production of proinflammatory cytokines (e.g., TNF-α and IL-6) and promotes leukocyte rolling in venules (13–15). The potential importance of a link between TF and PAR signaling is supported by the observation that anti-PAR strategies successfully blunt the inflammatory responses in different models of experimental colitis (16). Furthermore, mice that lack the cytoplasmic domain of TF exhibit an attenuated recruitment of rolling, adherent, and transmigrating leukocytes in postcapillary venules after LPS challenge (17). Similarly, a small molecule inhibitor of the TF-VIIa complex (BCX-3607) has been shown to attenuate the LPS-induced production of IL-6 and IL-8 in vitro (by human umbilical vein endothelial cells) and IL-6 in vivo (18). Despite the mounting evidence that implicates TF and other components of the coagulation pathway as potential mediators of acute and chronic inflammatory responses, the involvement of these coagulation pathways in the initiation and/or propagation of intestinal inflammation has not been systematically assessed in experimental models of IBD, nor has the feasibility of targeting the inflammation–coagulation interface to blunt inflammation and tissue injury been evaluated in such models.
The overall objective of this study was to determine whether TF contributes to the pathogenesis of intestinal inflammation. In addition, we determined whether the systemic procoagulant state and enhanced extraintestinal thrombus formation that are manifested in human IBD can be recapitulated in an animal model of experimental colitis. The role of TF in mediating these coagulation/thrombotic events was also evaluated.
RESULTS
Clinical indices of disease activity
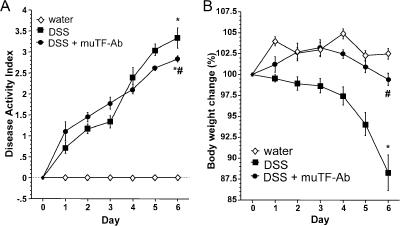
All animals survived the dextran sodium sulfate (DSS) protocol, and experiments were performed on day 6. Mice fed DSS had symptoms of colitis (diarrhea, weight loss, or perianal bleeding) 1–2 d after starting the DSS that progressed throughout the 6-d period. The time course of change in disease activity index (DAI) is shown in Fig. 1 A. Control mice receiving water showed no clinical signs (diarrhea, fecal occult blood, perianal bleeding, or weight loss) of spontaneous intestinal inflammation. Mouse TF-blocking antibody (muTF-Ab) treatment of mice receiving DSS resulted in a significantly lower DAI score on day 6 (Fig. 1 A), and the loss in body weight was minimal in the muTF-Ab–treated group (Fig. 1 B). Bleeding in the muTF-Ab–treated mice was not significantly altered when compared with untreated DSS controls.
Figure 1.
Inhibition of TF abrogates the clinical indices of colitis. (A) Changes in DAI on days 0–6 of DSS treatment. Some mice received muTF-Ab on days 0 and 3. (B) Changes in body weight from days 0 to 6 of DSS treatment. Values are reported as means ± SE. *, P < 0.05 versus water; #, P < 0.05 versus DSS.
Histopathology
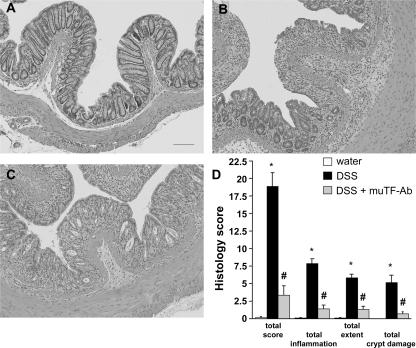
Blinded histological injury scoring was quantified in the distal colon after 6 d of DSS treatment. Control animals showed no signs of inflammation (Fig. 2, A and D). In contrast, mice receiving DSS exhibited histologic signs of severe colitis, as assessed by overall score as well as specific parameters (inflammation, extent, and crypt damage) (Fig. 2, B and D). Inflammation was mainly confined to the mucosa and the submucosa, with a dense cellular infiltrate and partial loss of epithelial integrity. All of the histologic variables were significantly blunted by the muTF-Ab treatment regimen (Fig. 2, C and D).
Figure 2.
TF contributes to the histological damage and inflammation observed in the colon during colitis. Representative images of histology (A–C) and blinded histological scoring of colonic mucosa (D) at day 6 in mice receiving (A) water, (B) DSS, or (C) DSS + muTF-Ab. Values are reported as means ± SE. *, P < 0.05 versus water; #, P < 0.05 versus DSS. Bar, 100 μm.
Colonic microvascular responses
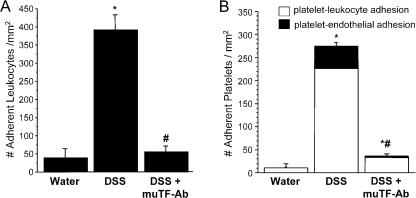
DSS treatment elicited an approximately sevenfold increase in the number of firmly adherent leukocytes in colonic venules when compared with controls (Fig. 3 A). This increased recruitment of adherent leukocytes was virtually abolished by muTF-Ab treatment. Increased platelet adhesion was also noted in colonic venules of DSS-treated mice (Fig. 3 B), with the majority of the CFSE-stained, exogenous platelets binding to adherent leukocytes and the remainder adhering directly to colonic endothelial cells. Both platelet–leukocyte and platelet–endothelial cell adhesion were greatly attenuated in DSS colitic mice treated with muTF-Ab.
Figure 3.
Ab blockade of TF virtually abolishes leukocyte and platelet recruitment in postcapillary venules of the colitic colon. Effects of treatment with muTF-Ab on the number of adherent leukocytes (A) and platelets (B) in the colonic microcirculation during DSS colitis. The shaded bar in B denotes the number of platelets bound directly to venular endothelium, whereas the open bar represents platelets binding to adherent leukocytes. Values are reported as means ± SE. *, P < 0.05 versus water; #, P < 0.05 versus DSS.
Light/dye-induced thrombus formation
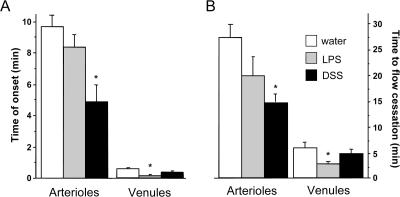
To characterize the contribution of TF to the extraintestinal coagulopathy associated with experimental colitis, we examined the effects of DSS-induced colonic inflammation on light/dye-induced thrombosis in cremaster muscle microvessels and compared the responses with those elicited by bacterial endotoxin. Fig. 4 examines the thrombotic responses, expressed as the time of onset of platelet deposition/aggregation (left) and the time required for complete flow cessation (right), in the light/dye-exposed microvessels of control, LPS-treated, and DSS colitic mice. The data confirm that much longer times are required for both the onset of thrombus formation and complete flow cessation in arterioles, compared with venules, of the control cremaster microcirculation exposed to light/dye, as previously reported (19). Although LPS treatment does appear to slightly affect both variables in arterioles, the reductions in both the time of onset and time for flow cessation in venules was more dramatic and significantly different. DSS colitis is also associated with a significantly altered thrombotic response that is consistent with a procoagulant state; however, this effect was largely manifested in arterioles and not in venules.
Figure 4.
DSS and LPS enhance thrombus formation in arterioles and venules, respectively. (A) Time of onset of thrombus formation in cremaster arterioles and venules exposed to light/dye. (B) Time to cessation of flow in the same vessels after light/dye exposure. Values are reported as means ± SE. *, P < 0.05 versus water.
Fig. 5 summarizes the changes in light/dye-induced thrombus formation in cremaster arterioles between untreated and muTF-Ab–treated mice on day 6 of DSS. The data show that the enhanced light/dye-induced thrombus formation in cremaster arterioles of DSS mice is significantly blunted by TF immunoneutralization. The figure also illustrates that treatment of colitic mice on day 6 of DSS (∼30 min before light/dye exposure) with the muTF-Ab (DSS + post–muTF-Ab) was as effective as the pretreatment regimen (DSS + pre–muTF-Ab), indicating that the presence of this reagent at the time of thrombus formation is sufficient to exert its beneficial effect.
Figure 5.
DSS accelerates the onset of thrombus formation via a TF-dependent mechanism. Time of onset of thrombosis and cessation of flow in arterioles of the cremaster after DSS and pretreatment (during the induction of colitis) or posttreatment (on the day of the experiment before thrombus induction) with muTF-Ab. Values are reported as means ± SE. *, P < 0.05 versus water; #, P < 0.05 versus DSS.
Fig. 6 shows the effect of muTF-Ab treatment on the changes in coagulation elicited by LPS in venules. It illustrates the complete normalization of light/dye-induced thrombus formation, which was significantly enhanced after LPS. Note that this effect is seen on the venular side in contrast to DSS, where this effect is seen on the arteriolar side.
Figure 6.
TF participates in LPS-induced enhancement of thrombotic responses. Time to onset of thrombosis and cessation of flow in venules of the cremaster after LPS and treatment with muTF-Ab. Values are reported as means ± SE. *, P < 0.05 versus water.
TAT measurements
The blood level of TAT complexes was significantly elevated in mice on DSS compared with control mice, which is consistent with studies in IBD patients showing increased TAT levels and a systemic procoagulant state (4). Mice pretreated with the muTF-Ab showed no elevation in plasma TAT concentration on day 6 of DSS colitis (Fig. 7).
Figure 7.
Colitis increases circulating TAT complexes through a pathway that requires TF. TAT levels in plasma samples of DSS colitic mice with or without muTF-Ab treatment. Values are reported as means ± SE. *, P < 0.05 versus water; #, P < 0.05 versus DSS.
Control Ab does not alter injury in DSS colitis
To confirm that the protective effect of muTF-Ab was not a byproduct of the administration of volume leading to a reduction in DSS intake and, therefore, colitis, a separate group of DSS mice was given an equivalent volume of an isotype-matched control Ab. This treatment did not alter drinking volume, and the DSS load of these animals was above the threshold of 30 mg/g required for induction of colitis (20). The DAI score (3.3 ± 0.13; P < 0.0001 vs. water and muTF-Ab groups) and weight loss (93 ± 0.95% at day 6; P < 0.005 vs. water) responses in the control Ab–treated mice were similar to the values detected in untreated DSS mice. Thrombosis in extraintestinal vessels was also assessed in control Ab–treated mice. The time of onset of thrombosis (5.8 ± 0.43 s; P < 0.05 vs. water and both muTF-Ab groups) and cessation of flow (14 ± 0.81 s) in arterioles did not differ from the responses noted in untreated DSS mice. These data confirm that the protective effect of muTF-Ab was caused by blockade of TF rather than a nonspecific effect mediated by administration of Ab.
DISCUSSION
Although studies of hemostasis and coagulation in different pathologic conditions have revealed an intimate link between coagulation and the inflammatory response, the relevance of this relationship to the pathophysiology of IBD remains poorly understood. There is a large amount of evidence that supports the notion that the coagulation cascade is activated in IBD and that this activation is manifested both in the involved segment of the inflamed bowel and at extraintestinal sites where potentially fatal thrombi are formed (2, 7, 8). Most of the evidence that implicates an activated coagulation cascade in IBD pathogenesis is derived from clinical studies, and there is relatively little information regarding the ability of animal models of IBD to recapitulate the hypercoagulable state that accompanies this disease. The results of this study provide novel insights into the link between gut inflammation and coagulation, with evidence implicating the procoagulant TF in the inflammatory cell recruitment and tissue injury that accompany colonic inflammation. We also provide evidence that is consistent with a procoagulant state in the systemic blood of colitic mice, as reflected by elevated levels of TAT complexes in plasma and an enhanced ability of an extraintestinal vascular bed (cremaster muscle) to produce microthrombi in response to colonic inflammation. TF also appears to play a major role in the formation of these extraintestinal thrombi. Hence, our findings indicate that TF is a key molecule that acts at the inflammation–coagulation interface to promote both inflammation and coagulation in experimental IBD.
Our observation that immunoneutralization of TF very effectively blunts the accumulation of adherent leukocytes and platelets in venules of the inflamed colon is consistent with a previous report describing the ability of TF to induce endothelial cells to assume a proadhesive phenotype (21). Whether this action of TF results from its ability to induce cytokine production via the PAR2 pathway or reflects a direct effect on the endothelial cell remains unclear. Although endothelial cells are an important source of TF in vivo (17, 22–24), circulating monocytes and neutrophils are also known to produce and express the procoagulant (23–26). Hence, it is conceivable that leukocyte-associated TF could contribute to the TF-dependent blood cell recruitment responses elicited in colonic venules by DSS. Studies on mouse chimeras wherein TF-deficient bone marrow is transplanted into WT recipients have revealed a major role for blood cell–associated TF in mediating the prothrombotic state and the generation of circulating inflammatory cytokines induced by LPS (27). This raises the possibility that exposure of blood cells to enteric bacteria-derived LPS (entering the interstitium through an injured mucosal membrane) during transit through the inflamed bowel may contribute to the adhesion of leukocytes and platelets elicited by DSS. This is supported by the finding that circulating cells such as neutrophils, eosinophils, and platelets are capable of TF expression (25). Because the colonic tissue injury associated with DSS treatment is known to be neutrophil dependent, it appears likely that the attenuated histopathological changes observed in the muTF-Ab–treated mice reflect the diminished recruitment of injury-causing neutrophils. This possibility is supported by a recent report describing an increased TF activity after renal ischemia-reperfusion that leads to neutrophil-mediated injury predominantly via thrombin-dependent PAR1 signaling (28).
Novel and potentially important observations in this study were the elevated TAT complex levels and enhanced light/dye-induced thrombosis formation in extraintestinal microvessels of mice with DSS-induced intestinal inflammation. These responses are consistent with clinical studies describing a hypercoagulable state in systemic blood and an increased incidence of thromboembolism in IBD patients (1, 2). The magnitude of the increased TAT levels detected in the DSS model was quantitatively similar to the changes reported in human IBD (4). Although both arterial and venous circulations appear to be involved in the thromboembolism that occurs in IBD patients, venous complications occur more frequently (1). In the present study, however, we noted that arterioles in the cremaster microcirculation, rather than venules, exhibit an enhanced thrombus formation in response to light/dye exposure. This is also in contrast to the pattern of microvessel susceptibility to thrombus formation observed after LPS administration wherein venules, but not arterioles, exhibit substantially enhanced thrombus formation, which has also been reported by others (19, 29, 30). The latter findings would argue against a role for LPS as a gut-derived mediator of the enhanced thrombus formation in cremaster arterioles during DSS-induced colonic inflammation.
Although the enhanced microthrombus formation observed in cremaster muscle arterioles during DSS colitis is consistent with the hypercoagulable state that has been repeatedly described in IBD patients, the pathophysiological relevance of these microvascular responses to the potentially fatal deep vein thrombosis and pulmonary vein thrombosis that occurs in some IBD patients remains unclear. It is conceivable that the thrombi that are found in large veins are initially formed in the microvasculature and seed the much larger thrombi that eventually develop in the large veins. This possibility is consistent with our observation that a large number of thrombi formed in both arterioles and venules dislodge from the vessel wall and eventually enter the blood stream (unpublished data).
Another major objective of this study was to determine whether TF contributes to the enhanced extraintestinal thrombus formation that accompanies DSS-induced colonic inflammation. Our data strongly support a role for TF in the enhanced thrombus formation and suggest that administration of the TF blocking Ab either as a pretreatment regimen during the development of DSS colitis or as a posttreatment on the final day (30 min before thrombus induction/formation) is equally effective in blunting the response. In addition to the therapeutic importance of these findings, it suggests that TF activation is not limited to the colonic microcirculation in this model. As noted above, this extraintestinal manifestation of a TF-dependent event may reflect the actions of inflammatory mediators released from the inflamed colon that activate TF both locally and at the distant site, or it may reflect the passage of blood cells (e.g., monocytes and neutrophils) that are activated to produce TF as they flow through the gut circulation and eventually transit through extraintestinal vascular beds, such as skeletal muscle. Additional work using bone marrow chimeras in TF-deficient mice is needed to resolve the contribution of endothelial versus blood cell TF to the local and distant tissue responses during colonic inflammation.
In conclusion, our study provides the first evidence for a role for TF in mediating the inflammatory cell recruitment and tissue injury in the colon, as well as enhanced extraintestinal thrombus formation during experimental colitis. These findings indicate that gut inflammation is accompanied by activation of a major coagulation pathway that, in turn, can propagate the inflammatory response in the bowel wall. Exactly how these two responses interact with each other remains unclear. As described earlier, TF can promote the activation and/or release of many proinflammatory and procoagulant mediators, whereas inflammatory cytokines and other factors can stimulate TF production (31). This intimate link between coagulation and inflammation makes it difficult to determine whether anti-TF is predominantly targeting one or both of these responses in our model. However, our findings are in agreement with other studies in which blocking coagulation pathways influenced inflammatory responses during IBD. For example, heparin has been shown to attenuate the inflammatory and injury responses in DSS colitis in mice (32) and trinitrobenzene sulphonic acid colitis in rats (33). Also, we have previously demonstrated a role for the CD40/CD40L dyad, which is known to participate in both coagulation and inflammatory processes, in the inflammatory and thrombogenic responses to DSS colitis (34). Furthermore, the triazolopyrimidine trapidil, which was developed for the prevention of restenosis after vascular injury, was shown to protect against DSS-induced colonic inflammation, suggesting that targeting coagulation may interfere with the development of the inflammatory response. Therapeutic strategies that target this vicious cycle of inflammation and coagulation may prove effective in reducing the morbidity and mortality of chronic inflammatory diseases such as IBD.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice were purchased from the Jackson Laboratory and were maintained under pathogen-free conditions, with ad libitum access to a standard diet and water until reaching the desired age (8–10 wk) and/or weight (20–25 g). All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were performed according to the criteria outlined by the National Institutes of Health.
Induction of colitis.
Mice received 3% DSS (40 kD; MP Biomedicals) in filter-purified drinking water for 6 d ad libitum (35). Control mice received filtered water alone.
Assessment of disease progression.
DAI, ranging from 0 to 4, was used for clinical assessment of disease severity and calculated using stool consistency, fecal blood, macroscopic evaluation of the anus, and weight loss, as previously described (36, 37).
Histological colitis score.
For each animal, three samples of the distal colon were evaluated histologically after staining with hematoxylin and eosin. Quantification of the histological changes was performed using a previously described scoring system (38).
Blood sampling and platelet preparation.
Platelets monitored in the intravital microscopy experiments were harvested from donor mice (not receiving DSS) for labeling with the fluorochrome CFSE (90-μM final concentration; Invitrogen), as previously described (39). Leukocytes accounted for <0.01% of the cells in the final platelet suspension.
Surgical preparation for intravital microscopy.
Mice were anesthetized with 150 mg/kg ketamine hydrochloride i.p. and 7.5 mg/kg xylazine i.p. The right carotid artery was cannulated for blood pressure measurements, and the right jugular vein was cannulated for infusion of rhodamine 6G (Sigma-Aldrich) for leukocyte labeling, followed by infusion of CFSE-labeled platelets. A laparotomy was performed, and the animal was placed on an adjustable acrylic microscope stage for visualization of venules in the proximal large bowel (40).
Intravital fluorescence microscopy.
Platelets and leukocytes were visualized with an inverted fluorescence microscope (DIAPHOT 300; Nikon) (40). The video images of five randomly selected postcapillary venules were captured with a CCD camera (C2400; Hamamatsu Photonics K.K.) and were digitally recorded, each for 1 min. Venular diameter (20–40 μm) was measured with a video caliper. Platelets and leukocytes were classified according to their interaction with the venular wall as either free flowing or adherent (cells remaining stationary for ≥30 s, expressed as the number of cells per square millimeter of venular surface).
TAT complex measurements.
TAT complex concentrations in plasma were measured spectrophotometrically (492 nm) using a sandwich enzyme immunoassay (Enzygnost TAT micro; Dade Behring) (17, 41). The blood samples were obtained from separate groups of mice not subjected to other manipulation, and withdrawn from a venous canula into heparin to avoid artificial activation.
Thrombus formation in cremaster muscle microcirculation.
Mice were anesthetized with 50 mg/kg sodium pentobarbital i.p., and the cremaster muscle was surgically prepared for intravital fluorescence microscopic observation, as previously described (42). Arterioles and venules (30–45-μm diameters) with shear rates of >400 s−1 were selected for study. Thrombosis formation in cremasteric microvessels was induced in mice receiving 10 ml/kg FITC-dextran i.v., which was allowed to circulate for 10–15 min before photoactivation (19, 29, 30). Blood cell velocities and shear rates were determined in arterioles and venules before photoactivation, which was initiated by exposing a 100-μm length of microvessel (venules first, followed by arterioles) to epiillumination with a 175-W xenon lamp and a fluorescein filter cube. Daily measurements of excitation power density were obtained to maintain a value within 1% of 1.4 W/cm2, as previously described (29). Epiillumination was continuously applied, and thrombus formation was quantified by determining (a) the time of onset of platelet deposition/aggregation within the microvessel and (b) the time required for complete flow cessation (>60 s) (19). Two to three thrombi were induced in each mouse, and the results of each vessel type (arterioles and venules) were averaged.
Experimental protocols.
In the first series of experiments (n = 6), we focused on the macroscopic and histologic responses of the colon to DSS in WT mice treated with 20 mg/kg of body weight of the muTF-mAb 1H1 (43), which was administered i.p. two times (200 μl/injection on days 0 and 3) during the course of colitis induction (DSS + TF-Ab group). A separate group of DSS mice was treated with 20 mg/kg of body weight of an isotype-matched control Ab (rat IgG2A; eBioscience), using the same protocol. On day 6, leukocyte and platelet adhesion in the colonic microvasculature was quantified. After recording the adhesion responses, the mice were killed with an overdose of anesthetic, and the colon was excised for measurement of bowel length and weight and then divided for histologic processing.
Separate experiments were performed to evaluate thrombus formation in the cremaster microcirculation of DSS-treated mice. Some of these mice were pretreated in a manner similar to that described for the gut inflammation experiments (DSS + pre–muTF-Ab), except that the cremaster muscle was evaluated on day 6 of DSS treatment for the onset of thrombus formation and cessation of flow after light/dye exposure. Another group of colitic mice received the muTF-Ab (DSS + post–muTF-Ab) on day 6 of DSS, ∼30 min before light/dye exposure, to examine the efficacy of TF immunoneutralization after the onset of colitis. Because bacterial endotoxin has been extensively used to evaluate thrombus formation in the cremaster muscle microcirculation, we also evaluated the thrombus formation in mice receiving 1.5 × 106 EU/kg of Escherichia coli endotoxin 0111:B4 (LPS; Sigma-Aldrich), as previously described (19). TAT measurements were obtained in a separate series of mice (n = 10 per group) to avoid any interference of the fluorescent dyes used in the intravital microscopy experiments with the color reaction in the TAT assay.
Data analysis.
Statistical analyses were performed using one-way analysis of variance, followed by the Scheffé post-hoc test. All values are reported as means ± SE. Statistical significance was set at P < 0.05.
Acknowledgments
This work was supported by grant R01 DK65649 from the National Institute of Diabetes and Digestive and Kidney Diseases.
D. Kirchhofer is an employee of Genentech, Inc. All other authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; DAI, disease activity index; DSS, dextran sodium sulfate; IBD, inflammatory bowel disease; muTF-Ab, mouse TF-blocking Ab; PAR, protease-activated receptor; TAT, thrombin–antithrombin; TF, tissue factor.
References
- 1.Irving, P.M., K.J. Pasi, and D.S. Rampton. 2005. Thrombosis and inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 3:617–628. [DOI] [PubMed] [Google Scholar]
- 2.Twig, G., G. Zandman-Goddard, M. Szyper-Kravitz, and Y. Shoenfeld. 2005. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann. NY Acad. Sci. 1051:166–173. [DOI] [PubMed] [Google Scholar]
- 3.Hatoum, O.A., H. Miura, and D.G. Binion. 2003. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am. J. Physiol. Heart Circ. Physiol. 285:H1791–H1796. [DOI] [PubMed] [Google Scholar]
- 4.Chamouard, P., L. Grunebaum, M.L. Wiesel, P.L. Frey, C. Wittersheim, R. Sapin, R. Baumann, and J.P. Cazenave. 1995. Prothrombin fragment 1 + 2 and thrombin-antithrombin III complex as markers of activation of blood coagulation in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 7:1183–1188. [DOI] [PubMed] [Google Scholar]
- 5.Esmon, C.T. 2004. The impact of the inflammatory response on coagulation. Thromb. Res. 114:321–327. [DOI] [PubMed] [Google Scholar]
- 6.Esmon, C.T. 2005. The interactions between inflammation and coagulation. Br. J. Haematol. 131:417–430. [DOI] [PubMed] [Google Scholar]
- 7.Solem, C.A., E.V. Loftus, W.J. Tremaine, and W.J. Sandborn. 2004. Venous thromboembolism in inflammatory bowel disease. Am. J. Gastroenterol. 99:97–101. [DOI] [PubMed] [Google Scholar]
- 8.van Bodegraven, A.A. 2003. Haemostasis in inflammatory bowel diseases: clinical relevance. Scand. J. Gastroenterol. Suppl. 239:51–62. [DOI] [PubMed] [Google Scholar]
- 9.Camerer, E., W. Huang, and S.R. Coughlin. 2000. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. USA. 97:5255–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coughlin, S.R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3:1800–1814. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, M.A., E. Rondeau, X. Chen, S.R. Coughlin, S.R. Holdsworth, and P.G. Tipping. 2000. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J. Exp. Med. 191:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major, C.D., R.J. Santulli, C.K. Derian, and P. Andrade-Gordon. 2003. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 23:931–939. [DOI] [PubMed] [Google Scholar]
- 13.Fan, Y., W. Zhang, and M. Mulholland. 2005. Thrombin and PAR-1-AP increase proinflammatory cytokine expression in C6 cells. J. Surg. Res. 129:196–201. [DOI] [PubMed] [Google Scholar]
- 14.Li, T., H. Wang, and S. He. 2006. Induction of interleukin-6 release from monocytes by serine proteinases and its potential mechanisms. Scand. J. Immunol. 64:10–16. [DOI] [PubMed] [Google Scholar]
- 15.Lindner, J.R., M.L. Kahn, S.R. Coughlin, G.R. Sambrano, E. Schauble, D. Bernstein, D. Foy, A. Hafezi-Moghadam, and K. Ley. 2000. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J. Immunol. 165:6504–6510. [DOI] [PubMed] [Google Scholar]
- 16.Vergnolle, N., L. Cellars, A. Mencarelli, G. Rizzo, S. Swaminathan, P. Beck, M. Steinhoff, P. Andrade-Gordon, N.W. Bunnett, M.D. Hollenberg, et al. 2004. A role for proteinase-activated receptor-1 in inflammatory bowel diseases. J. Clin. Invest. 114:1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Sharma, L., E. Melis, M.J. Hickey, C.D. Clyne, J. Erlich, L.M. Khachigian, P. Davenport, E. Morand, P. Carmeliet, and P.G. Tipping. 2004. The cytoplasmic domain of tissue factor contributes to leukocyte recruitment and death in endotoxemia. Am. J. Pathol. 165:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold, C.S., C. Parker, R. Upshaw, H. Prydz, P. Chand, P. Kotian, S. Bantia, and Y.S. Babu. 2006. The antithrombotic and anti-inflammatory effects of BCX-3607, a small molecule tissue factor/factor VIIa inhibitor. Thromb. Res. 117:343–349. [DOI] [PubMed] [Google Scholar]
- 19.Rumbaut, R.E., R.V. Bellera, J.K. Randhawa, C.N. Shrimpton, S.K. Dasgupta, J.F. Dong, and A.R. Burns. 2006. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am. J. Physiol. Heart Circ. Physiol. 290:H1671–H1679. [DOI] [PubMed] [Google Scholar]
- 20.Vowinkel, T., T.J. Kalogeris, M. Mori, C.F. Krieglstein, and D.N. Granger. 2004. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig. Dis. Sci. 49:556–564. [DOI] [PubMed] [Google Scholar]
- 21.Pawlinski, R., B. Pedersen, G. Schabbauer, M. Tencati, T. Holscher, W. Boisvert, P. Andrade-Gordon, R.D. Frank, and N. Mackman. 2004. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 103:1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlinski, R., and N. Mackman. 2004. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit. Care Med. 32:S293–S297. [DOI] [PubMed] [Google Scholar]
- 23.Osterud, B., and E. Bjorklid. 2006. Sources of tissue factor. Semin. Thromb. Hemost. 32:11–23. [DOI] [PubMed] [Google Scholar]
- 24.Mackman, N. 2006. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol. Dis. 36:104–107. [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt, C. 2007. New locations of intravascular tissue factor: indications. Hamostaseologie. 27:55–58. [PubMed] [Google Scholar]
- 26.Ritis, K., M. Doumas, D. Mastellos, A. Micheli, S. Giaglis, P. Magotti, S. Rafail, G. Kartalis, P. Sideras, and J.D. Lambris. 2006. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 177:4794–4802. [DOI] [PubMed] [Google Scholar]
- 27.Schoenmakers, S.H., A.P. Groot, S. Florquin, P.H. Reitsma, and C.A. Spek. 2004. Blood cell-derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol. Dis. 32:325–333. [DOI] [PubMed] [Google Scholar]
- 28.Sevastos, J., S.E. Kennedy, D.R. Davis, M. Sam, P.W. Peake, J.A. Charlesworth, N. Mackman, and J.H. Erlich. 2007. Tissue factor deficiency and PAR-1 deficiency are protective against renal ischaemia reperfusion injury. Blood. 109:577–583. [DOI] [PubMed] [Google Scholar]
- 29.Rumbaut, R.E., J.K. Randhawa, C.W. Smith, and A.R. Burns. 2004. Mouse cremaster venules are predisposed to light/dye-induced thrombosis independent of wall shear rate, CD18, ICAM-1, or P-selectin. Microcirculation. 11:239–247. [DOI] [PubMed] [Google Scholar]
- 30.Rumbaut, R.E., D.W. Slaff, and A.R. Burns. 2005. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 12:259–274. [DOI] [PubMed] [Google Scholar]
- 31.Danese, S., A. Papa, S. Saibeni, A. Repici, A. Malesci, and M. Vecchi. 2007. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am. J. Gastroenterol. 102:174–186. [DOI] [PubMed] [Google Scholar]
- 32.Wan, M.X., Q. Liu, Y. Wang, and H. Thorlacius. 2002. Protective effect of low molecular weight heparin on experimental colitis: role of neutrophil recruitment and TNF-alpha production. Inflamm. Res. 51:182–187. [DOI] [PubMed] [Google Scholar]
- 33.Fries, W., E. Pagiaro, E. Canova, P. Carraro, G. Gasparini, F. Pomerri, A. Martin, C. Carlotto, E. Mazzon, G.C. Sturniolo, and G. Longo. 1998. The effect of heparin on trinitrobenzene sulphonic acid-induced colitis in the rat. Aliment. Pharmacol. Ther. 12:229–236. [DOI] [PubMed] [Google Scholar]
- 34.Vowinkel, T., C. Anthoni, K.C. Wood, K.Y. Stokes, J. Russell, L. Gray, S. Bharwani, N. Senninger, J.S. Alexander, C.F. Krieglstein, et al. 2007. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 132:955–965. [DOI] [PubMed] [Google Scholar]
- 35.Okayasu, I., S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, and R. Nakaya. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98:694–702. [DOI] [PubMed] [Google Scholar]
- 36.Ebaugh, F.G., Jr., and W.L. Beeken. 1959. Quantitative measurement of gastrointestinal blood loss. II. Determination of 24-hour fecal blood loss by a chemical photospectrometric technique. J. Lab. Clin. Med. 53:777–788. [PubMed] [Google Scholar]
- 37.Cooper, H.S., S.N. Murthy, R.S. Shah, and D.J. Sedergran. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69:238–249. [PubMed] [Google Scholar]
- 38.Dieleman, L.A., M.J. Palmen, H. Akol, E. Bloemena, A.S. Pena, S.G. Meuwissen, and E.P. Van Rees. 1998. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper, D., K.D. Chitman, M.C. Williams, and D.N. Granger. 2003. Time-dependent platelet-vessel wall interactions induced by intestinal ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 284:G1027–G1033. [DOI] [PubMed] [Google Scholar]
- 40.Mori, M., J.W. Salter, T. Vowinkel, C.F. Krieglstein, K.Y. Stokes, and D.N. Granger. 2005. Molecular determinants of the prothrombogenic phenotype assumed by inflamed colonic venules. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G920–G926. [DOI] [PubMed] [Google Scholar]
- 41.Pamuk, G.E., O. Vural, B. Turgut, M. Demir, H. Umit, and A. Tezel. 2006. Increased circulating platelet-neutrophil, platelet-monocyte complexes, and platelet activation in patients with ulcerative colitis: a comparative study. Am. J. Hematol. 81:753–759. [DOI] [PubMed] [Google Scholar]
- 42.Stokes, K.Y., E.C. Clanton, K.P. Clements, and D.N. Granger. 2003. Role of interferon-gamma in hypercholesterolemia-induced leukocyte-endothelial cell adhesion. Circulation. 107:2140–2145. [DOI] [PubMed] [Google Scholar]
- 43.Kirchhofer, D., P. Moran, S. Bullens, F. Peale, and S. Bunting. 2005. A monoclonal antibody that inhibits mouse tissue factor function. J. Thromb. Haemost. 3:1098–1099. [DOI] [PubMed] [Google Scholar]