Abstract
Immunoglobulin (Ig) class switch recombination (CSR) is initiated by activation-induced cytidine deaminase (AID), which converts cytosines to uracils in switch (S) regions. Subsequent excision of dU by uracil DNA glycosylase (UNG) of the base excision repair (BER) pathway is required to obtain double-strand break (DSB) intermediates for CSR. Since UNG normally initiates faithful repair, it is unclear how the AID-instigated S region lesions are converted into DSBs rather than correctly repaired by BER. Normally, DNA polymerase β (Polβ) would replace the dC deaminated by AID, leading to correct repair of the single-strand break, thereby preventing CSR. We address the question of whether Polβ might be specifically down-regulated during CSR or inhibited from accessing the AID-instigated lesions, or whether the numerous AID-initiated S region lesions might simply overwhelm the BER capacity. We find that nuclear Polβ levels are induced upon activation of splenic B cells to undergo CSR. When Polβ−/− B cells are activated to switch in culture, they switch slightly better to IgG2a, IgG2b, and IgG3 and have more S region DSBs and mutations than wild-type controls. We conclude that Polβ attempts to faithfully repair S region lesions but fails to repair them all.
Ig class switch recombination (CSR) occurs by an intrachromosomal deletional recombination in B cells after activation by antigen in vivo and results in a switch from expression of IgM and IgD to expression of IgG, IgE, or IgA isotypes. CSR allows the generation of antibodies with the same antigen-binding variable region but with various constant regions, thereby enhancing the effectiveness of humoral immune responses. CSR requires the formation of DNA double-strand breaks (DSBs) within the donor μ gene switch (S) region (Sμ) and one of the downstream S regions, and occurs by an end-joining type of recombination (1–3). Mammalian S regions vary substantially in primary sequences but uniformly share the features of being highly repetitive and G-rich on the nontranscribed strand. CSR is a region-specific recombination, as it can occur anywhere within the S region tandem repeats (4).
CSR and somatic hypermutation (SHM) of Ig variable region genes are initiated by activation-induced cytidine deaminase (AID) (5), which converts cytosines in S regions and variable region genes to uracils (6–9). AID expression is induced in mouse splenic B cells activated to switch in culture, as well as in germinal center B cells that undergo CSR and SHM (5, 10, 11). Transcription through a particular S region is needed for CSR to the corresponding isotype, most likely to create a target for AID. The act of transcription creates single-strand DNA, the substrate for AID (8, 9, 12–15). Furthermore, transcription might increase chromatin accessibility by displacing nucleosomes and altering histone modifications (16, 17), and it has been shown that AID associates with RNA polymerase II, perhaps thereby recruiting AID to transcriptionally active loci (18, 19).
The uracil base resulting from AID activity can be removed by the ubiquitously expressed base excision repair (BER) enzyme uracil DNA glycosylase (UNG), leaving an abasic site (6, 20). Uracil excision by UNG is critical for CSR, as UNG deficiency dramatically reduces CSR and the formation of DSBs in S regions (7, 11, 21). These observations indicate that UNG is the predominant, and perhaps only, uracil-excision enzyme involved in CSR and that none of the other enzymes with similar activity provide a significant backup for UNG during CSR (22, 23). In the BER pathway, abasic sites are subsequently recognized by apurinic/apyrimidic endonucleases (APEs), which nick the DNA backbone to create DNA single-strand breaks (SSBs) (24). Recent evidence indicates that APE is important for DSB formation during CSR (unpublished data).
Closely spaced nicks on opposite strands could spontaneously lead to staggered DSBs. In addition, the U:G mismatches could be processed by the mismatch repair (MMR) machinery to create DSBs from distal SSBs on opposite strands (unpublished data) (25). During the canonical BER pathway, the single nucleotide gap generated by the action of UNG and APE is filled in by DNA polymerase β (Polβ) and then the 5′-deoxyribose phosphate (dRP) group remaining after APE activity is excised by the lyase activity of Polβ (20, 26). Subsequently, DNA ligase I or DNA ligase III-XRCC1 are recruited to seal the gap, restoring the original DNA sequence, which, however, would prevent CSR. Correct repair of the AID lesion would also prevent SHM.
Hence, an intriguing question arises as to how the S region nicks are spared from faithful repair so that they can be converted into DSBs to provide the essential intermediates for CSR. One appealing hypothesis is that BER components downstream of UNG and APE might be down-regulated in cells undergoing CSR or specifically prevented from accessing S region lesions. As Polβ is recruited by APE1, the major APE in cells, and the Polβ lyase activity is the rate-limiting step of BER (20, 27), it is possible that the levels of Polβ or its activity might be inhibited during CSR and SHM. Indeed, the recent finding that the amount of Polβ is inversely correlated with the frequency of SHM in subclones of the human BL2 cell line makes this hypothesis even more attractive (28). Alternatively, it is possible that the introduction of numerous S region lesions overwhelms the BER machinery, although BER activity is not inhibited during CSR. To address this issue, we have investigated the potential role that Polβ might have by examining the effect of Polβ deficiency on CSR in splenic B cells induced to undergo CSR in culture. We find that polβ−/− B cells manifest moderately increased CSR to IgG2a, IgG2b, and IgG3, but there was no effect on CSR to IgG1 and IgA, the S regions of which bear the greatest numbers of AID target (AGCT) hotspots. Ligation-mediated PCR (LM-PCR) experiments reveal that Polβ deficiency increases the induction of DSBs at both donor and acceptor S regions. Additionally, recombined Sμ–Sγ3 segments and unrearranged Sμ segments from stimulated polβ−/− splenic B cells show an elevated mutation frequency with a striking bias toward mutation of the A:T bp, compared with WT cells. In light of these observations, we propose that Polβ normally competes with CSR by performing faithful repair of S region lesions, thereby reducing S region DSBs; therefore, Polβ might inhibit CSR when AID-instigated breaks in S regions are limiting.
RESULTS
Expression of Polβ and its localization at S regions in switching B cells
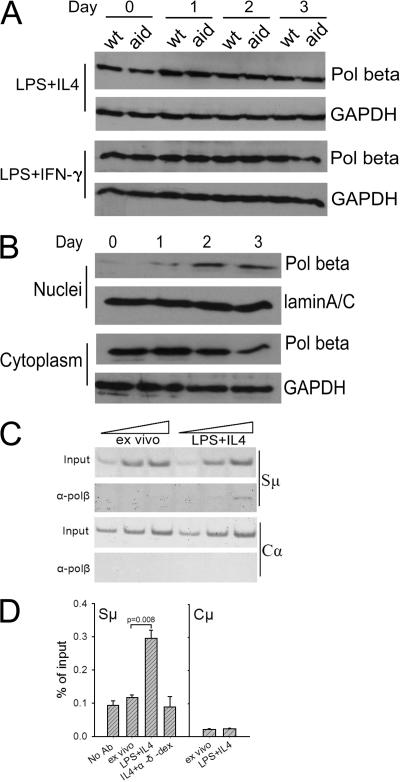
To gain some insight into the potential regulation of Polβ activity in switching B cells, we examined levels of Polβ protein in mouse splenic B cells induced to undergo CSR. B cells from WT and AID-deficient mice were treated with LPS plus IL-4 or with LPS plus IFN-γ for various time periods, and whole-cell lysates were prepared for Western blots. We postulated that if any mechanisms exist in switching B cells to specifically reduce Polβ levels, such mechanisms are likely to be AID dependent. AID expression is greatly induced in splenic B cells 2 d after stimulation to switch (11). Fig. 1 A shows that over the course of 3 d, the levels of Polβ in whole-cell extracts did not change in WT or aid −/− cells. To determine whether Polβ might be excluded from the nuclei of switching B cells, we examined nuclear and cytoplasmic extracts from the cultured B cells. However, we instead observed nuclear accumulation of Polβ in cells undergoing CSR, and cytoplasmic Polβ was coincidently reduced, suggesting that Polβ was redistributed from the cytoplasm to the nucleus in switching B cells (Fig. 1 B). Blots of the WT nuclear and cytoplasmic extracts incubated with antibody to GAPDH and lamin A/C, respectively, demonstrate that the extracts are not cross-contaminated (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070756/DC1). Polβ nuclear translocation was not AID dependent, as Polβ underwent similar translocation in AID-deficient B cells (unpublished data). This translocation might be caused by the requirement for BER to repair the large amount of oxidative DNA damage occurring in rapidly proliferating B cells (29).
Figure 1.
Polβ translocates to the nucleus and binds to the Ig Sμ region in B cells activated to undergo CSR. (A) Western blot of whole-cell extracts from splenic B cells from WT and aid −/− mice cultured for the indicated days in the presence of LPS + IL-4 or LPS + IFN-γ. Day 0 cells are ex vivo cells. Western blots were probed with anti-Polβ and anti-GAPDH antibodies. GAPDH was used as a loading control. (B) Western blot of nuclear and cytoplasmic extracts from WT splenic B cells cultured with LPS + IL-4 for the indicated days. Lamin A/C was used for nuclear loading control. (C) ChIP showing association of Polβ with Sμ, but not with Cα, from splenic B cells from WT mice, ex vivo or cultured for 3 d with LPS + IL-4. Threefold dilutions of the input and the immunoprecipitated DNA amplified by conventional PCR were detected by ethidium bromide. (D) ChIP of extracts from splenic B cells, ex vivo or cultured as indicated. α-δ-dex shows that Polβ associates with Sμ but not with Cμ. The ChIP was assayed by real-time PCR, and the values were calculated as in Materials and methods. The mean of five ChIPs is shown for Sμ, and the mean of triplicates from one ChIP is shown for Cμ. Error bars represent the SEM. The “no antibody” control was not performed for the Cμ ChIP. The significance of the difference between ex vivo cells and LPS + IL-4–treated cells was determined using the paired t test.
We considered the possibility that Polβ might be prevented from accessing S regions in switching B cells. To address this, we used chromatin immunoprecipitation (ChIP) to detect the association of Polβ with the Sμ region. As shown in Fig. 1 C, binding of Polβ to the Sμ region was detected in LPS plus IL-4–stimulated B cells but barely in ex vivo (day 0) B cells. As another control, binding of Polβ to the constant region of IgA heavy chain (Cα) gene, which is not involved in CSR, was not detectable under the same conditions. To obtain quantitative results, ChIP was also analyzed by real-time PCR (Fig. 1 D). Stimulation with LPS plus IL-4 for 3 d resulted in a 2.6-fold enrichment of Polβ association with the Sμ region compared with ex vivo B cells, whereas no significant enrichment was observed upon treatment with IL-4 plus anti-IgD conjugated to dextran (α-δ-dex), a treatment that induces B cell proliferation but not CSR. In fact, the latter treatment resulted in the same amount of association of Polβ with Sμ as the no antibody control. Fig. 1 D also shows that Polβ does not associate with the Cμ gene in either ex vivo or LPS plus IL-4–activated B cells, consistent with previous data showing that AID-dependent DSBs are found in S regions but not in the Cμ gene (11, 30, 31). Collectively, these results clearly indicate that Polβ localizes to nuclei and binds the Sμ region during CSR in cultured B cells.
Polβ-deficient splenic B cells have a moderately increased ability to undergo CSR
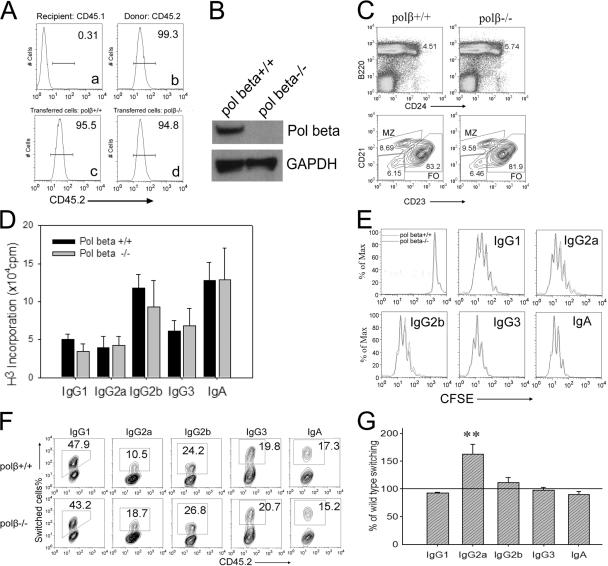
As Polβ-deficient mice die just before birth, to generate mice with polβ−/− B cells, 2 × 106 fetal liver cells (FLCs) from polβ−/− and from polβ+/+ day 18.5 postcoitum fetuses were injected intravenously into lethally irradiated recipient mice, as previously described (32). Because the recipient cells bear CD45.1 and the donor cells bear CD45.2, successful reconstitution could be verified by FACS analysis with antibodies recognizing CD45.1 and CD45.2. Splenic B cells from donor mice, but not from recipient mice, were recognized by anti-CD45.2, validating the feasibility of this approach (Fig. 2 A, a and b). The recipient mice were killed 6 wk after FLC injection; FACS analysis revealed that their splenic B cells were almost exclusively CD45.2+ (95–99%; Fig. 2 A, c and d), indicating successful transfer and reconstitution. Lack of Polβ protein in splenic B cells in recipients that received polβ−/− FLC was confirmed by Western blot analysis (Fig. 2 B). Analysis of splenic B cell subsets showed that the proportion of immature, marginal zone, and follicular B cells was similar between the polβ+/+ and polβ−/− spleens (Fig. 2 C).
Figure 2.
Polβ-deficient B cells show a moderate increase in CSR to IgG2a. (A) Splenic B cells from recipient (a) and donor (b) mice or from recipient mice that had received liver cells of polβ+/+ or polβ−/− fetuses (c and d) were cultured for 3 d and stained with anti-CD45.2 antibody and analyzed by flow cytometry. The horizontal lines represent the fluorescence intensity corresponding to positively staining (i.e., CD45.2+) cells. Fluorescence intensity (FI) values are shown. (B) Western blot of Polβ expression in splenic B cells, cultured with LPS + IL-4, from mice that had received polβ+/+ or polβ−/− FLCs. Whole-cell lysates were prepared from day-3 cultures. (C) Splenic cells from WT and polβ−/− mice were simultaneously stained with anti-B220, anti-CD24, anti-CD21, and anti-CD23 antibodies and analyzed by flow cytometry. (top) The gated cells represent immature cells, and (bottom) an analysis of B220+ cells is shown. FI values are shown. FO, follicular cells; MZ, marginal zone. (D) [H3]thymidine incorportation to measure DNA synthesis in splenic B cells cultured to induce switching to the indicated isotypes at 105 cells/ml for 3 d in a 96-well plate. During the final 4 h, each well was pulsed with 1 μCi [3H]thymidine (2 Ci/mmol; MP Biomedicals). Plates were harvested onto filter mats (LKB Wallac) and read on a 1205 Betaplate (LKB Wallac). Data shown are the mean cpm of quadruplicate wells. (E) CFSE-stained B cells were cultured for 3 d to induce switching to the indicated isotypes and assayed by flow cytometry. The graphs were analyzed by FlowJo software. (F) Representative FACS analysis for surface Ig isotype expression in transferred CD45.2+ cells. WT and polβ−/− splenic B cells were stimulated under optimal conditions, as described in Materials and methods for 4 d, and surface stained with anti-CD45.2 antibody and the antibodies recognizing different Ig isotypes. The stained cells were analyzed by flow cytometry. FI values are shown. (G) Percentage of switching to the indicated isotypes by polβ−/− cells relative to WT cells (set at 100%). The mean of four experiments is shown, and error bars represent the SEM. **, P < 0.01 using the paired t test to determine the significance of the difference between WT and polβ−/− switching for IgG2a.
We examined whether CSR in splenic B cells was affected by Polβ deficiency in an in vitro isotype switching assay. We initially determined whether Polβ deficiency could impair cellular proliferation, as CSR is coupled to cell division (33, 34). Splenic B cells were isolated from reconstituted mice and cultured in the presence of LPS and cytokines to induce CSR to IgG1, IgG2a, IgG2b, IgG3, and IgA, as described in Materials and methods. Surprisingly, polβ−/− B cells proliferated as well as polβ+/+ B cells under all of the conditions tested, as determined by tracking cell division with CFSE (Fig. 2 E) and by [3H]thymidine uptake (Fig. 2 D). Also, DNA content analysis showed that the cell-cycle distribution of polβ−/− cells was similar to WT cells in cultures treated with switch inducers, and no increase in apoptosis was detected (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070756/DC1).
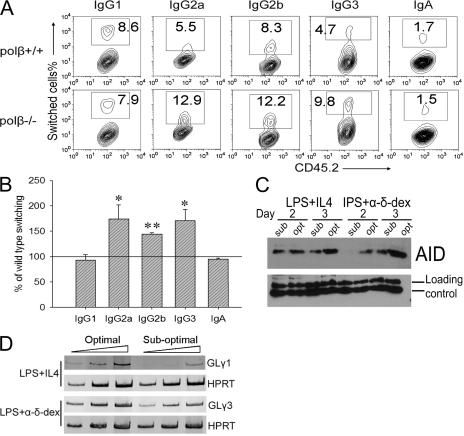
If Polβ repairs SSBs during CSR, its deletion might result in an increase of CSR. We examined CSR to several isotypes and found that switching to IgG2a was increased in the polβ−/− B cells, but no other isotype was significantly affected (Fig. 2, F and G). As isotype specificity is regulated by germline (GL) transcription, we asked if this specific stimulation of IgG2a CSR might be caused by increased levels of GL γ2a transcripts in polβ−/− cells but found they were not increased (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070756/DC1). Because Polβ has no known involvement in other cellular pathways except BER, it is unlikely that Polβ deficiency alters the signal transduction pathway specifically for IgG2a induction. S regions consist of tandem repeats that are unique to each isotype, although all contain numerous targets for AID (e.g., the hotspot motif WRC/GYW, where W = A or T and R = G or A) (14, 35, 36). We considered the possibility that IgG2a CSR might be inhibited by Polβ because of the fact that there are fewer AID hotspot targets in Sγ2a than in other S regions (Table S1), and thus, it was possible that SSBs might be limiting for IgG2a CSR but not for other isotypes. To test this hypothesis, we developed suboptimal conditions for CSR for each isotype, by reducing the concentration of LPS and cytokines in culture (see Materials and methods), and examined CSR under these conditions in polβ−/− cells and WT controls. We reasoned that under suboptimal conditions, DNA breaks might be limiting, and thus, Polβ might inhibit CSR. Under suboptimal conditions, lower levels of AID and GL γ1 and γ3 transcripts were induced in both WT and polβ−/− cells (Fig. 3, C and D). As expected, suboptimal conditions resulted in decreased CSR efficiency, but polβ−/− cells switched relatively better than polβ+/+ cells to IgG2b, IgG3, and IgG2a (Fig. 3, A and B). However, CSR to IgG1 and IgA still did not differ between WT and Polβ-deficient cells. This might be because of the fact that Sγ1 and Sα sequences have more of the hottest of the AID hotspots (AGCT) (37, 38) than any other S region except for Sμ (Table S1).
Figure 3.
Polβ−/− cells switch better to IgG1, IgG2a, and IgG3 than WT B cells under suboptimal conditions. (A) Representative FACS profile to assay Ig isotypes in transferred CD45.2 cells. WT and polβ−/− splenic B cells were induced to switch under suboptimal conditions, as described in Materials and methods, for 4 d. Cells were stained with anti-CD45.2 antibody and antibodies recognizing different Ig isotypes, followed by FACS analysis. FI values are shown. (B) Percentage of switching to the indicated isotypes by polβ−/− cells relative to WT cells (set at 100%) under suboptimal conditions. The mean of three experiments is shown, and error bars represent the SEM. *, P < 0.05; and **, P < 0.01 using the paired t test to determine the significance of the CSR difference between WT and polβ−/− cells. (C) Western blot of AID expression in total cell extracts from WT splenic B cells treated with LPS + IL-4 or LPS + α-δ-dex under optimal and suboptimal conditions for the indicated days. (D) Expression level of γ1 and γ3 GL transcripts in cells stimulated under optimal and suboptimal conditions for 3 d. RT-PCR was performed as described in Materials and methods.
More DSBs in S regions from Polβ-deficient B cells
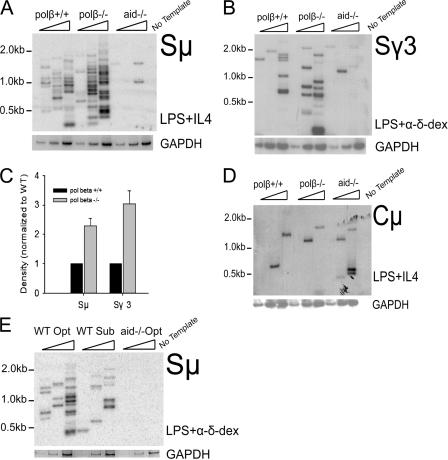
If Polβ possesses the ability to repair SSBs in S regions, as we hypothesize, its deletion should result in the accumulation of SSBs and, consequently, an increased formation of DSBs. We used LM-PCR to detect the DSBs in Sμ and Sγ3 regions from polβ−/− and WT B cells. Splenic B cells were activated to switch for 47–49 h, and genomic DNA was prepared for LM-PCR. In agreement with previous findings (11), abundant DSBs were detected in WT cells at this time point, with very few breaks detectable in identically treated AID-deficient cells (Fig. 4 A). Remarkably, 2.3-fold more Sμ DSBs were detected in polβ−/− than in WT cells (Fig. 4, A and C). A threefold increase in DSBs was observed in the acceptor Sγ3 region in polβ−/− cells (Fig. 4, B and C). To ascertain whether the increased DSBs in S regions of polβ−/− cells are relevant to CSR and are not caused by a nonspecific increase in DSBs, we assayed DSBs at the Cμ region. Very few breaks in the Cμ gene were detected, and no increase was detected in polβ−/− cells (Fig. 4 D). These results clearly demonstrate that Polβ is able to repair DSBs induced in Ig S regions during CSR, as its absence leads to increased S region DSBs. To show that suboptimal conditions indeed reduced the breaks in Sμ, LM-PCR was performed to assay DSBs under these conditions. The results in Fig. 4 E confirmed that fewer breaks were induced in Sμ under suboptimal conditions.
Figure 4.
Increased formation of DSBs at S regions in switching Polβ−/− splenic cells. (A and B) Splenic B cells from WT and polβ−/− mice were induced to switch under the optimal conditions indicated. At 47–49 h, viable cells were isolated, DNA was purified, and LM-PCR was performed to identify blunt DSBs. LM-PCR products were blotted and hybridized with the indicated probes. PCR amplification of the GAPDH gene is shown below the blots as an internal control for template input (threefold dilutions of 1,630 cell equivalents). (C) Quantification of LM-PCR results by densitometry of autoradiographic films, setting WT = 1 (n = 6 for Sμ and 3 for Sγ3); for both regions, the differences between WT and polβ−/− cells are statistically significant (P < 0.01). The densities of bands from all three lanes for each mouse were measured (GeneTools; Syngene). Error bars represent the SEM. (D) Cμ LM-PCR products from WT and polβ−/− B cells detected with the Cμ probe. These blots are from the same experiment shown in A. (E) LM-PCR assay shows that fewer DSBs are induced in WT splenic B cells incubated for 2 d under suboptimal rather than under optimal conditions.
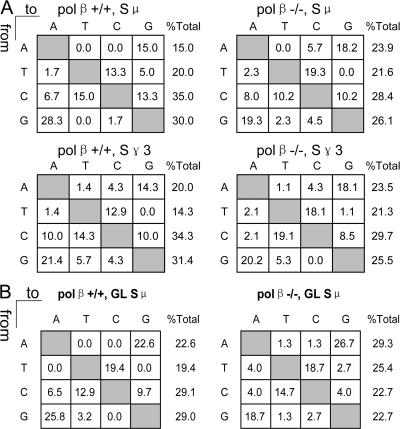
It was previously shown that AID-dependent DSBs in S regions occur preferentially at the G:C bp in WRC/GYW AID hotspots (11). We asked if Polβ deficiency caused deviation of breakpoints from those hotspots. The products of LM-PCR were cloned and sequenced to locate the nucleotides at which the DSBs occurred (Table I). In the absence of Polβ, nearly all the DSBs are located at the G:C bp, as in WT cells, consistent with their introduction by the AID–UNG–APE pathway. The fraction of DSBs at the G:C bp was even greater than in WT cells, but the difference was not significant. Although this pathway produces SSBs, resulting in staggered DSBs, these and previous data suggest that the breaks we detect by LM-PCR have been converted to blunt DSBs in vivo by fill-in DNA synthesis or by excision (11, 25).
Table I.
Blunt DSBs in Sμa from pol β−/− and WT littermate splenic B cells occur preferentially at the G:C bp and at WRC/GYW hotspots
| nt at DSB | polβ+/+ | polβ−/− | Sequenceb |
|---|---|---|---|
| A:T | 20%c | 6.5% | 43.3% |
| C:G | 80% | 93.5% | 56.7% |
| Hotspots | 50% | 41.9% | 23.2% |
| Totald | 30 breaks | 31 breaks | 2,000 nt's |
Sites of DSBs determined with both the 5′ Sμ and 3′ Sμ primers are combined.
Frequency at which the nt or motif occurs in the Sμ sequence analyzed.
Percentage of DSBs located at the indicated bp or at the underlined C or G in the WRC/GYW motif.
Total number of DSBs analyzed.
Polβ deficiency results in an increased mutation frequency and alters the mutation spectrum in S regions
To further assess the function of Polβ during Ig switch, we analyzed mutations in recombined Sμ–Sγ3 junctions from Polβ-deficient B cells and compared them with the WT controls. Splenic B cells were activated with LPS plus α-δ-dex to induce CSR to IgG3, and cells were harvested for genomic DNA preparation 4 d later. Recombined Sμ–Sγ3 DNA junctions were amplified and cloned for sequencing. The overall frequency of mutations in the recombined Sμ segments from polβ+/+ mice was 29.6 × 10−4, comparable to previous observations (11). However, the mutation frequency in the same segment of polβ−/− littermate cells was significantly higher (51.2 × 10−4; Table II). Particularly striking is the finding that the increased mutations in polβ−/− mice predominantly occurred at the A:T bp, with no significant increase at G:C bp (Table II). The same tendency is also true for the recombined Sγ3 segment, although the difference is of borderline significance. We also examined the positions of the mutations relative to Sμ–Sγ3 junctions (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070756/DC1). Polβ deficiency increased mutations in the regions proximal to the junction, as well as distal to the junction on the Sμ side, suggesting that the function of Polβ during CSR is not restricted to processing DNA ends.
Table II.
Mutation frequency in recombined Sμ–Sγ3 segments from splenic B cells induced for 4 d with LPS + α-δ-dex
|
|
polβ+/+
|
polβ−/−
|
|
|---|---|---|---|
| Mutation frequency ×10−4 (number) | p-valuea | ||
| Sμ | |||
| Total mutations | 29.6 (60) | 51.2 (88) | 0.001 |
| Mutations at A:T | 10.4 (21) | 23.3 (40) | 0.003 |
| Mutations at G:C | 19.2 (39) | 27.9 (48) | 0.085 |
| Mutations at AID hotspotb | 15.8 (32) | 18.6 (32) | 0.533 |
| nt's analyzed | 20,274 | 17,202 | |
| Sγ3 | |||
| Total mutations | 19.8 (70) | 25.1 (94) | 0.138 |
| Mutations at A:T | 6.8 (24) | 11.2 (42) | 0.05 |
| Mutations at G:C | 13 (46) | 13.9 (52) | 0.762 |
| Mutations at AID hotspotb | 8.8 (31) | 8.3 (31) | 0.899 |
| nt's analyzed | 35,364 | 37,437 | |
Significance of difference between WT and polβ−/− mutations (Fisher's exact test).
Mutations within the underlined C or G in the WRC/GYW hotspot motifs.
Mutations observed in the segment 5′ to unrearranged (GL) Sμ segments have been taken to reflect AID targeting to S regions (39–41). If Polβ participates in repairing SSBs introduced by the AID–UNG–APE pathway, one would predict that Polβ deficiency will result in increased mutations in the GL Sμ segment. We examined mutations in the GL 5′ Sμ segments from activated polβ−/− B cells compared with WT B cells. Similar to our results with recombined Sμ–Sγ3 junctions, Polβ deficiency resulted in increased mutations, especially at the A:T bp (Table III). The mutation spectra for recombined Sμ–Sγ3 and GL 5′ Sμ segments are shown in Fig. 5. There are no remarkable differences in the ratios of transitions/transversions at either the A:T or G:C bp between WT and polβ−/− sequences, suggesting that the absence of Polβ does not change the specific translesion polymerases involved in repairing S regions. Together with the LM-PCR results, these data unambiguously indicate that Polβ is able to repair AID-initiated SSBs in S regions during CSR.
Table III.
Mutation frequency in GL 5′ Sμ segments from splenic B cells induced for 4 d with LPS + α-δ-dex
|
|
polβ+/+
|
polβ−/−
|
|
|---|---|---|---|
| Mutation frequency ×10−4(number) | p-valuea | ||
| Total mutations | 16 (31) | 33.1 (75) | <0.001 |
| Mutations at A:T | 6.7 (13) | 18.1 (41) | 0.001 |
| Mutations at G:C | 9.3 (18) | 15 (34) | 0.126 |
| Mutations at AID hotspotb | 4.1 (8) | 9.7 (22) | 0.042 |
| nt's analyzed | 19,388 | 22,634 | |
Significance of difference between WT and polβ−/− mutations (Fisher's exact test).
Mutations within the underlined C or G in the WRC/GYW hotspot motifs.
Figure 5.
The mutation spectra of S regions do not differ between WT and polβ−/− B cells. (A) Mutation spectra in recombined Sμ–Sγ3 segments. (B) Mutation spectra in 5′ GL Sμ cells. The numbers shown are the percentages of total mutations. The numbers of mutations analyzed are as shown in Tables II and III.
We examined the effect of Polβ deficiency on the use of microhomologies and the presence of insertions at Sμ–Sγ3 junctions (Table S2, available at http://www.jem.org/cgi/content/full/jem.20070756/DC1). Although the overall distribution of microhomologies and the average microhomology did not notably differ from that in WT junctions, there was a tendency toward a reduction in microhomology lengths, and there were considerably decreased insertions at the Sμ–Sγ3 junctions. The greater frequency of DSBs in the absence of Polβ might result in shorter single-stranded tails at the DSBs, which might in turn result in decreased junctional microhomologies and insertions.
DISCUSSION
Polβ is able to repair AID-initiated lesions in S regions during CSR
Uracil in DNA is a common form of DNA damage, and efficient pathways in cells are dedicated to its repair. Normally, uracil in DNA is excised by UNG or by single-strand selective monofunctional uracil glycosylase (SMUG1), and less frequently by other uracil DNA glycosylases, initiating high-fidelity repair by the BER pathway (20). A multi-protein complex that can perform BER and contains UNG2 (the nuclear form of UNG), APE1, Polβ, replicative DNA polymerases δ and ɛ, XRCC1, and DNA ligase I has been isolated from both proliferating and growth-arrested HeLa cells, as well as from human peripheral blood lymphocytes (42, 43). Furthermore, physical interactions among BER enzymes have been shown to increase repair efficiency (44–46). These findings suggest that BER will proceed to completion once it is initiated by UNG. As UNG has been firmly established to be required for CSR (7, 23, 47), an interesting question arises as to whether the AID-instigated lesions in S regions of B cells might be specifically prevented from being correctly repaired. The results presented in this paper do not support this hypothesis. We find that Polβ expression is induced in nuclei and is associated with the Ig Sμ region in mouse splenic B cells induced to switch in culture, and that Polβ deficiency results in increased mutations and DSBs in S regions. Our data clearly indicate that, at least at the level of Polβ, the BER pathway remains competent to repair AID-induced SSBs in B cells induced to switch in culture. It is possible, however, that B cells in vivo might show different results, as immunohistochemical data suggest that Polβ is down-regulated in human tonsil germinal centers (28). Furthermore, Polβ deficiency did not appear to have a clear effect on SHM of mouse V genes during a response to 4-hydroxy-3-nitrophenyl acetyl-chicken gamma globulin, although there was a reduction of the most highly selected amino acid change resulting from SHM of the Vh gene (32).
Increased mutations in S regions of Polβ−/− mice
Incubation of the BER complexes from HeLa cells with an inhibitory antibody to Polβ decreases their ability to perform short-patch (single-nucleotide) repair in vitro (mediated by Polβ) and increases long-patch repair mediated by replicative polymerases (43). As replicative polymerases are high-fidelity polymerases, this substitution would not increase mutation frequency. However, we found that the overall frequency of mutations in the absence of Polβ increased by 1.7–2.1-fold. The mutations increase primarily at the A:T bp but also slightly at the G:C bp (Tables II and III). These data are consistent with the possibility that there is an increase in the activity of error-prone translesion polymerases at Sμ in the absence of Polβ, and that the polymerases do not simply add a single nucleotide as Polβ usually does but instead perform long-patch displacement synthesis that continues beyond the original excised nucleotide.
Polη is the predominant translesion polymerase that mutates the A:T bp in Ig S regions and is recruited by Msh2-Msh6 (37, 48–53). Polη can also mutate V genes and Sμ in the absence of Msh2, although the mutation frequency is much reduced, and it is hypothesized that Polη can also be recruited by UNG (53). We hypothesize that Polη is substituting for Polβ, initiating DNA synthesis from the SSBs created by APE activity. Furthermore, because Polη does not have the dRP lyase activity that Polβ has, Polη cannot perform small-patch repair; i.e., it cannot simply insert one nucleotide, because the dRP group would need to be excised to complete the repair. Therefore, Polη would most likely perform displacement synthesis, explaining the increased mutations at the A:T bp (54). We hypothesize that a translesion polymerase substitutes for Polβ rather than a high-fidelity polymerase because of the presence of numerous abasic sites in the S regions, which will arrest DNA synthesis by high-fidelity polymerases.
Although we hypothesize that Polη is substituting for Polβ in the polβ−/− cells, it is possible that Polλ might also be substituting. Like Polβ, Polλ is a member of the X family of DNA polymerases, the only other member known to have dRP lyase activity (55). Like Polβ, Polλ protects mouse embryonic fibroblast (MEF) cells against oxidative DNA damage (56). Furthermore, Polλ is able to substitute for Polβ in the repair of uracils in extracts prepared from MEFs, as well as in assays where BER is reconstituted with purified proteins (55, 57). However, Polλ coimmunoprecipitates with SMUG1 from MEFs and functions after SMUG1 (56), whereas SMUG1 does not normally function during CSR (23, 58). Furthermore, neither SMUG1 nor Polλ are found in the large BER complex isolated from HeLa cells described earlier in this section (42, 43). Although Polλ is an error-prone polymerase, it predominately generates single-nucleotide insertion and deletion mutations (59), and we did not observe an increase of such mutations in the S regions of polβ−/− mice (unpublished data). Collectively, it is unlikely that Polλ substitutes for Polβ to repair S region lesions.
The mild CSR phenotype of Polβ-deficient mice suggests that SSBs in S regions are not limiting
The DSBs in both Sμ and Sγ3 regions were increased 2.3–3-fold in polβ−/− cells relative to WT cells. However, CSR was not increased as much. When we stimulated B cells to switch under suboptimal conditions, switching to IgG2a, IgG2b, and IgG3 were increased by 1.4–1.8-fold in the absence of Polβ, whereas under optimal induction conditions only CSR to IgG2a was consistently higher (1.6-fold) in Polβ-deficient B cells. We suggest that Polβ only inhibits CSR when SSBs in S regions are limiting. The S regions of the two isotypes that are not affected by Polβ deficiency, IgG1 and IgA, have more AID hotspots than any of the other acceptor S regions (Table S1). Sγ1 has the longest section of tandem repeats, ∼9 kb (60), and Zarrin et al. (61) have provided evidence that CSR efficiency is proportional to S region length. Sα is ∼4 kb in length (62) but is highly homologous to Sμ (63) and has the highest density of AID target hotspots of all acceptor S regions, especially of the AGCT motif (Table S1), which is the hottest hotspot (37, 38). In addition, in MMR-deficient B cells, switching to IgG2a is the most reduced (fivefold), and IgG1 and IgA switching are the least reduced of all isotypes (64). MMR has been shown to be important for converting SSBs to DSBs and is especially important for switching when there are very few AID hotspot targets (unpublished data) (25, 65). These results are consistent with our hypothesis that DNA breaks in Sγ2a are limiting and that Polβ deficiency increases switching to isotypes in which S region breaks are limiting.
Polβ possesses two enzymatic activities: polymerase and lyase activities. The polymerase function of Polβ can be substituted by other cellular polymerases, whereas the lyase function is essential for cell survival from methyl methanesulphonate treatment (66). It is therefore expected that in polβ−/− cells, DNA breaks in S regions should bear the dRP moiety before their ligation. The implication would be that Polβ deficiency increases breaks to potentiate CSR, yet because of remaining dRP moieties, this deficiency might impair ligation efficiency during end joining. Although the dRP moiety can be removed during long-patch repair by the flap-endonuclease Fen1 (55, 67, 68), the dual activities of Polβ complicate the interpretation of the CSR phenotype of polβ−/− mice. It is likely that the LM-PCR assay is not impaired by the presence of dRP groups, as they are extremely labile in vitro (69).
Conclusions
If B cells were to down-regulate BER during CSR, this could be dangerous, given the great amount of reactive oxygen species produced during B cell activation and proliferation. Therefore, it instead seems plausible that a mechanism is adopted that endows S regions with such numerous AID targets that the ability of BER to repair them is overwhelmed, rather than abrogating overall BER ability and thus jeopardizing the integrity of the B cell genome. In fact, examination of mutations in ung −/− msh2 −/− mice demonstrated that AID introduces many more lesions into the Sμ region than result in actual mutations, most likely because of their being correctly repaired in WT mice (47). The finding that artificially introduced I-SceI sites in Sμ and Sγ1 regions mediate CSR to IgG1 suggests that only a single DSB in the donor and acceptor S regions is sufficient for CSR (70). Introduction of numerous dU residues might be required to obtain DSBs in the donor and acceptor S regions simultaneously. These considerations and the experimental data in this study suggest that Polβ functions normally during CSR to repair AID-initiated DNA lesions but that the numerous AID lesions overwhelm it, and thus, some breaks remain unrepaired.
MATERIALS AND METHODS
Fetal liver transfers.
C57BL/6 mice heterozygous for the Polβ gene (CD45.2 allotype) were bred to obtain homozygous embryos. 2-mo-old C57BL/6 mice with the CD45.1 allotype (strain B6.SJL-Ptprca Pepcb/BoyJ; The Jackson Laboratory) were used as recipients for fetal liver transfers. For timed pregnancies, the appearance of a vaginal plug after overnight mating was labeled as day 0.5 of gestation. Polβ−/−, polβ+/−, and WT FLCs were isolated from fetuses of the CD45.2 allotype at day 18.5 postcoitum. Fetuses were obtained by intercrossing polβ+/− mice (provided by K. Rajewsky, Harvard Medical School, Boston, MA; and S.H. Wilson, National Institute of Environmental Health Sciences, Research Triangle Park, NC) of the CD45.2 allotype. Mice and fetuses were genotyped by a PCR-based assay. Single-cell suspensions of fetal livers were obtained, and 2 × 106 FLCs in HBSS (Invitrogen) in a volume of 100 μl were injected intravenously into CD45.1 recipient mice, which were lethally irradiated (650 rads) the previous day. Recipients were treated with antibiotics in their drinking water for 4 wk after irradiation. Reconstitution of transferred cells was examined 6 wk after fetal liver transfer by staining peripheral blood with anti-CD45.1 and anti-CD45.2 antibodies, followed by FACS analysis, and was consistently ≥95%. The mice were bred and used according to the guidelines of the University of Massachusetts Animal Care and Use Committee.
B cell isolation and stimulation.
Splenic B cells were isolated by T cell depletion with antibody and complement, as described previously (71), and cultured at 105 cells/ml. For optimal induction conditions, 50 μg/ml LPS (for all isotypes; Sigma-Aldrich) and 800 U/ml IL-4 were used to induce switch recombination to IgG1; 10 U/ml LPS and IFN-γ were used to induce IgG2a switching; 30 μg/ml LPS and dextran sulfate (GE Healthcare) were used to induce IgG2b switching; 0.3 ng/ml LPS and α-δ-dex were used to induce IgG3 switching; and LPS, 2 ng/ml TGF-β, 800 U/ml IL-4, 1.5 ng/ml IL-5 (BD Biosciences), and 0.3 ng/ml α-δ-dex were used to induce IgA switching. 100 ng/ml BAFF/BLyS (Human Genome Sciences) was also included in all cultures. These optimal induction conditions were used for induction of CSR in all experiments, except where suboptimal conditions are indicated; this includes the LM-PCR, mutation studies, and Western blotting experiments. For CFSE labeling, cells were washed in serum-free HBSS and resuspended at 40 × 106 cells/ml. An equal volume of 2.4 μM CFSE was added, and cells were incubated at 37°C for 12 min and washed with medium containing 10% FCS. Cells were diluted and cultured as described. The suboptimal conditions used were as follows: IgG1 (5 μg/ml LPS, 80 U/ml IL-4, and 100 ng/ml BAFF), IgG2a (5 μg/ml LPS, 1 U/ml IFN-γ, and 100 ng/ml BAFF), IgG2b (1 μg/ml LPS and 0.6 μg/ml dextran sulfate), IgG3 (1 μg/ml LPS and 0.012 ng/ml α-δ-dex), and IgA (0.5 μg/ml LPS, 1 ng/ml TGF-β, and 50 ng/ml BAFF). FACS was performed as previously described (11) and analyzed by FlowJo software (TreeStar, Inc.).
B cell extracts and Western blotting.
The whole-cell lysates and nuclear/cytoplasmic fractions were prepared and analyzed as described previously, with some modifications (72). For whole-cell lysates, cells were lysed in solution A (50 mM Tris-HCl [pH 7.8], 420 mM NaCl, 1 mM EDTA, 0.5% nonidet P-40, 0.34 M sucrose, 10% glycerol, 1 mM Na3VO4, 10 mM NaF and β-glycerophosphate, 1 mM PMSF, and protease inhibitor cocktail), followed by a brief sonication. Lysates were cleared by centrifugation, and protein concentration was determined by the Bradford assay (Bio-Rad Laboratories). For nuclear extract preparation, cells were first lysed in buffer B (10 mM Hepes [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 0.1% Triton X-100, protease, and phosphatase inhibitors). Cytoplasmic proteins were separated from nuclei by low-speed centrifugation (1,300 g for 4 min). Isolated nuclei were washed once with solution B and further lysed in solution A, as described. Proteins were separated on SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with TBST buffer containing 5% powdered milk and probed using the following primary antibodies: anti-Polβ (Abcam), GAPDH (Santa Cruz Biotechnology, Inc.), lamin A/C (Cell Signaling), and anti-AID antibody (11). The membranes were incubated with horseradish peroxidase–linked secondary anti–mouse (rabbit) antibodies, and bound antibodies were visualized using enhanced chemiluminescence.
ChIP.
The reagents used for ChIP were purchased from Upstate Biotechnology, and the procedures were previously described (73). In brief, one million cell equivalents were used per immunoprecipitation with 4 μg anti-Polβ (Abcam), and 105 cells were used for the input sample. A mixture of BSA- and salmon sperm DNA–coated protein A/G agarose beads was used for preclearing and for immunoprecipitation. The recovered DNA was either amplified by real-time PCR with a Light Cycler (Roche) and SYBR Green I (Invitrogen) or by conventional PCR and ethidium bromide staining. The PCR primers for 5′ of Sμ amplified the segment corresponding to positions 137,276–137,376 (available from GenBank/EMBL/DDBJ under accession no. AC073553). Primers for Cα are 5′-ATCCCACCATCTACCCACTGA-3′ (forward) and 5′-CGTGCCGGAAGGGAAGTA-3′ (reverse). Primers for Cμ are 5′-GTCAGTCCTTCCCAAATGTCTTCC-3′ (forward) and 5′-CTGGAATGGGCACATGCAGATCTTT-3′ (reverse). The binding of Polβ with DNA was calculated by dividing the signal intensity from densitometry analysis or the relative quantity obtained from real-time PCR by 10 times the value obtained for the input sample.
RT-PCR.
RNA was isolated from cultured splenic B cells using TriReagent (Ambion) and primed with oligo(dT) for reverse-transcription with M-MLV Reverse Transcriptase (Promega). Hypoxanthine phosphoribosyltransferase primers include 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ (forward) and 5′-TACTAGGCAGATGGCCACAGGACTA-3′ (reverse). GL γ1 primers are 5′-CAGCCTGGTGTCAACTAG-3′ (forward) and 5′-CTGTACATATGCAAGGCT-3′ (reverse). GL γ2a primers are 5′-GTCCACCTTGGTGCTGCTT-3′ (forward) and 5′-GCTGATGTACCTACCTGAGAGAG-3′ (reverse). GL γ3 primers are 5′-CAAGTGGATCTGAACACA-3′ (forward) and 5′-GGCTCCATAGTTCCATT-3′ (reverse).
LM-PCR.
Genomic DNA was prepared, and LM-PCR was performed as previously described (11). In brief, after 2 d of culture, viable cells were isolated by flotation on Ficoll/Hypaque gradients and embedded in agarose plugs. The plugs were treated with proteinase K and washed to purify genomic DNA. For ligation, LM-PCR1 (5′-GCGGTGACCCGGGAGATCTGAATTC-3′) and LM-PCR2 (5′-GAATTCAGATC-3′) oligonucleotides were used to make the linker. The following primers were used in conjunction with the linker-primer (LM-PCR1) to amplify DNA breaks: 5′ Sμ, 5′-GCAGAAAATTTAGATAAAATGGATACCTCAGTGG-3′; 3′ Sμ, 5′-GCTCATCCCGAACCATCTCAACCAGG-3′; Sγ3, 5′-AACATTTCCAGGGACCCCGGAGGAG-3′; and Cμ, 5′-CTGCGAGAGCCCCCTGTCTGATAAG-3′. Three-fold dilutions of input DNA were amplified by Hotstar Taq (QIAGEN) using a touchdown PCR program. The following probes were used for Southern blotting: Sμ probe, 5′-AGGGACCCAGGCTAAGAAGGCAAT-3′; Sγ3 probe, 5′-GGACCCCGGAGGAGTTTCCATGATCCTGGG-3′; and Cμ probe, 5′-TGGCCATGGGCTGCCTAGCCCGGGACTTCCTG-3′. PCR products were cloned into the vector pCR4-TOPO (Invitrogen) and sequenced by Macrogen using T3 and T7 primers. Cloned breaks in Sμ were aligned with the GL Sμ sequence from C57BL/6 chromosome 12 (available from GenBank/EMBL/DDBJ under accession no. AC073553), with numbering starting at nt 136,645 (=1) to locate the breakpoints.
PCR amplification of Sμ–Sγ3 junctions and GL Sμ segments.
PCR amplification was performed as previously described (41). In brief, genomic DNA was isolated from purified splenic B cells cultured for 4 d in the presence of LPS plus α-δ-dex. Sμ–Sγ3 junctions were amplified from genomic DNA by PCR using the Expand Long Template Taq and Pfu polymerase mix (Roche) with the primers μ3-H3 (5′-AACAAGCTTGGCTTAACCGAGATGAGCC-3′) and g3-2 (5′-TACCCTGACCCAGGAGCTGCATAAC-3′). The primers used for GL 5′ Sμ amplification were 5μ3 (5′-AATGG- ATACCTCAGTGGTTTTTAATGGTGGGTTTA-3′) and 3μ2 (5′-AGAGGCCTAGATCCTGGCTTCTCAAGTAG-3′). The PCR products were cloned into the pCR4-TOPO vector (Invitrogen) and sequenced by Macrogen. The statistical significance of differences between sequences from WT and Polβ-deficient cells was calculated using Fisher's exact t test.
Online supplemental material.
Table S1 shows the lengths and AGCT densities of different mouse S regions. Table S2 compares the microhomology lengths of Sμ–Sγ3 junctions in Polβ+/+ and Polβ−/− cells. Fig. S1 is a Western blot to show that cytoplasmic and nuclear extracts are not cross-contaminated. Fig. S2 shows an example of a DNA content analysis of Polβ+/+ and Polβ−/− cells during CSR. Fig. S3 presents RT-PCR analyses of GL γ2a and γ1 transcripts in Polβ+/+ and Polβ−/− cells induced to switch to the corresponding isotype. Fig. S4 shows the distribution of mutations across Sμ–Sγ3 junctions in Polβ+/+ and Polβ−/− cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070756/DC1.
Supplemental Material
Acknowledgments
We thank Drs. Klaus Rajewsky and Samuel H. Wilson for providing us with polβ+/− mice for this study, and we thank Drs. Wilson and Rajendra Prasad for useful information about BER. We thank Dr. Robert Woodland for showing us how to do tail innoculations and for advice regarding lymphocyte reconstitution. We thank Drs. Carol Schrader, Jeroen Guikema, and Rachel Gerstein for helpful advice and discussions. We thank Anna Ucher and Erin Linehan for help with cloning and the statistical analysis.
This research was supported by grant RO1 AI 632026 from the National Institutes of Health (to J. Stavnezer).
The authors have no conflicting financial interests.
Abbreviations used: α-δ-dex, anti-IgD conjugated to dextran; AID, activation-induced cytidine deaminase; APE, apurinic/apyrimidic endonuclease; BER, base excision repair; Cα, constant region of IgA heavy chain; ChIP, chromatin immunoprecipitation; CSR, class switch recombination; dRP, 5′-deoxyribose phosphate; DSB, double-strand break; FI, fluorescence intensity; FLC, fetal liver cell; GL, germline; LM-PCR, ligation-mediated PCR; MEF, mouse embryonic fibroblast; MMR, mismatch repair; Polβ, DNA polymerase β; S, switch; SHM, somatic hypermutation; Sμ, μ gene S region; SMUG, single-strand selective monofunctional uracil glycosylase; SSB, single-strand break; UNG, uracil DNA glycosylase.
References
- 1.Honjo, T., K. Kinoshita, and M. Muramatsu. 2002. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 20:165–196. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri, J., and F.W. Alt. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 4:541–552. [DOI] [PubMed] [Google Scholar]
- 3.Li, Z., C.J. Woo, M.D. Iglesias-Ussel, D. Ronai, and M.D. Scharff. 2004. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev. 18:1–11. [DOI] [PubMed] [Google Scholar]
- 4.Dunnick, W., G.Z. Hertz, L. Scappino, and C. Gritzmacher. 1993. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 21:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563. [DOI] [PubMed] [Google Scholar]
- 6.Petersen-Mahrt, S.K., R.S. Harris, and M.S. Neuberger. 2002. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 418:99–104. [DOI] [PubMed] [Google Scholar]
- 7.Rada, C., G.T. Williams, H. Nilsen, D.E. Barnes, T. Lindahl, and M.S. Neuberger. 2002. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 12:1748–1755. [DOI] [PubMed] [Google Scholar]
- 8.Bransteitter, R., P. Pham, M.D. Scharff, and M.F. Goodman. 2003. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl. Acad. Sci. USA. 100:4102–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickerson, S.K., E. Market, E. Besmer, and F.N. Papavasiliou. 2003. AID mediates hypermutation by deaminating single stranded DNA. J. Exp. Med. 197:1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muramatsu, M., V.S. Sankaranand, S. Anant, M. Sugai, K. Kinoshita, N.O. Davidson, and T. Honjo. 1999. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 274:18470–18476. [DOI] [PubMed] [Google Scholar]
- 11.Schrader, C.E., E.K. Linehan, S.N. Mochegova, R.T. Woodland, and J. Stavnezer. 2005. Inducible DNA breaks in Ig S regions are dependent upon AID and UNG. J. Exp. Med. 202:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhuri, J., M. Tian, C. Khuong, K. Chua, E. Pinaud, and F.W. Alt. 2003. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 422:726–730. [DOI] [PubMed] [Google Scholar]
- 13.Yu, K., F. Chedin, C.L. Hsieh, T.E. Wilson, and M.R. Lieber. 2003. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat. Immunol. 4:442–451. [DOI] [PubMed] [Google Scholar]
- 14.Bransteitter, R., P. Pham, P. Calabrese, and M.F. Goodman. 2004. Biochemical analysis of hypermutational targeting by wild type and mutant activation-induced cytidine deaminase. J. Biol. Chem. 279:51612–51621. [DOI] [PubMed] [Google Scholar]
- 15.Ronai, D., M.D. Iglesias-Ussel, M. Fan, Z. Li, A. Martin, and M.D. Scharff. 2007. Detection of chromatin-associated single-stranded DNA in regions targeted for somatic hypermutation. J. Exp. Med. 204:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Workman, J.L. 2006. Nucleosome displacement in transcription. Genes Dev. 20:2009–2017. [DOI] [PubMed] [Google Scholar]
- 17.Woo, C.J., A. Martin, and M.D. Scharff. 2003. Induction of somatic hypermutation is associated with modifications in immunoglobulin variable region chromatin. Immunity. 19:479–489. [DOI] [PubMed] [Google Scholar]
- 18.Nambu, Y., M. Sugai, H. Gonda, C.G. Lee, T. Katakai, Y. Agata, Y. Yokota, and A. Shimizu. 2003. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 302:2137–2140. [DOI] [PubMed] [Google Scholar]
- 19.Wang, L., N. Whang, R. Wuerffel, and A.L. Kenter. 2006. AID-dependent histone acetylation is detected in immunoglobulin S regions. J. Exp. Med. 203:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes, D.E., and T. Lindahl. 2004. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 38:445–476. [DOI] [PubMed] [Google Scholar]
- 21.Imai, K., N. Catalan, A. Plebani, L. Marodi, O. Sanal, S. Kumaki, V. Nagendran, P. Wood, C. Glastre, F. Sarrot-Reynauld, et al. 2003. Hyper-IgM syndrome type 4 with a B lymphocyte-intrinsic selective deficiency in Ig class-switch recombination. J. Clin. Invest. 112:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardwell, P.D., A. Martin, E. Wong, Z. Li, W. Edelmann, and M.D. Scharff. 2003. Cutting edge: the G-U mismatch glycosylase methyl-CpG binding domain 4 is dispensable for somatic hypermutation and class switch recombination. J. Immunol. 170:1620–1624. [DOI] [PubMed] [Google Scholar]
- 23.Di Noia, J.M., C. Rada, and M.S. Neuberger. 2006. SMUG1 is able to excise uracil from immunoglobulin genes: insight into mutation versus repair. EMBO J. 25:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christmann, M., M.T. Tomicic, W.P. Roos, and B. Kaina. 2003. Mechanisms of human DNA repair: an update. Toxicology. 193:3–34. [DOI] [PubMed] [Google Scholar]
- 25.Stavnezer, J., and C.E. Schrader. 2006. Mismatch repair converts AID-instigated nicks to double-strand breaks for antibody class-switch recombination. Trends Genet. 22:23–28. [DOI] [PubMed] [Google Scholar]
- 26.Beard, W.A., and S.H. Wilson. 2006. Structure and mechanism of DNA polymerase Beta. Chem. Rev. 106:361–382. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava, D.K., B.J. Berg, R. Prasad, J.T. Molina, W.A. Beard, A.E. Tomkinson, and S.H. Wilson. 1998. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 273:21203–21209. [DOI] [PubMed] [Google Scholar]
- 28.Poltoratsky, V., R. Prasad, J.K. Horton, and S.H. Wilson. 2007. Down-regulation of DNA polymerase beta accompanies somatic hypermutation in human BL2 cell lines. DNA Repair (Amst.). 6:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, K., K. Takubo, F. Arai, H. Satoh, S. Matsuoka, M. Ohmura, K. Naka, M. Azuma, K. Miyamoto, K. Hosokawa, et al. 2007. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J. Immunol. 178:103–110. [DOI] [PubMed] [Google Scholar]
- 30.Rush, J.S., S.D. Fugmann, and D.G. Schatz. 2004. Staggered AID-dependent DNA double strand breaks are the predominant DNA lesions targeted to S mu in Ig class switch recombination. Int. Immunol. 16:549–557. [DOI] [PubMed] [Google Scholar]
- 31.Catalan, N., F. Selz, K. Imai, P. Revy, A. Fischer, and A. Durandy. 2003. The block in immunoglobulin class switch recombination caused by activation-induced cytidine deaminase deficiency occurs prior to the generation of DNA double strand breaks in switch mu region. J. Immunol. 171:2504–2509. [DOI] [PubMed] [Google Scholar]
- 32.Esposito, G., G. Texido, U.A. Betz, H. Gu, W. Muller, U. Klein, and K. Rajewsky. 2000. Mice reconstituted with DNA polymerase beta-deficient fetal liver cells are able to mount a T cell-dependent immune response and mutate their Ig genes normally. Proc. Natl. Acad. Sci. USA. 97:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rush, J.S., M. Liu, V.H. Odegard, S. Unniraman, and D.G. Schatz. 2005. Expression of activation-induced cytidine deaminase is regulated by cell division, providing a mechanistic basis for division-linked class switch recombination. Proc. Natl. Acad. Sci. USA. 102:13242–13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deenick, E.K., J. Hasbold, and P.D. Hodgkin. 1999. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J. Immunol. 163:4707–4714. [PubMed] [Google Scholar]
- 35.Pham, P., R. Bransteitter, J. Petruska, and M.F. Goodman. 2003. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 424:103–107. [DOI] [PubMed] [Google Scholar]
- 36.Yu, K., F.T. Huang, and M.R. Lieber. 2004. DNA substrate length and surrounding sequence affect the activation-induced deaminase activity at cytidine. J. Biol. Chem. 279:6496–6500. [DOI] [PubMed] [Google Scholar]
- 37.Martomo, S.A., W.W. Yang, and P.J. Gearhart. 2004. A role for Msh6 but not Msh3 in somatic hypermutation and class switch recombination. J. Exp. Med. 200:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohm-Laursen, L., and T. Barington. 2007. Analysis of 6912 unselected somatic hypermutations in human VDJ rearrangements reveals lack of strand specificity and correlation between phase II substitution rates and distance to the nearest 3′ activation-induced cytidine deaminase target. J. Immunol. 178:4322–4334. [DOI] [PubMed] [Google Scholar]
- 39.Petersen, S., R. Casellas, B. Reina-San-Martin, H.T. Chen, M.J. Difilippantonio, P.C. Wilson, L. Hanitsch, A. Celeste, M. Muramatsu, D.R. Pilch, et al. 2001. AID is required to initiate Nbs1/gamma-H2AX focus formation and mutations at sites of class switching. Nature. 414:660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaoka, H., M. Muramatsu, N. Yamamura, K. Kinoshita, and T. Honjo. 2002. Activation-induced deaminase (AID)–directed hypermutation in the immunoglobulin Sμ region: implication of AID involvement in a common step of class switch recombination and somatic hypermutation. J. Exp. Med. 195:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrader, C.E., S.P. Bradley, J. Vardo, S.N. Mochegova, E. Flanagan, and J. Stavnezer. 2003. Mutations occur in the Ig Sμ region but rarely in Sγ regions prior to class switch recombination. EMBO J. 22:5893–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbari, M., M. Otterlei, J. Pena-Diaz, P.A. Aas, B. Kavli, N.B. Liabakk, L. Hagen, K. Imai, A. Durandy, G. Slupphaug, and H.E. Krokan. 2004. Repair of U/G and U/A in DNA by UNG2-associated repair complexes takes place predominantly by short-patch repair both in proliferating and growth-arrested cells. Nucleic Acids Res. 32:5486–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parlanti, E., G. Locatelli, G. Maga, and E. Dogliotti. 2007. Human base excision repair complex is physically associated to DNA replication and cell cycle regulatory proteins. Nucleic Acids Res. 35:1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong, D., and B. Demple. 2004. Modulation of the 5′-deoxyribose- 5-phosphate lyase and DNA synthesis activities of mammalian DNA polymerase beta by apurinic/apyrimidinic endonuclease 1. J. Biol. Chem. 279:25268–25275. [DOI] [PubMed] [Google Scholar]
- 45.Parsons, J.L., I.I. Dianova, S.L. Allinson, and G.L. Dianov. 2005. DNA polymerase beta promotes recruitment of DNA ligase III alpha-XRCC1 to sites of base excision repair. Biochemistry. 44:10613–10619. [DOI] [PubMed] [Google Scholar]
- 46.Liu, Y., R. Prasad, W.A. Beard, P.S. Kedar, E.W. Hou, D.D. Shock, and S.H. Wilson. 2007. Coordination of steps in single-nucleotide base excision repair mediated by apurinic/apyrimidinic endonuclease 1 and DNA polymerase beta. J. Biol. Chem. 282:13532–13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue, K., C. Rada, and M.S. Neuberger. 2006. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. J. Exp. Med. 203:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng, X., D.B. Winter, C. Kasmer, K.H. Kraemer, A.R. Lehmann, and P.J. Gearhart. 2001. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nat. Immunol. 2:537–541. [DOI] [PubMed] [Google Scholar]
- 49.Faili, A., S. Aoufouchi, S. Weller, F. Vuillier, A. Stary, A. Sarasin, C.-A. Reynaud, and J.-C. Weill. 2004. DNA polymerase η is involved in hypermutation occurring during immunoglobulin class switch recombination. J. Exp. Med. 199:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delbos, F., A. De Smet, A. Faili, S. Aoufouchi, J.C. Weill, and C.A. Reynaud. 2005. Contribution of DNA polymerase η to immunoglobulin gene hypermutation in the mouse. J. Exp. Med. 201:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson, T.M., A. Vaisman, S.A. Martomo, P. Sullivan, L. Lan, F. Hanaoka, A. Yasui, R. Woodgate, and P.J. Gearhart. 2005. MSH2–MSH6 stimulates DNA polymerase η, suggesting a role for A:T mutations in antibody genes. J. Exp. Med. 201:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, Z., C. Zhao, M.D. Iglesias-Ussel, Z. Polonskaya, M. Zhuang, G. Yang, Z. Luo, W. Edelmann, and M.D. Scharff. 2006. The mismatch repair protein Msh6 influences the in vivo AID targeting to the Ig locus. Immunity. 24:393–403. [DOI] [PubMed] [Google Scholar]
- 53.Delbos, F., S. Aoufouchi, A. Faili, J.C. Weill, and C.A. Reynaud. 2007. DNA polymerase η is the sole contributor of A/T modifications during immunoglobulin gene hypermutation in the mouse. J. Exp. Med. 204:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald, J.P., E.G. Frank, B.S. Plosky, I.B. Rogozin, C. Masutani, F. Hanaoka, R. Woodgate, and P.J. Gearhart. 2003. 129-derived strains of mice are deficient in DNA polymerase ι and have normal immunoglobulin hypermutation. J. Exp. Med. 198:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Diaz, M., K. Bebenek, T.A. Kunkel, and L. Blanco. 2001. Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase lambda: a possible role in base excision repair. J. Biol. Chem. 276:34659–34663. [DOI] [PubMed] [Google Scholar]
- 56.Braithwaite, E.K., P.S. Kedar, L. Lan, Y.Y. Polosina, K. Asagoshi, V.P. Poltoratsky, J.K. Horton, H. Miller, G.W. Teebor, A. Yasui, and S.H. Wilson. 2005. DNA polymerase lambda protects mouse fibroblasts against oxidative DNA damage and is recruited to sites of DNA damage/repair. J. Biol. Chem. 280:31641–31647. [DOI] [PubMed] [Google Scholar]
- 57.Braithwaite, E.K., R. Prasad, D.D. Shock, E.W. Hou, W.A. Beard, and S.H. Wilson. 2005. DNA polymerase lambda mediates a back-up base excision repair activity in extracts of mouse embryonic fibroblasts. J. Biol. Chem. 280:18469–18475. [DOI] [PubMed] [Google Scholar]
- 58.Kavli, B., S. Andersen, M. Otterlei, N.B. Liabakk, K. Imai, A. Fischer, A. Durandy, H.E. Krokan, and G. Slupphaug. 2005. B cells from hyper-IgM patients carrying UNG mutations lack ability to remove uracil from ssDNA and have elevated genomic uracil. J. Exp. Med. 201:2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bebenek, K., M. Garcia-Diaz, L. Blanco, and T.A. Kunkel. 2003. The frameshift infidelity of human DNA polymerase lambda. Implications for function. J. Biol. Chem. 278:34685–34690. [DOI] [PubMed] [Google Scholar]
- 60.Mowatt, M.R., and W.A. Dunnick. 1986. DNA sequence of the murine γ1 switch segment reveals novel structural features. J. Immunol. 136:2674–2683. [PubMed] [Google Scholar]
- 61.Zarrin, A.A., M. Tian, J. Wang, T. Borjeson, and F.W. Alt. 2005. Influence of switch region length on immunoglobulin class switch recombination. Proc. Natl. Acad. Sci. USA. 102:2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arakawa, H., T. Iwasato, H. Hayashida, A. Shimizu, T. Honjo, and H. Yamagishi. 1993. The complete murine immunoglobulin class switch region of the α heavy chain gene-hierarchic repetitive structure and recombination breakpoints. J. Biol. Chem. 268:4651–4655. [PubMed] [Google Scholar]
- 63.Stanton, L.W., and K.B. Marcu. 1982. Nucleotide sequence and properties of the murine γ3 immunoglobulin heavy chain gene switch region: implications for successive Cγ gene switching. Nucleic Acids Res. 10:5993–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrader, C., J. Vardo, and J. Stavnezer. 2003. Mlh1 can function in antibody class switch recombination independently of Msh2. J. Exp. Med. 197:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Min, I., C. Schrader, J. Vardo, N. D'Avirro, T. Luby, J. Stavnezer, and E. Selsing. 2003. Sμ tandem repeat region is required for isotype switching in the absence of Msh2. Immunity. 19:515–524. [DOI] [PubMed] [Google Scholar]
- 66.Sobol, R.W., R. Prasad, A. Evenski, A. Baker, X.P. Yang, J.K. Horton, and S.H. Wilson. 2000. The lyase activity of the DNA repair protein beta-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 405:807–810. [DOI] [PubMed] [Google Scholar]
- 67.Lees-Miller, S.P., and K. Meek. 2003. Repair of DNA double strand breaks by non-homologous end joining. Biochimie. 85:1161–1173. [DOI] [PubMed] [Google Scholar]
- 68.Lieber, M.R., Y. Ma, U. Pannicke, and K. Schwarz. 2003. Mechanism and regulation of human non-homologous DNA end-joining. Nat. Rev. Mol. Cell Biol. 4:712–720. [DOI] [PubMed] [Google Scholar]
- 69.Prasad, R., W.A. Beard, P.R. Strauss, and S.H. Wilson. 1998. Human DNA polymerase beta deoxyribose phosphate lyase. Substrate specificity and catalytic mechanism. J. Biol. Chem. 273:15263–15270. [DOI] [PubMed] [Google Scholar]
- 70.Zarrin, A.A., C. Del Vecchio, E. Tseng, M. Gleason, P. Zarin, M. Tian, and F.W. Alt. 2007. Antibody class switching mediated by yeast endonuclease-generated DNA breaks. Science. 315:377–381. [DOI] [PubMed] [Google Scholar]
- 71.Schrader, C.E., W. Edelmann, R. Kucherlapati, and J. Stavnezer. 1999. Reduced isotype switching in splenic B cells from mice deficient in mismatch repair enzymes. J. Exp. Med. 190:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu, X., S.M. Shell, and Y. Zou. 2005. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene. 24:4728–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaminski, D.A., and J. Stavnezer. 2007. Stimuli that enhance IgA class switching increase histone 3 acetylation at S alpha, but poorly stimulate sequential switching from IgG2b. Eur. J. Immunol. 37:240–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.