Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the production of autoantibodies. However, the underlying cause of disease appears to relate to defects in T cell tolerance or T cell help to B cells. Transgenic (Tg) mice overexpressing the cytokine B cell–activating factor of the tumor necrosis factor family (BAFF) develop an autoimmune disorder similar to SLE and show impaired B cell tolerance and altered T cell differentiation. We generated BAFF Tg mice that were completely deficient in T cells, and, surprisingly, these mice developed an SLE-like disease indistinguishable from that of BAFF Tg mice. Autoimmunity in BAFF Tg mice did, however, require B cell–intrinsic signals through the Toll-like receptor (TLR)–associated signaling adaptor MyD88, which controlled the production of proinflammatory autoantibody isotypes. TLR7/9 activation strongly up-regulated expression of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), which is a receptor for BAFF involved in B cell responses to T cell–independent antigens. Moreover, BAFF enhanced TLR7/9 expression on B cells and TLR-mediated production of autoantibodies. Therefore, autoimmunity in BAFF Tg mice results from altered B cell tolerance, but requires TLR signaling and is independent of T cell help. It is possible that SLE patients with elevated levels of BAFF show a similar basis for disease.
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disorder that affects various tissues, particularly the skin and kidney (1, 2). Disease pathogenesis is characterized by the production of a range of autoantibodies, in particular antinuclear antibodies (ANA) (1). Treatment of SLE still relies on broad immunosuppressants, such as corticosteroids and hydroxychloroquine sulfates (3, 4). Although these treatments have been improved, they remain inefficacious in some SLE patients, suggesting that alternative or unappreciated immune mechanisms may be operating.
Autoantibody-producing B cells appear central to the pathogenesis of SLE. Many mechanisms have been proposed to explain their appearance, from impaired survival/apoptosis signals preventing negative selection (5), to dysfunctional complement or inhibitory Fc receptors (6, 7), to activation of Toll-like receptors (TLRs) in response to accumulation of apoptotic bodies (8, 9). However, substantial evidence indicates that T cells are important players in SLE (2, 10, 11). Inhibition of T cell activation or T–B cell interaction is an effective way to prevent autoimmunity in several animal models of SLE (12, 13). More recently, mutation of the Roquin gene, which specifically perturbs the function of follicular helper T cells, induces development of SLE in mice (14). In humans, various HLA haplotypes have been associated with susceptibility to SLE (2, 10). T cell signaling in SLE patients can be abnormal, and analysis of anti-DNA autoantibodies has revealed somatic mutations, which are suggestive of T cell–dependent affinity maturation (2, 10). B cell–depleting reagents, such as Rituximab, have shown promising therapeutic efficacy in SLE clinical trials (15). It is still unclear whether this efficacy is caused by reduced autoantibody production or by the depletion of B cells with potent antigen-presenting cell (APC) function for T cells, or both. Despite the obvious pathogenic role of antibodies and B cells, the corruption of T cell tolerance has been considered an important underlying defect in antibody-mediated systemic autoimmune diseases (2, 16).
B cell–activating factor of the TNF family (BAFF; also termed TNFSF13b and BLyS) is a TNF-like cytokine that is essential for the maturation and survival of peripheral B cells (17, 18). BAFF transgenic (Tg) mice develop autoantibodies, leading to nephritis and salivary gland destruction, features that are reminiscent of SLE and Sjögren's syndrome (SS), respectively (19, 20). Elevated BAFF levels are detected in some human autoimmune conditions, including SLE (18, 20). Like most SLE mouse models, disease in BAFF Tg mice correlates with clear abnormalities of the B cell compartent, leading to inappropriate BAFF-induced survival of self-reactive B cells (21). Moreover, we have shown that marginal zone (MZ) and B1 B cells may play tissue-specific roles in the pathogenesis of SLE in BAFF Tg mice (20, 22, 23). We have studied the effect of excess BAFF on B cell tolerance using hen egg lysozyme (HEL) B cell receptor (BCR) knock-in mice, combined with HEL/BAFF Tg mice. In this system, negative selection of self-reactive B cells was mostly normal, with expansion of only a subset of low-affinity, HEL-specific B cells, with many of these being MZ B cells (24). Thus, the relevance of low-affinity, self-reactive B cells in BAFF-mediated autoimmunity is questionable, suggesting an additional pathogenic contribution from T cells and/or from innate immune signals. BAFF costimulates T cells and promotes T cell differentiation to effector cells (25), and increased numbers of effector T cells are present in BAFF Tg mice (26). Therefore, we hypothesized that the BAFF Tg model is similar to many mouse models of SLE, with the likely combined involvement of both B and T cells in the disease process.
To determine the role of T cells in BAFF-driven autoimmune disease, we crossed BAFF Tg mice onto mice lacking both α/β and γ/δ T cells (27) and looked for signs of autoimmunity. Surprisingly, the resulting TΔ-BTg mice were capable of producing proinflammatory autoantibodies and developed autoimmunity with the same severity as BAFF Tg mice. Although IgA autoantibody production was T cell dependent, it was dispensable for disease progression. We found that BAFF promoted TLR7/9 expression in B cells and TLR-induced production of autoantibodies, and that disease in BAFF Tg mice required B cell–intrinsic TLR-associated MyD88 signaling. Therefore, BAFF-induced SLE-like disease in BAFF Tg mice is a T cell–independent process, but requires innate immune signals through MyD88 for B cell activation and production of pathogenic autoantibodies.
RESULTS
Altered T cell constitution in BAFF Tg mice
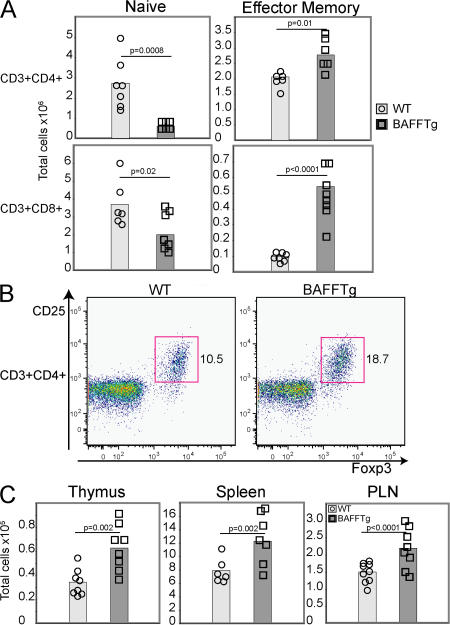
In BAFF Tg mice, total numbers of T cells are not changed (19), although there is an increased proportion of effector T cells (19, 26). The CD8+ T cell compartment is also significantly affected (Fig. 1 A). Effector T cell expansion occurs before disease onset and depends on the presence of B cells (26). Therefore, we hypothesized that in BAFF Tg mice alteration of the B cell compartment leads to a change in the makeup of the T cell compartment and, combined, these changes contribute to the development of high-affinity autoantibodies and, ultimately, autoimmune disease. Changes in T cells that might affect peripheral tolerance include inappropriate activation of autoreactive T cells, or impaired regulatory T cell (T reg cell) function. Unexpectedly, we found that the proportion of T reg cells was significantly elevated in the peripheral LNs (PLNs) of BAFF Tg mice (Fig. 1 B). Absolute numbers of T reg cells were increased in the spleen, PLN, and thymus, which is suggestive of a change in T reg cell production (Fig. 1 C). The consequence of increased numbers of effector and T reg cell numbers in BAFF Tg mice is uncertain, but it does suggest a role of T cells in BAFF-mediated autoimmune disease.
Figure 1.
T cell abnormalities in BAFF Tg mice. T cell subsets were determined by FACS, as described in the Materials and methods. (A) Total splenic T cell numbers of CD4+ (top) and CD8+ (bottom). Naive (left) or effector–memory (right) T cells were assessed from 12-mo-old WT (circle) and BAFF Tg (square) mice. (B) Representative FACS plots of PLN FoxP3+ T reg cells from WT (left) and BAFF Tg (right) mice. The T reg cell population is indicated with a box. (C) Total numbers of T reg cells from WT (circle) and BAFF Tg (square) mice from thymus, spleen, and PLN. In A and C, symbols represent individual mice, and the mean for each group is indicated by a column. Significant P values are shown. n ≥ 7 per group.
Expansion of the B cell compartment in BAFF Tg mice is T cell independent
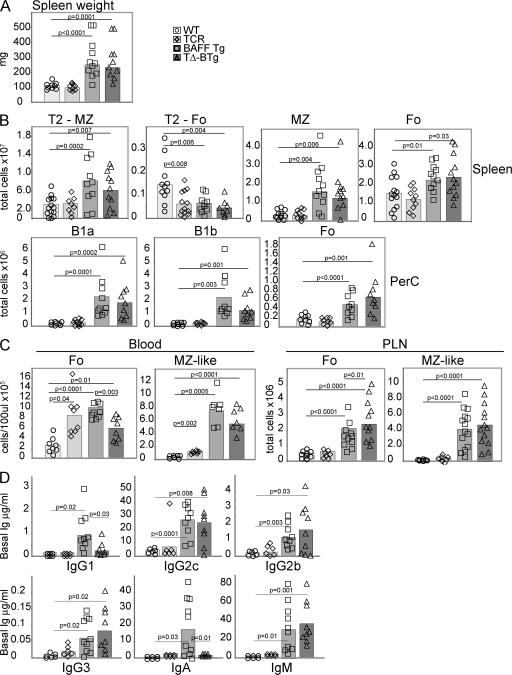
To address the role of T cells in the SLE disease seen in BAFF Tg mice, we crossed BAFF Tg mice onto TCR−/− mice (27) and generated BAFF Tg mice lacking T cells (TΔ-BTg; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1). Splenomegaly, which is a typical feature of BAFF Tg mice, was unchanged in TΔ-BTg mice (Fig. 2 A). Analysis of absolute numbers of transitional type 2 MZ (T2-MZ), T2 follicular (T2-Fo), MZ, and Fo B cells in the spleen, as well as B1a, B1b, and Fo B cells in the peritoneal cavity (PerC), B cell subsets that are particularly expanded in BAFF Tg mice (21), revealed no difference between BAFF Tg and TΔ-BTg mice (Fig. 2 B). In BAFF Tg mice, a high proportion of MZ-like B cells have been detected in the blood and PLNs (21), and we saw a similar expansion of this population in TΔ-BTg mice (Fig. 2 C). Levels of total serum IgG2c, IgG2b, IgG3, and IgM were increased similarly in TΔ-BTg mice, as in BAFF Tg mice (Fig. 2 D). IgG1 levels and, most importantly, IgA levels are elevated in BAFF Tg mice (28), possibly as a result of BAFF-induced isotype switching to IgA (29, 30). Interestingly, the levels of these two isotypes were normal in TΔ-BTg mice (Fig. 2 D), suggesting a role for T cells in addition to that of BAFF in the augmentation of IgA and IgG1 levels seen in BAFF Tg mice. Similarly, fecal IgA levels, which are elevated in BAFF Tg mice (19, 23), were normal in TΔ-BTg mice (unpublished data). As expected, germinal centers were absent in TΔ-BTg mice, which is similar to T cell–deficient control mice (unpublished data). In conclusion, expansion of the B cell compartment in BAFF Tg mice was T cell independent, resulting from a direct survival effect of BAFF on B cells, followed by B cell activation, whereas increased total serum IgG1 and IgA levels in BAFF Tg mice depended on T cell help.
Figure 2.
Similar splenomegaly, B cell hyperplasia, and elevated Ig levels in TΔ-BTg and Baff Tg mice. 12-mo-old WT (circle), TCR−/− (diamond), BAFF Tg (square), and TΔ-BTg (triangle) mice were assessed for spleen size (A) and total numbers of T2-MZ, T2-Fo, MZ, and Fo B cell subsets in spleen and B1a, B1b, and Fo B cells in peritoneal lavage (PerC; B). (C) Fo and MZ B cells in peripheral blood (left) and PLN (right). Subsets were gated by FACS analysis as described in Materials and methods. (D) Basal Ig levels in TΔ-BTg mice and control mice. Symbols represent individual mice, and columns indicate the mean for each group. Significant P values are shown. n ≥ 7 per group.
Elevated IgG2c and IgG2b levels in TΔ-BTg mice in response to T cell–dependent soluble antigen
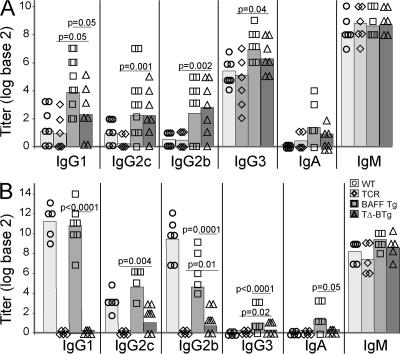
BAFF promotes B cell activation (31) and BAFF Tg mice mount enhanced responses to immunization with both T cell–independent and –dependent antigens (22, 23). BAFF is involved in isotype class switching, although this effect is often weak without additional T cell–dependent signals, such as CD40 ligand (CD40L) or T cell–derived cytokines (29, 30). Using our TΔ-BTg mouse model, we addressed the contribution of T cell help in BAFF-mediated isotype class switching in vivo. As expected, antibody responses to the T cell–independent antigen nitrophenyl (NP)-Ficoll is essentially similar in BAFF Tg and TΔ-BTg mice, and increased compared with WT and TCR−/− mice, respectively (Fig. 3 A). The higher levels of NP-specific IgG1, IgG2c, and IgG2b seen in NP-Ficoll–immunized BAFF Tg and TΔ-BTg mice is intriguing because switching to these isotypes is characteristic of a response to T cell–dependent antigens (32).
Figure 3.
Increased switching to T cell–independent antigens and low-level switching to T cell–dependent antigens in TΔ-BTg mice. Titers of antigen-specific Ig from 12-wk-old WT (circle), TCR−/− (diamond), BAFF Tg (square), and TΔ-BTg (triangle) mice were determined, 5 d after T cell–independent NP-Ficoll immunization (A) and 28 d after initial T cell–dependent immunization NP-OVA (B). Symbols represent individual mice, and the mean for each group is indicated by a column. n ≥ 5 per group. Significant P values are shown between TΔ-BTg v BAFF Tg and TΔ-BTg v TCR−/− groups.
To test whether excess BAFF may, in part, help B cells overcome the need for the T cell–derived signals required for isotype switching, we tested the response of TΔ-BTg mice to immunization with a soluble T cell–dependent antigen. The antibody response to the T cell–dependent soluble antigen NP-OVA given with adjuvant was impaired in TΔ-BTg mice (Fig. 3 B). However, significantly elevated levels of NP-specific IgG2c and IgG2b were detected in TΔ-BTg mice compared with TCR−/− mice (Fig. 3 B), indicating that excess BAFF in TΔ-BTg mice was able to partially support isotype switching to NP-specific IgG2c and IgG2b, a process that is normally dependent on the presence of T cells (32) and totally impaired in TCR−/− mice (Fig. 3 B). However, similar to TCR−/− mice, affinity maturation was impaired in TΔ-BTg mice, as tested by measuring antibody titers in a low-density, hapten-coated BSA ELISA (unpublished data).
BAFF promotes isotype switching to IgA (29, 30), and BAFF Tg mice produce higher levels of IgA in response to both T cell–independent and –dependent antigens (Fig. 3, A and B). TΔ-BTg mice, in contrast, retained an IgA response to a T cell–independent antigen (Fig. 3 A), but not to the T cell–dependent antigen NP-OVA. Thus, in the absence of T cells, excess production of BAFF can allow partial class switching to Ig isotypes that are normally dependent on the presence of T cell help. In addition, BAFF-induced switching to IgA in response to T cell–dependent, but not T cell–independent, antigens relies on T cell help in vivo.
Production of IgA, but not IgG and IgM, autoantibodies is T cell dependent in BAFF Tg mice
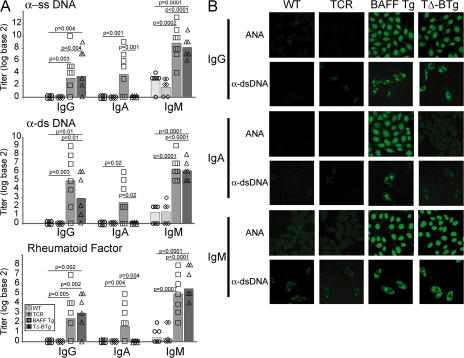
Antibody reactivity with double-stranded (ds) DNA is associated with development of SLE (33, 34). Switching to IgG2a and IgG2b autoantibodies is linked to pathogenicity (35–37), whereas IgM anti-DNA antibodies, per se, are insufficient for disease (38). Studies that have described a pathogenic role for IgG2a in C57BL/6 animal models (35) were most likely measuring IgG2c (an isotype recognized by most commercial anti-IgG2a reagents) because in C57BL/6 mice the IgG2a gene is deleted (39). As T cells are thought necessary for development of the switched anti-DNA antibodies in SLE (10, 34), we investigated whether TΔ-BTg mice developed autoantibodies similar to those seen in BAFF Tg mice. Levels of IgM and IgG anti–single-stranded (ss) DNA, dsDNA, and rheumatoid factor (RF) autoantibodies were comparable in TΔ-BTg and BAFF Tg mice (Fig. 4 A). Within the IgG repertoire, autoantibodies in TΔ-BTg mice were predominantly IgG2c, IgG2b, and IgG3 isotypes (Fig. S2, A and B, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1). IgA autoantibodies were not detected in TΔ-BTg mice (Fig. 4 A). Specificities of ANA and anti-dsDNA antibodies were further characterized using serum samples to stain slides carrying Hep-2 and Crithidia luciliae, respectively (Fig. 4 B). This staining confirmed the absence of IgA autoantibodies in TΔ-BTg mice, in contrast to BAFF Tg mice. The staining pattern of BAFF Tg–derived IgA autoantibodies on Hep-2 slides was granular and cytoplasmic, indicating reactivity to RNA complexes (Fig. 4 B) (40). The staining pattern of all autoantibody isotypes from BAFF Tg mice on C. luciliae slides was characteristic of kinetoplast staining, confirming detection of dsDNA (Fig. 4 B). Although IgA autoantibody production was absent in TΔ-BTg mice, the staining pattern observed with the remaining autoantibody isotypes was unchanged (Fig. 4 B). Collectively, data in Fig. 2 D and Fig. 4 suggest that elevated production of IgA seen in BAFF Tg mice is not solely related to the role of BAFF in IgA class-switching (29, 30), but also requires the presence of T cells. Nevertheless, activation of autoreactive B cells in BAFF Tg mice is a T cell–independent process.
Figure 4.
Production of IgM and IgG, but not IgA, autoantibodies in TΔ-BTg mice. (A) ELISA was used to determine IgG, IgA, and IgM anti-ssDNA (top), anti-dsDNA (middle), and RF (bottom) in serum from 8-mo-old WT (circle), TCR−/− (diamond), BAFF Tg (square), and TΔ-BTg (triangle) mice. Symbols represent individual mice, and the mean for each group is indicated by columns. Significant P values are shown. n ≥ 6 per group. (B) Representative staining of isotype-specific ANA and anti-dsDNA were determined by staining Hep-2 and C. luciliae slides, respectively, with serum from indicated mice.
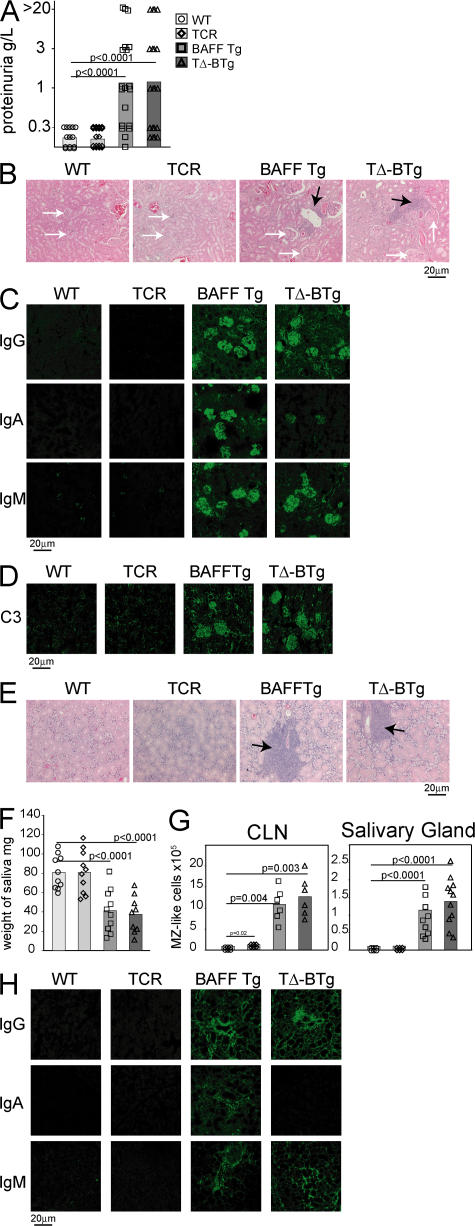
TΔ-BTg mice develop severe autoimmune features indistinguishable from those of BAFF Tg mice
As TΔ-BTg mice are capable of producing autoantibodies of isotypes known to correlate with pathogenicity (Fig. 4) (35, 37), we monitored these mice for features of autoimmunity and tissue destruction, as previously described for BAFF Tg mice (19, 20). At 12 mo of age, similar elevated levels of proteinuria were detected in both BAFF Tg and TΔ-BTg mice (Fig. 5 A), which is indicative of progressing nephritis. Histochemical staining of kidney sections from both BAFF Tg and TΔ-BTg mice revealed nephritis, which is characterized by abnormally enlarged and segmented glomeruli and large infiltrates of mononuclear cells (Fig. 5 B). Examination of Ig deposits in the kidneys of BAFF Tg mice revealed abundant IgM, IgA, and IgG deposits (Fig. 5 C). IgG deposits were predominantly IgG2c, IgG2b, and IgG3 (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1). In contrast, kidneys from TΔ-BTg mice contained IgM, IgG2c, and IgG2b deposits, but no IgG1 or IgA (Fig. 5 C and Fig. S3 A). In addition, both BAFF Tg and TΔ-BTg mice had deposits of C3 complement in glomeruli (Fig. 5 D), which is indicative of an ongoing inflammatory process (41). Therefore, nephritis resulting from excessive BAFF production does not require T cells or deposits of IgA and IgG1 in the kidneys.
Figure 5.
TΔ-BTg and BAFF Tg mice develop indistinguishable kidney and salivary gland pathology. In A, F, and G, the mice are WT (circle), TCR−/− (diamond), BAFF Tg (square), and TΔ-BTg (triangle). Symbols represent individual mice, and the mean for each group is indicated by a column. (A) Proteinuria analysis of 12-mo-old animals. (B) HE staining of kidney tissue sections. BAFF Tg and TΔ-BTg sections show glomeruli separation (white arrows) and mononuclear cell infiltrate (black arrows). (C) IgG, IgA, and IgM (D) C3 deposition in the kidney of 8-mo-old mice. (E) HE staining of salivary gland tissue sections from BAFF Tg and TΔ-BTg sections show salivary gland destruction and lymphocyte infiltrate (black arrows). (F) Saliva flow after pilocarpine injection in 8-mo-old animals. (G) Total numbers of MZ-like B cells detected in CLN and salivary glands. (H) Isotype-specific Ig deposition in salivary gland of 8-mo-old mice. Significant P values are shown.
In BAFF Tg mice, sialadenitis correlates with the aberrant accumulation of MZ-like B cells in the salivary glands (20, 23). TΔ-BTg mice suffer from the same signs of inflammation and tissue destruction (Fig. 5 E), which are characterized by decreased saliva production (Fig. 5 F), as was previously shown in BAFF Tg mice (20). Infiltration of MZ-like B cells into the cervical LNs (CLNs) and salivary gland (20) was identical in both BAFF Tg and TΔ-BTg mice (Fig. 5 G). IgA, IgM, and IgG deposition were also detected in the salivary gland of BAFF Tg mice, but only IgM and IgG deposits were detected in the same tissues from TΔ-BTg mice (Fig. 5 H). IgG deposits were primarily IgG2c, IgG2b, and IgG3, with no IgG1 seen in salivary glands from TΔ-BTg mice (Fig. S3 B). Thus, BAFF Tg mice have severe autoimmune pathology that develops independently of T cells, but concomitantly with production and deposition of autoantibodies in affected tissues.
B cell activation and autoantibody production in BAFF Tg mice requires MyD88-dependent signaling
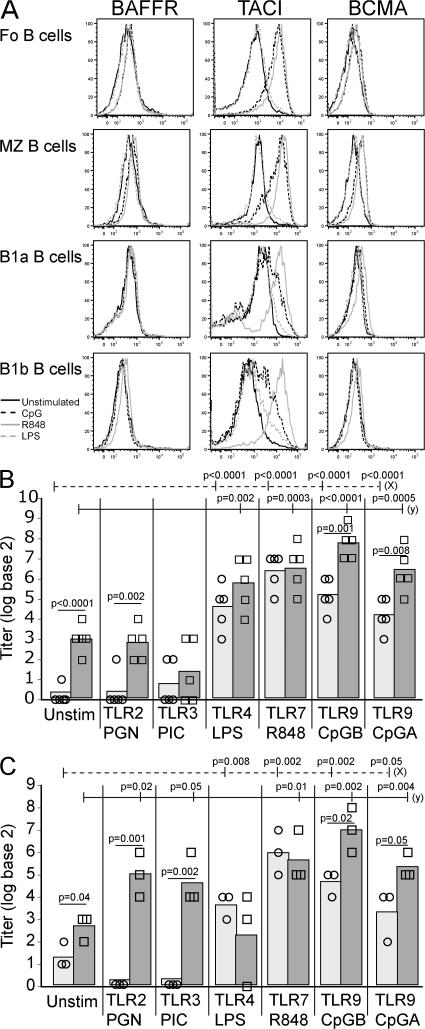
Because a severe autoimmune disease develops in T cell–deficient BAFF Tg mice, alternative pathogenic mechanisms must be responsible for self-reactive B cell activation. We previously showed an expansion of the MZ and B1 B cell compartment in BAFF Tg mice (21, 23), and found the same for TΔ-BTg mice (Fig. 3 B). MZ and B1 B cells are considered innate B cells with self-reactive properties, and they are particularly responsive to activation via TLRs (42–45). In addition, BAFF can enhance TLR-mediated B cell activation and Ig class switching in human B cells (45). Recent studies revealed that TLR7/9 may play a dominant role in SLE, although their role varies depending on the model under investigation (8, 46, 47). Using FACS-sorted, unswitched mouse B cells, we found that BAFF promoted TLR9-induced, but not TLR7-induced, isotype switching from IgM to IgG in vitro (Fig. S4, A and B, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1). This may be a mechanism whereby BAFF specifically exacerbates self-reactive B cell activation via TLR9.
Stimulation of FACS-sorted WT Fo, MZ, B1a, and B1b B cells with TLR ligands showed that both TLR7 (R848) and TLR9 (CpG), but not TLR4 (LPS), activation led to a strong up-regulation of one of the three BAFF receptors, TACI, on Fo and MZ B cells (Fig. 6 A). TLR7/9 stimulation also increased TACI expression on B1 B cells, although TLR7 activation was more potent than that of TLR9 (Fig. 6 A). TLR7/9 activation also led to up-regulation of the most widely expressed BAFF receptor (BAFF-R), although this was weaker and mostly restricted to Fo and MZ B cells (Fig. 6 A). These results confirm a recent independent study (48). Therefore, TLR7/9 activation in B cells strongly up-regulates the expression of TACI, a receptor that is essential for the response of B cells to T cell–independent antigens (49). Moreover, BAFF stimulation of sorted Fo, MZ, and B1 B cells in culture leads to a strong up-regulation of TLR7/9 expression on Fo and MZ, but not B1, B cells (Fig. S4 C).
Figure 6.
TLR7/9, but not TLR7, ligands increase BAFF-R expression, and BAFF enhances TLR-mediated autoantibody secretion. (A) FACS-sorted Fo, MZ, B1a, and B1b B cells were stimulated with the following: unstimulated (solid black), CpG (TLR9; dashed black), R848 (TLR7; solid gray), and LPS (TLR4; dashed gray) for 24 h and analyzed for surface expression of BAFF-Rs by FACS, as indicated. Histograms are representative of three individual sorting and culture experiments. MZ (B) and B1 (C) BAFF Tg B cells were cultured with TLR ligands with (squares) or without (circles) BAFF for 6 d. Total anti-dsDNA antibody levels in culture supernatants were measured by ELISA. Symbols indicate individual cultures. In C and D, significant P values are shown between TLR-stimulated cultures with or without BAFF, between unstimulated and TLR stimulated (dashed line [x]) and between unstimulated with BAFF and TLR-stimulated with BAFF (solid line [y]).
Work by us and others demonstrated a role for MZ B cells in the pathogenesis of autoimmunity in BAFF Tg mice (20, 23, 50). We sorted splenic Fo, MZ, and peritoneal B1 B cells from BAFF Tg mice and cultured these cells with various TLR ligands, which were supplemented or not with exogenous recombinant BAFF, and measured total anti-dsDNA autoantibody levels in the supernatant. BAFF Tg–derived Fo B cells failed to produce anti-dsDNA autoantibodies under any of the conditions tested (unpublished data). Both BAFF Tg-derived MZ and B1 B cell populations contained autoantibody-producing cells in response to TLR stimulation (Fig. 6, B and C). Activation of anti-DNA–producing, BAFF Tg–derived MZ and B1 B cells was increased in vitro in response to TLR4-, TLR9-, and TLR7-specific ligands. The addition of BAFF to these cultures further stimulated TLR9-induced production of anti-dsDNA autoantibodies by both MZ and B1 B cell cultures (Fig. 6, B and C), and TLR2- and TLR3-induced autoantibody production in B1 B cell cultures (Fig. 6 C). Thus, MZ and B1, but not Fo, B cells are the source of anti-dsDNA autoantibodies in BAFF Tg mice, and BAFF-enhanced autoantibody production occurs in response to stimulation through TLR9 in particular. Collectively, these results suggest cooperation between BAFF and TLR9 as the dominant driver of self-reactive MZ B cell activation in BAFF Tg mice. A more complex BAFF–TLR2/3/9 cooperation exists for B1 B cells.
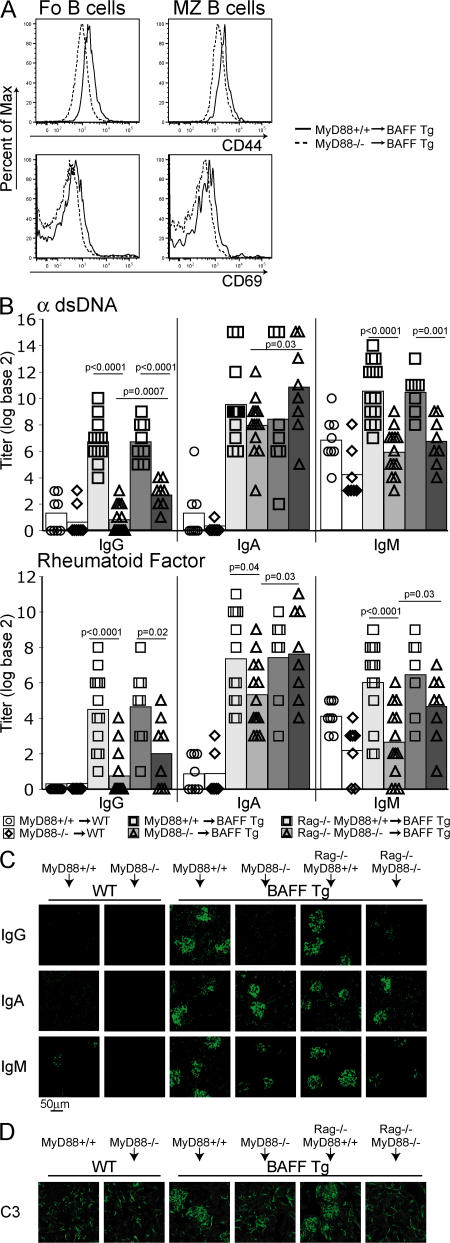
Most TLRs, as well as receptors for IL-1–related cytokines, recruit the signaling adaptor MyD88 upon activation (51–53). The BAFF transgene is liver-specific, allowing us to adoptively transfer BM from WT or MyD88−/− mice into lethally irradiated WT or BAFF Tg mice. 12 wk after reconstitution, a clear reduction in B cell activation was observed in BAFF Tg mice reconstituted with MyD88−/− BM compared with MyD88+/+ BM (Fig. 7 A). We also observed a loss of all autoreactive IgG isotypes (Fig. 7 B) and reduced IgM, but similar IgA, levels (Fig. 7 B), which is consistent with the T cell–dependent nature of autoreactive IgA production seen in TΔ-BTg mice (Fig. 2 D and Fig. 4). Total IgG2c and IgG2b levels in the blood of MyD88−/− BM–reconstituted mice were restored to normal, but IgA levels remained elevated (Fig. S5 B, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1). We examined Ig deposition in the kidneys of these animals and observed pronounced IgA and some IgM deposits, but no IgG, including the pathogenic IgG2c and IgG2b isotypes (Fig. 7 C and Fig. S5 A). Interestingly, lack of IgG deposition in the kidneys correlated with greatly reduced C3 deposition in the glomeruli and suggested an inhibition of complement-activated inflammation in the kidneys (Fig. 7 D). Although IgM alone is not pathogenic (38), the C3-fixing ability of the remaining IgA deposits remains unclear. BAFF Tg mice reconstituted with MyD88−/− BM failed to develop proteinuria (unpublished data). In conclusion, MyD88-mediated signaling was essential for the production of autoantibodies of the IgG2c/b isotype, which were previously described as pathogenic in murine SLE (35, 37).
Figure 7.
MyD88 signaling is essential for B cell activation and IgG autoantibody production in BAFF Tg mice. Lethally irradiated 6-wk-old BAFF Tg mice were reconstituted with MyD88+/+ or MyD88−/− or mixed Rag1−/−MyD88+/+ or Rag1−/−MyD88−/− BM, as indicated. (A) Histogram of B cell activation markers CD44 (top) and CD69 (bottom) of Fo (left) and MZ (right) splenic B cells from MyD88+/+ (solid line) and MyD88−/− (dashed line) BAFF Tg BM chimeras. (B) Anti-dsDNA and RF antibody production in WT mice reconstituted with MyD88+/+ (circles) or MyD88−/− (diamonds); BAFF Tg mice reconstituted with MyD88+/+ (squares; light gray bar); or MyD88−/− (triangles; light gray bar); or BAFF Tg mice reconstituted with Rag1−/−MyD88+/+ (squares; dark gray bar); or Rag1−/−MyD88−/− (triangles; dark gray bar). IgG, IgA, and IgM (C) and C3 (D) deposition in the kidney of BAFF Tg mice reconstituted with MyD88+/+, MyD88−/−, Rag1−/−MyD88+/+, or Rag1−/−MyD88−/− BM, as indicated. Significant P values are shown.
Two TLR-dependent mechanisms have been advanced to explain autoimmunity in SLE (8). The first involves direct activation of TLRs in self-reactive B cells. The second involves activation of TLRs in plasmacytoid DCs (pDCs), which in turn exacerbate autoimmune responses through production of type I IFNs (8, 9). Numbers of splenic myeloid DCs (mDC) and pDCs were increased in BAFF Tg mice compared with control mice (Fig. S5 C). However, these numbers correlated with the extent of splenomegaly (unpublished data). In addition, we saw no augmentation of mDC or pDC activation (expression of MHC class II and CD80) in BAFF Tg mice when compared with WT control cells (Fig. S5 C). The same observation was made for mDCs and pDCs in BAFF Tg mice reconstituted with MyD88+/+ or MyD88−/− BM (Fig. S5 D). We were also unable to detect IFNα in the serum of all of these mice (unpublished data). The effector T cell compartment was not expanded in MyD88−/−-reconstituted BAFF Tg mice (Fig. S5 E), suggesting that this effect was not merely B cell–dependent (26), but also dependent on MyD88 signaling in B cells. BAFF production was not impaired in MyD88−/− mice; in fact, BAFF levels were slightly elevated in the serum of these mice compared with WT animals (Fig. S4 D). This result is consistent with the elevated expression of CD23 seen on MyD88−/− B cells (54), a molecule known to require BAFF for its expression (55).
Lack of MyD88 can affect the function of innate cells in addition to that of B cells. To address this point, we reconstituted irradiated BAFF Tg mice with a 50:50 mix of MyD88−/− and Rag1−/− BM to repopulate the immune system of recipient BAFF Tg mice with MyD88-sufficient innate cells in the presence of MyD88−/− B cells. Macrophages derived from the BM of animals reconstituted with the Rag1−/−/MyD88−/− BM mix, but not those derived from MyD88−/−-reconstituted BAFF Tg mice, produced TNF in response to LPS ex vivo (unpublished data), confirming development of these cells from the Rag1−/− BM source. Animals reconstituted with the Rag1−/−/MyD88−/− BM mix had slightly more IgG autoantibodies in the serum than MyD88−/− BM–reconstituted mice (Fig. 7 B). This suggests that MyD88 expression in innate cells plays some role in autoantibody production. However, similar to MyD88−/− BM-reconstituted mice, animals reconstituted with the Rag1−/−/MyD88−/− BM mix did not show any IgG deposition or C3 fixation in the kidney (Fig. 7, C and D). Thus, MyD88 expression in B cells is likely an important element contributing to the pathogenic role of B cells in BAFF Tg mice.
Our in vitro work implicated a role for TLR9 and BAFF in autoantibody production. To address this point, irradiated BAFF Tg mice were reconstituted with TLR9−/− or control TLR9+/+ BM. However, we observed no protection or differences in autoantibody production (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20062567/DC1), or signs of kidney inflammation (unpublished data), suggesting that disease in BAFF Tg mice does not rely solely on TLR9 signaling. In view of the equally important role of TLR7 in our system (Fig. 6 A), extensive genetic approaches will need to be used to fully dissect TLR signaling in this system.
In conclusion, there was no evidence of mDC or pDC dysregulation in BAFF Tg mice; rather, lack of MyD88-mediated signaling directly affected B cell function, preventing pathogenic autoantibody-mediated kidney disease and effector T cell expansion.
DISCUSSION
Various mechanisms have been suggested as drivers of SLE. These include dysregulation of the innate immune system, overproduction of inflammatory cytokines, and impaired B and T cell tolerance/regulation (2, 4, 56). These mechanisms may be linked. For instance, accumulation of apoptotic cell–derived nucleic acids can lead to TLR-dependent activation of pDCs, production of type I IFNs, which stimulate DC maturation and self-reactive T and B cell activation (8, 9, 57). In addition, the activity of B cells as APCs for T cells may be important in corrupting immune tolerance in SLE (58). Finally, the pathology in SLE appears closely linked to the costimulatory role of self-reactive T cells (10, 14, 16). This model suggests a complex conspiracy between innate immunity, T cells, and B cells in SLE, and that disarming one contributor should influence disease pathogenesis. For instance, the B cell–depleting mAb Rituximab ameliorates disease in many patients with SLE (15) and underscores the importance of B cells, either as initiators or as end-stage effectors, in the pathogenesis of human SLE.
In this study, we showed that overexpression of BAFF in mice led to the development of a T cell–independent form of SLE. This was a surprising result because the initial analysis of BAFF Tg mice was consistent with the accepted idea of T and B cell involvement in SLE, with excessive autoantibody production (19) and an expanded effector T cell compartment (19, 26). These results are supported by other studies that showed that CD40 and germinal center formation were not required for disease in BAFF Tg mice (22, 23, 50).
Although disease in BAFF Tg mice was T cell independent, T cells did augment IgA levels. BAFF can induce switching of B cells to IgA in a T cell–dependent and –independent fashion, particularly in MZ and B1 B cells, which are two expanded B cell subsets in BAFF Tg mice (18, 59). However, this effect is potentiated by the addition of T cell–derived factors, such as CD40L and IL-4 (29, 59). Therefore, it is likely that high IgA levels in BAFF Tg mice are the result of BAFF-induced switching to IgA potentiated by endogenous T cell–derived factors. It is important to note that although serum IgA levels are reduced in TΔ-BTg mice compared with BAFF Tg mice, these levels are normal compared with WT animals, supporting the notion that T cell–dependent, but not T cell–independent, production of IgA is exacerbated by excess BAFF production. Switching to IgG2c and IgG2b in response to T cell–dependent antigens occurs at low levels in TΔ-BTg mice. BAFF combined with IL-21 can stimulate switching of B cells to IgG as efficiently as T cell–dependent signals, such as CD40L or IL-4 (60). Therefore, it is possible that excess BAFF in the presence of other endogenous cytokines, such as IL-21, may provide T cell–like signals, driving low levels of switching to IgG2c and IgG2b in T cell–deficient BAFF Tg mice.
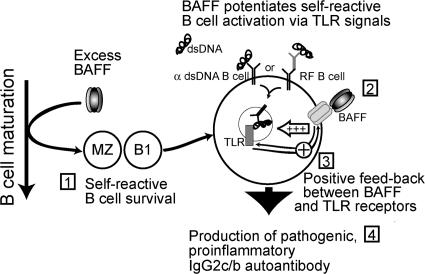
In view of the emerging role of TLR7/9 in SLE (8, 9, 57), TLR signaling was a candidate mechanism for disease progression in BAFF Tg mice. MZ and B1 B cells are often referred to as “innate” B cells and are particularly responsive to TLR activation (42–44). We showed that BAFF stimulation of Fo and MZ, but not B1, B cells strongly up-regulated TLR7/9 expression. In turn, activation of TLR7/9, but not of TLR4, strongly up-regulated TACI expression on B cells. TACI is a receptor for BAFF that is essential for B cell responses to T cell–independent antigens (49). Thus, a tight connection between TACI expression and TLR activation may be a central pathogenic mechanism in BAFF Tg mice (Fig. 8). In fact, TACI may play a role in the production of anti-chromatin autoantibodies (61).
Figure 8.
Model of BAFF-induced, T cell–independent autoimmune disease. (Stage 1) Excess levels of BAFF expand self-reactive MZ and B1 B cells (24). (Stage 2) BAFF signals promote TLR activation after internalization of autoreactive B cell receptors bound to either dsDNA or to immune complexes containing nucleic acids. (Stage 3) Positive feedback of this activation exists, with BAFF increasing TLR7/9 expression and TLR7/9 ligands stimulating BAFF-R expression. (Stage 4) BAFF enhances TLR signals, which lead to the production of IgG2c and IgG2b antibodies. Autoantibodies deposit in the kidney and promote inflammation through complement fixation.
TLR9-mediated promotion of autoantibodies was exacerbated by BAFF in both BAFF Tg–derived MZ and B1 B cells. However, reconstitution of BAFF Tg mice with TLR9−/− BM did not prevent the production of IgG autoantibodies and kidney inflammation. As TLR7 activation has similar effects to TLR9 on TACI expression, it is possible that ablation of TLR9 has been compensated for by TLR7 activation. TLR9 is involved in the pathology of several, but not all, mouse models of SLE (50, 62). For instance, in the MRL-lpr model, TLR9 has a protective role, and deficiency in TLR9 exacerbates SLE (47). This protection may occur, in part, via enhanced IL-10 production from TLR9-activated MZ B cells (44, 63), a population that is also expanded in MRL-lpr mice (64). In MRL-lpr mice lacking BAFF-R signaling, aspects of nephritis were more severe (65). Whether this effect is linked to lack of MZ B cells in these mice is unclear.
In contrast to TLR9, TLR7 deficiency has a protective effect in the MRL-lpr model (46, 47). Yet, there is a major difference between this model and BAFF Tg mice. The MRL-lpr model is T cell dependent (12), whereas SLE in BAFF Tg mice, shown here, is not. In support of this difference, MRL-lpr mice crossed onto BAFF-R mutant mice show impaired B cell development, but develop SLE (65). In the MRL-lpr SLE model, the pathogenic mechanism through TLR7 is linked to its role in T cell activation (47). In BAFF Tg mice, we showed that pDC activation was not altered and that IFNα production was undetectable, leaving pDC involvement as an unlikely mechanism for potentiating the autoimmune process. We did not see an alteration of other immune cells, such as NK cells or granulocytes, in BAFF Tg mice (unpublished data). Interestingly, we have reported that B1 B cells are particularly associated with nephritis in BAFF Tg mice (23), and our results here showed that TLR7 activation, more than TLR9, up-regulated TACI expression on B1 B cells. Therefore, it is possible that TLR7 may play a more dominant pathogenic role in the BAFF Tg model of nephritis, via the promotion of TACI-dependent responses by autoreactive B cells to T cell–independent signals. Generating mice with multiple TLR mutations will be needed to address this point.
We confirmed the importance of TLR-associated MyD88-dependent signaling in driving B cell activation in BAFF Tg mice, using MyD88−/− BM–reconstituted BAFF Tg mice in which B cell activation, secretion of pathogenic autoantibody isotypes, and C3 fixation in the kidneys were all reduced. We also showed that MyD88 expression in B cells is important to drive kidney disease in BAFF Tg mice. The MyD88 adaptor is also recruited by receptors of IL-1, -18, and -33 (52, 53). We have no evidence that expression of these cytokines is abnormal in BAFF Tg mice (unpublished data). The biology of IL-18 and its role in SLE is strongly associated with activation of Th1 T cells (66), and as disease in BAFF Tg mice is clearly T cell independent, IL-18 production is an unlikely link. IL-33 is not a likely candidate either, as it promotes Th2 cytokine release (53), whereas BAFF has been shown to oppose it (26). IL-1 can costimulate B cell activation (67) and plays an important proinflammatory role in SLE (68). Therefore, an IL-1–mediated proinflammatory role in the glomerulonephritis of BAFF Tg mice is not excluded.
An intriguing question is how SLE-like disease is triggered in BAFF Tg mice. Cell death is an ongoing process in healthy individuals as part of cellular turnover, immune tolerance, and regulation, and also occurs as a result of infections. Therefore, cell-derived nuclear autoantigens are continuously present. In BAFF Tg mice, three events lead to autoimmunity: (a) failure of B cell tolerance during splenic maturation and accumulation of self-reactive B cells (24), (b) up-regulation of TLR expression in B cells, and (c) MyD88-dependent B cell activation in the presence of high BAFF levels, promoting production of proinflammatory autoantibodies (Fig. 8). The production of IgG2a/c and IgG2b autoantibody isotypes is linked to kidney disease in our SLE model and others (35, 36), and their production requires MyD88 in BAFF Tg mice.
Understanding the exact role of individual TLRs in mouse SLE is very much dependent on the SLE model under investigation. Of the many known mouse SLE models, the MRL-lpr, the Yaa, and the Roquin models have an important T cell component driving disease (14, 69), and in the first two models, TLR7, which stimulates IFNα production by pDC, exacerbates T/B cell responses (47). In contrast, in FcγRIIB−/− mice or mice expressing the Ly108.1 isoform, and in BAFF Tg mice, disease is linked directly to impaired B cell tolerance (38, 70). Of the B cell–dependent SLE models, BAFF Tg mice are unique because self-reactive B cells are selected into the MZ B cell compartment (21, 24) and, possibly, the B1 B cell pools, both of which are particularly responsive to TLR activation, especially in the presence of BAFF (43, 44, 63).
Our results describe a new mechanism leading to SLE-like disease in mice, and highlight the diversity of separate disorders that can, by themselves, generate the same SLE-like syndrome. It will be a challenge to identify the various forms of SLE in humans, and whether they are T or B cell dominant, to allow new diagnostic approaches that are likely to help stratify SLE patients into groups with predictable responses to different therapies. A logical extension of these findings is to investigate whether human SLE patients with high serum BAFF levels have a similar MyD88-dependent and T cell–independent form of disease.
MATERIALS AND METHODS
Mice.
BAFF Tg mice on a C57BL/6 background have been previously described (19). Mice homozygous for both the TCRβtm/Mom and the TCRδtm/Mom-targeted mutations on a C57BL/6 background were purchased from The Jackson Laboratory (27). BAFF Tg x βδTCR−/− (TΔ-BTg) mice were generated by breeding homozygous BAFF Tg and βδTCR−/− mice and F1 interbreeding. Experimental animals were double-homozygous for the BAFF transgene and the βδTCR−/− mutation. The BAFF transgene was determined using PCR and Southern blot assays on genomic tail snip DNA, as previously described (21). βδTCR−/− status was determined by FACS analysis of peripheral blood to confirm the absence of T cells (Fig. S1). Littermates, WT, βδTCR−/− (TCR−/−), and BAFF Tg were used as controls in all experiments. Rag1−/−, MyD88+/+, and MyD88−/− (52) BM samples were obtained from the Peter MacCallum Cancer Centre (Melbourne, Australia). Irradiation and reconstitution was performed as previously described (24). Mixed BM chimeras received 50% Rag1−/− and 50% MyD88+/+ or MyD88−/− BM using previously detailed protocols (24). Animals were housed under conventional barrier protection and handled in accordance with the Garvan Institute of Medical Research and St. Vincent's Hospital Animal Experimentation and Ethics Committee, which comply with the Australian code of practice for the care and use of animals for scientific purposes. Proteinuria was measured using Multistix 10 SG reagent strips (Bayer). Saliva flow was assessed as previously described (20), with 30 μg i.p. pilocarpine in PBS (Sigma-Aldrich) per 10 g body weight.
Flow cytometry.
Mice were killed by cervical dislocation. Lymphocyte suspensions were obtained from tissues by mechanical disruption. Peripheral blood lymphocytes were isolated by density gradient centrifugation of EDTA-treated blood over Ficoll-Paque PLUS (GE Healthcare). Erythrocytes were removed from spleen samples by osmotic cell lysis solution (8.34 mg/ml ammonium chloride, 0.84 mg/ml sodium bicarbonate, and 1 mM EDTA, pH 8.0). Leukocytes were resuspended in FACS buffer (1% BSA and 0.02% sodium azide in PBS). Fluorescent-labeled anti–mouse antibodies CD1d, CD4, CD5, CD8, CD11c, CD21/CD35, CD25, CD44, CD45R/B220, CD62L, CD69, CD80, MHC class II, IgD, IgM, IgA, IgG subclasses (BD Biosciences) CD3, CD23, CD93 (eBioscience), BAFFR, TACI, BCMA (R&D Systems), and PDCA-1 (Miltenyi Biotec) were used for FACS and/or microscopy analysis. Intranuclear staining for FoxP3 was performed according to the manufacturer's instructions (eBioscience). Data was collected on a LSRII flow cytometer (BD Biosciences), and analyzed using FlowJo software (Tree Star, Inc.). T cell subsets were determined as follows: double-negative thymocytes (CD3−CD4−CD8−); naive (CD44−CD62L+); effector (CD44+CD62L−); T reg cells (CD3+CD4+CD25+FoxP3+). B cell populations were gated as follows: T2-MZ (B220+CD23+CD21hiIgMhiCD93intIgD+); T2-Fo (B220+CD23+CD21dullIgMhiCD93hiIgD+); Fo (B220+CD23+IgM+CD21intIgD+); MZ (B220+CD23−/loCD21hiIgM+CD1dhi); B1a (B220intIgM+CD5+IgDint); B1b (B220intIgM+CD5−IgDint). DC populations were gated as follows: mDCs (CD11chiPDCA01−B220−) and pDCs (CD11cintPDCA-1+B220int).
Immunizations.
For T cell–independent type 2 (TI-2) antigen, mice were immunized i.v. with 30 μg NP-coupled Ficoll (NP59-aminoethyl carboxymethyl-Ficoll; Biosearch Technology). T cell–dependent antibody responses in control littermates and TΔ-BTg mice were examined by i.p. immunization with 5 μg NP-OVA (Biosearch) emulsified in CFA (Sigma-Aldrich), followed by a boost immunization with 5 μg NP-OVA in IFA 21 d later.
ELISA.
ELISA plates were coated with 2 μg/ml of goat anti–mouse Ig (H+L; Jackson Immunoresearch Laboratories), or normal goat Ig (Jackson Immunoresearch Laboratories), or 10 μg/ml methylated BSA (Calbiochem) for total Ig, RF, and anti-DNA levels, respectively. For anti-DNA assays, 2 μg/ml dsDNA or ssDNA prepared from grade I calf thymus (Sigma-Aldrich), as previously described (19), was used to coat ELISA plates. Purified mouse Ig (Southern Biotechnology) was used as standards in total Ig isotype analysis. NP-specific antibodies were determined by ELISA (22). ELISA plates were coated with 2 μg/ml NP30-BSA (Biosearch). Titer (log base 2) is defined as the serum dilution giving an OD reading 4 times higher than background (where 1 = 1/50). ELISAs were detected with anti–mouse isotype-specific alkaline phosphatase–labeled antibodies (Southern Biotechnology), and revealed by P-NP phosphate substrate (Sigma-Aldrich).
ANA and anti-dsDNA immunofluorescence.
Serum obtained by tail bleed from 8-mo-old mice was diluted in PBS 1:50 and used for indirect immunofluorescence on fixed Hep-2 or C. luciliae slides (Antibodies, Inc.) for ANA or anti-dsDNA antibody detection, respectively. FITC anti–mouse Ig (BD Biosciences) was used to detect mouse antibodies. Slides were analyzed by fluorescent microscopy (Leica) at 128× magnification.
Organ pathology.
Kidneys and salivary glands were fixed with 4% paraformaldehyde in PBS and embedded in paraffin. 4 μm paraffin-embedded sections were dewaxed and stained with hematoxylin and eosin (HE). For Ig and complement C3 deposition, tissues were snap frozen embedded in Tissue-Teck OCT compound (Sakura). 5-μm sections were fixed with acetone and blocked with 5 μg/ml purified rat anti–mouse CD16/CD32 (BD Biosciences) and 5 μg/ml polyclonal human IgG (Biogen-Idec) in PBS for 10 min before staining with FITC-conjugated anti–mouse Ig isotypes (BD Biosciences) or FITC-conjugated anti–mouse C3 (Cedarlane Laboratories).
TLR stimulation.
TLR ligands used were as follows: 1 μg/ml peptidoglycan (stimulating TLR2); 1 μg/ml polyinosinic-polycytidylic acid (poly[I:C]; TLR3); 0.25 μg/ml LPS (TLR4); 0.5 μg/ml R848 (TLR7); and the CpG sequences ODN 1826 (0.5 μg/ml) and ODN 2216 (1 μg/ml; CpGB and CpGA, respectively; TLR9; InvivoGen). 1 μg/ml of recombinant murine BAFF (Apotech) was added or not with TLR ligands in B cell cultures. MZ (B220+CD23−CD21hi) B cells and peritoneal B1a (CD5+B220int) and B1b (CD5+B220int) B cells were sorted using a FACSAria (BD Biosciences).
Statistical analysis.
Statistical significance was determined using a Student's t test. Values are shown for data that reached a significance of P > 0.05.
Online supplemental material.
Fig. S1 confirms lack of T cells in the TΔ-BTg mouse line. Fig. S2 shows autoreactive IgG subclasses produced in TΔ-BTg mice. Fig. S3 shows IgG subclass deposition in kidneys and salivary glands of BAFF Tg and TΔ-BTg mice. Fig. S4 shows that BAFF enhanced TLR-mediated IgG switching, increased TLR7/9 mRNA expression in Fo and MZ, but not B1, B cells after BAFF stimulation, and increased levels of BAFF in the serum of MyD88−/− mice. Fig. S5 shows MyD88+/+ and MyD88−/− BAFF Tg BM chimeras and the DC phenotype in BAFF Tg mice. Fig. S6 shows that anti-dsDNA antibody production is similar in BAFF Tg mice reconstituted with TLR9+/+ or TLR9−/− BM. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062567/DC1.
Acknowledgments
We would like to thank Robert Brink and Ian Mackay for critical review of this manuscript. We thank Eric Schmied, Nancy Lui, and the staff at the Biological Testing Facility (Garvan Institute, Sydney, Australia) for assistance with animal care. We also thank Jerome Darakdjian for cell sorting.
F. Mackay is supported by a Wellcome Trust senior research fellowship and a National Health and Medical Research Council (NH & MRC) program grant. J.R. Groom and C.A. Fletcher are supported by NH & MRC PhD scholarships. S.V. Watt and M.J. Smyth are supported by a NH & MRC program grant.
The authors declare having no conflict of interest.
Abbreviations used: ANA, antinuclear antibodies; BAFF, B cell–activating factor of the TNF family; BAFF-R, BAFF receptor; CLN, cervical LN; ds, double-stranded; HE, hematoxylin and eosin; HEL, hen egg lysozyme; mDC, myeloid DC; MZ, marginal zone; NP, nitrophenyl; pDC, plasmacytoid DC; PerC, peritoneal cavity; PLN, peripheral LN; RF, rheumatoid factor; ss, single-stranded; SLE, systemic lupus erythematosus; SS, Sjögren's syndrome; TACI, transmembrane activator and calcium-modulator and cyclophilin ligand (CAML) interactor; Tg, transgenic.
References
- 1.Mills, J.A. 1994. Systemic Lupus Erythematosus. N. Engl. J. Med. 330:1871–1879. [DOI] [PubMed] [Google Scholar]
- 2.Kang, I., and J. Craft. 2006. Systemic lupus erythematosus: immunological features. In The Autoimmune Diseases. Fourth edition. N.R. Rose and I.R. Mackay, editors. Elsevier Academic Press Publication. 357–368 pp
- 3.Davidson, A., and C. Aranow. 2006. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr. Opin. Rheumatol. 18:468–475. [DOI] [PubMed] [Google Scholar]
- 4.Isenberg, D., and A. Rahman. 2005. Systemic lupus erythematosus-2005 annus mirabillis. Nat. Clin. Pract. Rheumatol. 2:145–152. [DOI] [PubMed] [Google Scholar]
- 5.Mackay, F., P. Schneider, P. Rennert, and J.L. Browning. 2003. BAFF and APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21:231–264. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, M.C. 2004. A protective role for innate immunity in systemic lupus erythematosus. Nat. Rev. Immunol. 4:825–831. [DOI] [PubMed] [Google Scholar]
- 7.McGaha, T.L., B. Sorrentino, and J.V. Ravetch. 2005. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 307:590–593. [DOI] [PubMed] [Google Scholar]
- 8.Marshak-Rothstein, A. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6:823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman, A.H., and R.A. Eisenberg. 2006. The role of toll-like receptors in systemic lupus erythematosus. Springer Semin. Immunopathol. 28:131–143. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman, R.W. 2004. T cells in the pathogenesis of systemic lupus erythematosus. Clin. Immunol. 113:4–13. [DOI] [PubMed] [Google Scholar]
- 11.Singh, R.R. 2005. SLE: translating lessons from model systems to human disease. Trends Immunol. 26:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson, A., X. Wang, M. Mihara, M. Ramanujam, W. Huang, L. Schiffer, and J. Sinha. 2003. Co-stimulatory blockade in the treatment of murine systemic lupus erythematosus (SLE). Ann. N. Y. Acad. Sci. 987:188–198. [DOI] [PubMed] [Google Scholar]
- 13.Kalled, S.L., A.H. Cutler, S.K. Datta, and D.W. Thomas. 1998. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J. Immunol. 160:2158–2165. [PubMed] [Google Scholar]
- 14.Vinuesa, C.G., M.C. Cook, C. Angelucci, V. Athanasopoulos, L. Rui, K.M. Hill, D. Yu, H. Domaschenz, B. Whittle, T. Lambe, et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 435:452–458. [DOI] [PubMed] [Google Scholar]
- 15.Thatayatikom, A., and A.J. White. 2006. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun. Rev. 5:18–24. [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa, C.G., S.G. Tangye, B. Moser, and C.R. Mackay. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 5:853–865. [DOI] [PubMed] [Google Scholar]
- 17.Mackay, F., and S.G. Tangye. 2004. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr. Opin. Pharmacol. 4:347–354. [DOI] [PubMed] [Google Scholar]
- 18.Mackay, F., F. Sierro, S.T. Grey, and T.P. Gordon. 2005. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr. Dir. Autoimmun. 8:243–265. [DOI] [PubMed] [Google Scholar]
- 19.Mackay, F., S.A. Woodcock, P. Lawton, C. Ambrose, M. Baetscher, P. Schneider, J. Tschopp, and J.L. Browning. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groom, J., S.L. Kalled, A.H. Cutler, C. Olson, S.A. Woodcock, P. Schneider, J. Tschopp, T.G. Cachero, M. Batten, J. Wheway, et al. 2002. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J. Clin. Invest. 109:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batten, M., J. Groom, T.G. Cachero, F. Qian, P. Schneider, J. Tschopp, J.L. Browning, and F. Mackay. 2000. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 192:1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batten, M., C. Fletcher, L. Ng, J. Groom, J. Wheway, Y. Laâbi, X. Xin, P. Schneider, J. Tschopp, C.R. Mackay, and F. Mackay. 2004. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphomas. J. Immunol. 172:812–822. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher, C.A., A.P. Sutherland, J.R. Groom, M.L. Batten, L.G. Ng, J. Gommerman, and F. Mackay. 2006. Development of nephritis but not sialadenitis in autoimmune-prone BAFF transgenic mice lacking marginal zone B cells. Eur. J. Immunol. 36:2504–2514. [DOI] [PubMed] [Google Scholar]
- 24.Thien, M., T.G. Phan, S. Gardam, M. Amesbury, A. Basten, F. Mackay, and R. Brink. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 20:785–798. [DOI] [PubMed] [Google Scholar]
- 25.Ng, L.G., A. Sutherland, R. Newton, F. Qian, T.G. Cachero, M.L. Scott, J.S. Thompson, J. Wheway, T. Chtanova, J. Groom, et al. 2004. BAFF-R is the principal BAFF receptor facilitating BAFF co-stimulation of B and T cells. J. Immunol. 173:807–817. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland, A.P., L.G. Ng, C.A. Fletcher, B. Shum, R.A. Newton, S.T. Grey, M.S. Rolph, F. Mackay, and C.R. Mackay. 2005. BAFF augments certain Th1-associated inflammatory responses. J. Immunol. 174:5537–5544. [DOI] [PubMed] [Google Scholar]
- 27.Mombaerts, P., E. Mizoguchi, M.J. Grusby, L.H. Glimcher, A.K. Bhan, and S. Tonegawa. 1993. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 75:274–282. [DOI] [PubMed] [Google Scholar]
- 28.Khare, S.D., and H. Hsu. 2001. The role of TALL-1 and APRIL in immune regulation. Trends Immunol. 22:61–63. [DOI] [PubMed] [Google Scholar]
- 29.Litinskiy, M., B. Nardelli, B.M. Hilbert, H. Bing, A. Schaffer, P. Casali, and A. Cerutti. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castigli, E., S.A. Wilson, S. Scott, F. Dedeoglu, S. Xu, K.P. Lam, R.J. Bram, H. Jabara, and R.S. Geha. 2005. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider, P., F. Mackay, V. Steiner, K. Hofmann, J.L. Bodmer, N. Holler, C. Ambrose, P. Lawton, S. Bixler, H. Acha-Orbea, et al. 1999. BAFF, a novel ligand of the tumor necrosis factor (TNF) family, stimulates B-cell growth. J. Exp. Med. 189:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray, D., K. Siepmann, and G. Wohlleben. 1994. CD40 ligation in B cell activation, isotype switching and memory development. Semin. Immunol. 6:303–310. [DOI] [PubMed] [Google Scholar]
- 33.Deshmukh, U.S., H. Bagavant, and S.M. Fu. 2006. Role of anti-DNA antibodies in the pathogenesis of lupus nephritis. Autoimmun. Rev. 5:414–418. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman, I.E., I. Peene, L. Meheus, T.W. Huizinga, L. Cebecauer, D. Isenberg, K. De Bosschere, F. Hulstaert, E.M. Veys, and F. De Keyser. 2004. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann. Rheum. Dis. 63:1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehlers, M., H. Fukuyama, T.L. McGaha, A. Aderem, and J.V. Ravetch. 2006. TLR9/MyD88 signalling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J. Exp. Med. 203:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng, S.L., S.J. Szabo, and L.H. Glimcher. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 99:5545–5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob, C.O., L. Pricop, C. Putterman, M.N. Koss, Y. Liu, M. Kollaros, S.A. Bixler, C.M. Ambrose, M.L. Scott, and W. Stohl. 2006. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J. Immunol. 177:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuyama, H., F. Nimmerjahn, and J.V. Ravetch. 2005. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 6:99–106. [DOI] [PubMed] [Google Scholar]
- 39.Martin, R.M., and A.M. Lew. 1998. Is IgG2a a good Th1 marker in mice? Immunol. Today. 19:49. [DOI] [PubMed] [Google Scholar]
- 40.Muro, Y. 2005. Antinuclear antibodies. Autoimmunity. 38:3–9. [DOI] [PubMed] [Google Scholar]
- 41.Bao, L., I. Osawe, M. Haas, and R.J. Quigg. 2005. Signalling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J. Immunol. 175:1947–1955. [DOI] [PubMed] [Google Scholar]
- 42.Viau, M., and M. Zouali. 2005. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 114:17–26. [DOI] [PubMed] [Google Scholar]
- 43.Ng, L.G., C.H. Ng, B. Woehl, A.P. Sutherland, J. Huo, S. Xu, F. Mackay, and K.P. Lam. 2006. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur. J. Immunol. 36:1837–1846. [DOI] [PubMed] [Google Scholar]
- 44.Brummel, R., and P. Lenert. 2005. Activation of marginal zone B cells from lupus mice with type A(D) CpG-oligodeoxynucleotides. J. Immunol. 174:2429–2434. [DOI] [PubMed] [Google Scholar]
- 45.He, B., X. Qiao, and A. Cerutti. 2004. CpG DNA induces IgG class switch DNA recombination by activating human B cells through innate pathways that require TLR9 and cooperates with IL-10. J. Immunol. 173:4479–4491. [DOI] [PubMed] [Google Scholar]
- 46.Berland, R., L. Fernandez, E. Kari, J.H. Han, I. Lomakin, S. Akira, H.H. Wortis, J.F. Kearney, A.A. Ucci, and T. Imanishi-Kari. 2006. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 25:429–440. [DOI] [PubMed] [Google Scholar]
- 47.Christensen, S.R., J. Shupe, K. Nickerson, M. Kashgarian, R.A. Flavell, and M.J. Shlomchik. 2006. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 25:417–428. [DOI] [PubMed] [Google Scholar]
- 48.Acosta-Rodriguez, E.V., A. Craxton, D.W. Hendricks, M.C. Merino, C.L. Montes, E.A. Clark, and A. Gruppi. 2007. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. Eur. J. Immunol. 37:990–1000. [DOI] [PubMed] [Google Scholar]
- 49.von Bulow, G.-U., J.M. van Deursen, and R.J. Bram. 2001. Regulation of the T-independent humoral response by TACI. Immunity. 14:573–582. [DOI] [PubMed] [Google Scholar]
- 50.Enzler, T., G. Bonizzi, G.J. Silverman, D.C. Otero, G.F. Widhopf, A. Anzelon-Mills, R.C. Rickert, and M. Karin. 2006. Alternative and classical NF-kappa B signalling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 25:403–415. [DOI] [PubMed] [Google Scholar]
- 51.West, A.P., A.A. Koblansky, and S. Ghosh. 2006. Recognition and signalling by Toll-like receptors. Annu. Rev. Cell Dev. Biol. 22:409–437. [DOI] [PubMed] [Google Scholar]
- 52.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T.K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, et al. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 23:479–490. [DOI] [PubMed] [Google Scholar]
- 54.Gavin, A.L., K. Hoebe, B. Duong, T. Ota, C. Martin, B. Beutler, and D. Nemazee. 2006. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 314:1936–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorelik, L., A.H. Cutler, G. Thill, S.D. Miklasz, D.E. Shea, C. Ambrose, S.A. Bixler, L. Su, M.L. Scott, and S.L. Kalled. 2004. BAFF regulates CD21/35 and CD23 expression independently of its B cell survival function. J. Immunol. 172:762–766. [DOI] [PubMed] [Google Scholar]
- 56.Banchereau, J., and V. Pascual. 2006. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 25:383–392. [DOI] [PubMed] [Google Scholar]
- 57.Means, T.K., and A.D. Luster. 2005. Toll-like receptor activation in the pathogenesis of systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1062:242–251. [DOI] [PubMed] [Google Scholar]
- 58.Grammer, A.C., and P.E. Lipsky. 2003. B cell abnormalities in systemic lupus erythematosus. Arthritis Res. Ther. 5:S22–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaminski, D.A., and J. Stavnezer. 2006. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J. Immunol. 177:6025–6029. [DOI] [PubMed] [Google Scholar]
- 60.Ettinger, R., G.P. Sims, R. Robbins, D. Withers, R.T. Fischer, A.C. Grammer, S. Kuchen, and P.E. Lipsky. 2007. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J. Immunol. 178:2872–2882. [DOI] [PubMed] [Google Scholar]
- 61.Hondowicz, B.D., S.T. Alexander, W.J. Quinn III, A.J. Pagan, M.H. Metzgar, M.P. Cancro, and J. Erikson. 2007. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. Int. Immunol. 19:465–475. [DOI] [PubMed] [Google Scholar]
- 62.Christensen, S.R., M. Kashgarian, L. Alexopoulou, R.A. Flavell, S. Akira, and M.J. Shlomchik. 2005. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J. Exp. Med. 202:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lenert, P., R. Brummel, E.H. Field, and R.F. Ashman. 2006. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J. Clin. Immunol. 25:29–40. [DOI] [PubMed] [Google Scholar]
- 64.Nijnik, A., H. Ferry, G. Lewis, E. Rapsomaniki, J.C. Leung, A. Daser, T. Lambe, C.C. Goodnow, and R.J. Cornall. 2006. Spontaneous B cell hyperactivity in autoimmune-prone MRL mice. Int. Immunol. 18:1127–1137. [DOI] [PubMed] [Google Scholar]
- 65.Ju, Z.L., G.Y. Shi, J.X. Zuo, and J.W. Zhang. 2007. Unexpected development of autoimmunity in BAFF-R-mutant MRL-lpr mice. Immunology. 120:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calvani, N., M. Tucci, H.B. Richards, P. Tartaglia, and F. Silvestris. 2005. Th1 cytokines in the pathogenesis of lupus nephritis: the role of IL-18. Autoimmun. Rev. 4:542–548. [DOI] [PubMed] [Google Scholar]
- 67.Durum, S.K., J.A. Schmidt, and J.J. Oppenheim. 1985. Interleukin-1: an immunological perspective. Annu. Rev. Immunol. 3:263–287. [DOI] [PubMed] [Google Scholar]
- 68.Ostendorf, B., C. Iking-Konert, K. Kurz, G. Jung, O. Sander, and M. Schneider. 2005. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann. Rheum. Dis. 64:630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodnow, C.C. 2006. Discriminating microbe from self suffers a double toll. Science. 312:1606–1608. [DOI] [PubMed] [Google Scholar]
- 70.Pisitkun, P., J.A. Deane, M.J. Difilippantonio, T. Tarasenko, A.B. Satterhwaite, and S. Bolland. 2006. RNA-related antigens due to TLR7 gene duplication. Science. 312:1669–1672. [DOI] [PubMed] [Google Scholar]