Abstract
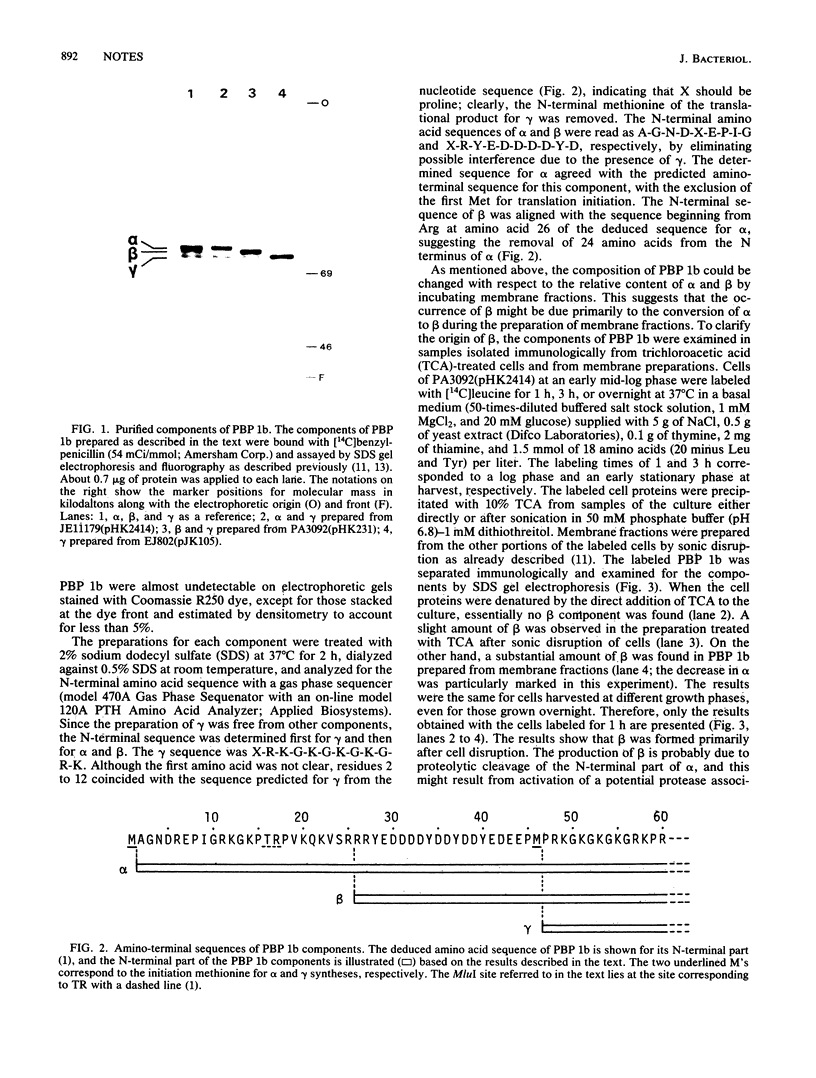
Among components alpha, beta, and gamma of penicillin-binding protein 1b, the alpha and gamma components were confirmed to represent the primary gene products by agreement of their N-terminal amino acid sequences with those predicted from the nucleotide sequence of the ponB (penicillin-binding protein 1b) gene with exclusion of the first methionine in each component. The generation of beta occurred primarily after cell disruption, and the simultaneous loss of alpha suggested the conversion of alpha to beta. The N-terminal amino acid sequence analyzed for beta showed that the conversion was due to the removal of 24 amino acids from the N terminus of alpha.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broome-Smith J. K., Edelman A., Yousif S., Spratt B. G. The nucleotide sequences of the ponA and ponB genes encoding penicillin-binding protein 1A and 1B of Escherichia coli K12. Eur J Biochem. 1985 Mar 1;147(2):437–446. doi: 10.1111/j.1432-1033.1985.tb08768.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E. Topographical distribution of penicillin-binding proteins in the Escherichia coli membrane. J Bacteriol. 1981 Mar;145(3):1293–1298. doi: 10.1128/jb.145.3.1293-1298.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Engström P., Wolf-Watz H., Iino T., Hazelbauer G. L. Cloning of trg, a gene for a sensory transducer in Escherichia coli. J Bacteriol. 1982 Oct;152(1):372–383. doi: 10.1128/jb.152.1.372-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol Gen Genet. 1985;200(2):272–277. doi: 10.1007/BF00425435. [DOI] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Overlapping of the coding regions for alpha and gamma components of penicillin-binding protein 1 b in Escherichia coli. Mol Gen Genet. 1984;196(3):449–457. doi: 10.1007/BF00436192. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Matsuhashi M. Molecular divergence of a major peptidoglycan synthetase with transglycosylase-transpeptidase activities in Escherichia coli --- penicillin-binding protein 1Bs. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1546–1553. doi: 10.1016/0006-291x(82)90964-0. [DOI] [PubMed] [Google Scholar]
- Nicholas R. A., Suzuki H., Hirota Y., Strominger J. L. Purification and sequencing of the active site tryptic peptide from penicillin-binding protein 1b of Escherichia coli. Biochemistry. 1985 Jul 2;24(14):3448–3453. doi: 10.1021/bi00335a009. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Iino T. In vitro synthesis of phase-specific flagellin of Salmonella. J Mol Biol. 1973 Nov 25;81(1):57–70. doi: 10.1016/0022-2836(73)90247-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., van Heijenoort Y., Tamura T., Mizoguchi J., Hirota Y., van Heijenoort J. In vitro peptidoglycan polymerization catalysed by penicillin binding protein 1b of Escherichia coli K-12. FEBS Lett. 1980 Feb 11;110(2):245–249. doi: 10.1016/0014-5793(80)80083-4. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Suzuki H., Nishimura Y., Mizoguchi J., Hirota Y. On the process of cellular division in Escherichia coli: isolation and characterization of penicillin-binding proteins 1a, 1b, and 3. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4499–4503. doi: 10.1073/pnas.77.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif S. Y., Broome-Smith J. K., Spratt B. G. Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985 Oct;131(10):2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]





