Abstract
Interleukin (IL) 25 (IL-17E), a distinct member of the IL-17 cytokine family, plays important roles in evoking T helper type 2 (Th2) cell–mediated inflammation that features the infiltrations of eosinophils and Th2 memory cells. However, the cellular sources, target cells, and underlying mechanisms remain elusive in humans. We demonstrate that human Th2 memory cells expressing distinctive levels of IL-25 receptor (R) are one of the responding cell types. IL-25 promotes cell expansion and Th2 cytokine production when Th2 central memory cells are stimulated with thymic stromal lymphopoietin (TSLP)–activated dendritic cells (DCs), homeostatic cytokines, or T cell receptor for antigen triggering. The enhanced functions of Th2 memory cells induced by IL-25 are associated with sustained expression of GATA-3, c-MAF, and JunB in an IL-4–independent manner. Although keratinocytes, mast cells, eosinophils, and basophils express IL-25 transcripts, activated eosinophils and basophils from normal and atopic subjects were found to secrete bioactive IL-25 protein, which augments the functions of Th2 memory cells. Elevated expression of IL-25 and IL-25R transcripts was observed in asthmatic lung tissues and atopic dermatitis skin lesions, linking their possible roles with exacerbated allergic disorders. Our results provide a plausible explanation that IL-25 produced by innate effector eosinophils and basophils may augment the allergic inflammation by enhancing the maintenance and functions of adaptive Th2 memory cells.
Allergic diseases, such as asthma and atopy, can be characterized by the accumulation of memory-like Th2 T cells and eosinophils at sites of inflammation (1, 2). During chronic asthma, the accumulation and functions of eosinophils are often correlated with the activation of local Th2 T cells through their production of IL-5 and GM-CSF, which prolong the viability and enhance the effector responses of eosinophils (3, 4). Conversely, recruitment of eosinophils to the airway by eotaxin and IL-5 promotes Th2 cell–mediated features of allergy, suggesting a positive role for recruited eosinophils in regulating the functions of local Th2 cells. Although there is abundant evidence for the regulation of eosinophil recruitment and activation by T cells and DCs in Th2 cell–type inflammation, there is relatively scant evidence for a role of eosinophils in regulating the function of Th2 cells, and the molecular mechanisms of such collaborative interactions remain unclear.
Distinct from other IL-17 cytokine family members, IL-25 (IL-17E) has been shown to evoke Th2 cell–mediated inflammatory responses in animal studies (5–10). In the mouse, IL-25 was first described as a Th2 cell–derived cytokine (9) and was also found to be expressed by mast cells (11). However, the cell types responding to IL-25 and the mechanism by which IL-25 amplifies allergic immune responses remain elusive. Moreover, the source of IL-25 and the cell types expressing IL-25R (or IL-17BR) involved in human allergic inflammation have not been defined.
Strong expression of thymic stromal lymphopoietin (TSLP), an IL-7–like cytokine, by skin or bronchial epithelium can activate DCs to express a Th2 cell–polarizing signal, OX40 ligand, and create a Th2 cell–permissive microenvironment in the absence of IL-12 (12, 13), which may result in atopic dermatitis or allergic asthma in humans and parallel changes in the mouse (14–18). DCs activated by TSLP (TSLP-DCs) can induce the differentiation of inflammatory Th2 cells (19) and play important roles in the maintenance and regulation of Th2 memory cells (20). Interestingly, TSLP-DCs further strengthen the allergy-inducing properties of Th2 memory cells by up-regulating their expression of proallergic genes, particularly IL-17RB, the receptor for the cytokine IL-25, suggesting a possible role of IL-25 in the regulation of Th2 memory cells (20). In this paper, we report a distinct expression pattern of human IL-25 and its cognate receptor within hematopoietic cell lineages. We found that eosinophils and basophils are one of the primary sources of IL-25, whereas TSLP-DC–activated Th2 memory cells are the major responders in humans. IL-25 enhances the expansion of Th2 memory cells induced by TSLP-DCs or homeostatic cytokines and further augments their Th2 cytokine production. Our findings suggest that eosinophils, through the production of IL-25, may exert a critical role in promoting the functional capacity of allergen-specific Th2 memory cells during allergic inflammation.
RESULTS
TSLP-DCs induce strong IL-25R expression by Th2 memory cells
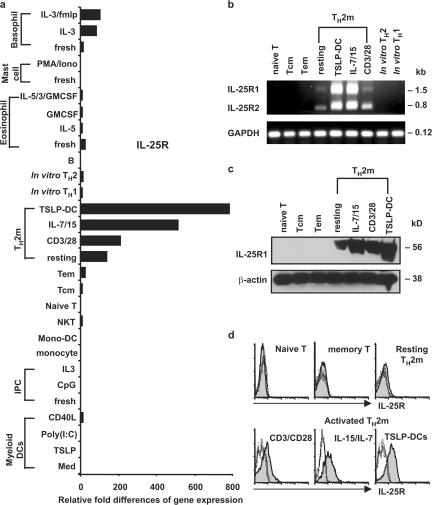
To characterize the expression pattern of human IL-25R in detail, we examined the cDNA archives of all human immune cell types using real-time PCR analysis. Among the entire repertoire of immune cells tested, only in vivo–derived resting Th2 memory cells, but not other T cell subsets, or in vitro–derived Th1 or Th2 cells expressed substantial levels of IL-25R transcripts. Although both resting and activated Th2 memory cells expressed the full-length and spliced isoforms of the IL-25R gene with ∼1.5- or 0.8-kb transcripts (Fig. 1 b), activated Th2 memory cells stimulated by CD3/CD28, the homeostatic cytokines IL-7/IL-15, or, in particular, TSLP-DCs strongly up-regulated their expression of IL-25R transcript (Fig. 1, a and b), corresponding to a 56-kD protein revealed by Western blot analysis (Fig. 1 c). Interestingly, only activated Th2 memory cells express detectable surface IL-25R by immunofluorescence analyses (Fig. 1 d). These composite molecular analyses reveal a distinct expression profile of IL-25R within hematopoietic immune cells and suggest that the activated Th2 memory cells may be the principle cells responding to the cytokine IL-25 in humans.
Figure 1.
Distinctive expression of IL-25R by human Th2 memory cells. (a) Expression of IL-25R was measured by quantitative PCR on a panel of human cDNA templates made from various cell types, as described in Materials and methods. Different T cell subsets were isolated as previously described (reference 20). (b) Expression of IL-25R isoforms by activated Th2 memory cells was demonstrated by RT-PCR using primers, as described in Materials and methods. Sorted resting T cell subsets, or activated Th2 memory cells stimulated by autologous TSLP-DCs, the homeostatic cytokine IL-7/15, and anti-CD3/CD28 for 7 d were isolated for cell lysate preparation (c) or flow cytometry analysis (d). Quantified cell lysates were subjected to Western blot analysis using biotinylated goat anti–human IL-17RB polyclonal antibody (c). Surface expression of IL-25R of activated Th2 memory cells or resting T cell subsets were examined using flow cytometry, as described in Materials and methods (d). Shaded histograms represent IL-25 R staining; open histograms represent the isotype control. Relative fold differences in IL-25R gene expression between samples listed in panel a are indicated on the x axis. Data are from one of three independent experiments.
IL-25 promotes the expansion and further polarization of Th2 central memory cells
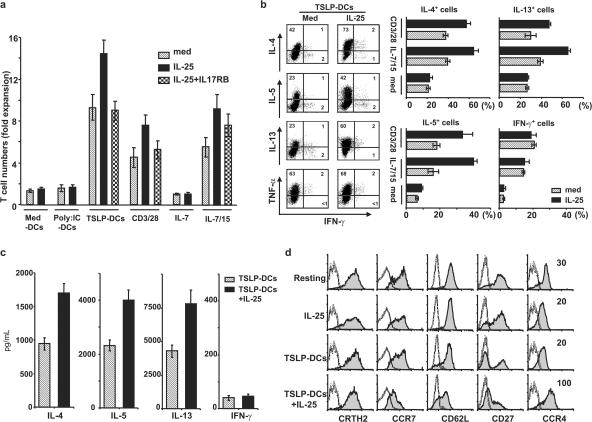
To test the effect of IL-25 on the maintenance of Th2 memory cells, sorted Th2 memory cells were co-cultured in the presence or absence of IL-25 for 7 d with IL-15 plus IL-7, anti-CD3/CD28, or autologous CD11c+ DCs in medium (med-DCs), or activated by TSLP or poly(I:C) for 24 h. Consistent with a previous study (20), TSLP-DCs induced the most robust expansion of Th2 memory cells, with a 10-fold increase in total T cell numbers compared with a 6-fold increase by IL-15 plus IL-7 or a 4.5-fold increase by anti-CD3/CD28, whereas no substantial proliferation was driven by poly(I:C)-DCs or med-DCs (Fig. 2 a). Interestingly, IL-25 further enhanced the proliferation of Th2 memory cells induced by TSLP-DCs, IL-15 plus IL-7, or anti-CD3/CD28, leading to 15-, 9-, or 7.5-fold increases, respectively, in total T cell numbers (Fig. 2 a). Although soluble IL-25R (IL-17RB-Fc) could efficiently abrogate the IL-25–induced enhancement of Th2 memory cell expansion by TSLP-DCs or anti-CD3/CD28, the additive effect of IL-25 on IL-15 plus IL-7–induced cell expansion could be partially neutralized (Fig. 2 a). To determine whether IL-25 regulates the differentiation of Th2 central memory cells during expansion, we examined Th2 cytokine production by the expanded cells after culture with TSLP-DCs, IL-15 plus IL-7, or anti-CD3/CD28, or medium only, for 7 d in the presence or absence of IL-25. We found that IL-25 induced an approximately twofold increase in the production of IL-4, IL-5, and IL-13, but not TNF-α or IFN-γ, by activated Th2 memory cells using intracellular cytokine analyses after PMA and ionomycin stimulations (Fig. 2 b), or ELISA analyses after CD3/CD28 restimulations (Fig. 2 c). In addition, IL-25 induced the expanded Th2 central memory cells driven by TSLP-DCs to acquire effector–memory phenotype by down-regulating CC chemokine receptor (CCR) 7 and CD27 expression, while maintaining CRTh2 and CD62L, or up-regulating CCR4, IL-4Rα, and IL-7Rα surface expression (Fig. 2 d; and not depicted). However, IL-25 alone could induce neither Th2 polarization nor phenotypic changes of Th2 memory cells cultured in medium only or with med-DCs or poly(I:C)-DCs for 7 d (Fig. 2, b and d; and not depicted), suggesting that only activated Th2 memory cells can respond to IL-25. Collectively, these results suggest that IL-25 may co-stimulate the proliferation and further polarization of Th2 memory cells induced by TSLP-DCs or other stimuli, leading to their acquired effector–memory phenotype and augmented Th2 cell functional attributes.
Figure 2.
IL-25 enhances the proliferation and Th2 polarization of stimulated Th2 memory cells. Sorted Th2 memory T cells were cultured with the cytokines IL-7 only, IL-15 plus IL-7, anti-CD3/CD28, or autologous CD11c+ DCs activated by various stimuli (x axis) at a DC/T cell ratio of 1:2 for 7 d in the presence or absence of IL-25. The neutralizing recombinant protein IL-17RB-Fc was used in culture. Their proliferative responses were compared, and graphic bars represent the times of expansion by dividing the final cell number by the initial T cell number in six individual experiments (a). To characterize the effect of IL-25 on Th2 memory cells, sorted cells were cultured in medium only or expanded by IL-15 plus IL-7, anti-CD3/CD28, or TSLP-DCs for 7 d in the presence or absence of IL-25. The proliferated cells were collected and restimulated with PMA plus ionomycin for analysis of intracellular cytokine production (b), or Th2 memory cells expanded by TSLP-DCs in the presence or absence of IL-25 for 7 d were collected and restimulated with anti-CD3/CD28 for 24 h before the measurements of cytokines in the culture supernatants by ELISA (c). For phenotypic analyses, sorted Th2 memory cells cultured in IL-25 alone, or expanded by TSLP-DCs, or TSLP-DCs plus IL-25 were examined using flow cytometry (d). Shaded histograms represent staining of Th2 memory T cells with the markers indicated below histograms; open histograms represent the isotype control. Numbers within the quadrants indicate the percentage of expanded cells that stained positive for each respective cytokine. The results in panels a, b, c, and d are for separate experiments. Data represent the mean ± SD of five experiments.
IL-25 enhance Th2 cytokine production by TSLP-DC–activated Th2 memory cells
Because TSLP-DCs induced strong surface IL-25R expression on activated Th2 memory cells (Fig. 1 d), we tested whether IL-25 can directly enhance Th2 cytokine production by TSLP-DC–activated Th2 memory cells. Freshly isolated or TSLP-DC–activated Th2 memory cells were washed and cultured with or without IL-25 stimulation for 24 h, and culture supernatants were harvested and examined for cytokine production by ELISA analyses. Although activated Th2 memory cells were capable of autonomously producing moderate amount of IL-5 and IL-13 in medium, IL-25 stimulation led to a more than twofold increase of Th2 cytokine production, particularly in IL-5 and IL-13 in the absence of TCR triggering, but not in IFN-γ or TNF-α (Fig. 3). In contrast, IL-25 has no effect on resting Th2 memory cells (Fig. 3), which can be explained by the very low level of surface IL-25R expression on resting Th2 memory cells (Fig. 1 d). The effect of IL-25 stimulation could be neutralized by soluble IL-25R, but not anti–IL-4 mAb, suggesting that the induction of the increased Th2 cytokine production by IL-25 is independent of IL-4 (Fig. 3). These results demonstrate that, in addition to promoting Th2 memory cell expansion and further Th2 polarization, IL-25 may directly enhance cytokine secretion by activated Th2 memory cells.
Figure 3.
IL-25 induces elevated cytokine production by TSLP-DC–activated Th2 memory cells. Freshly isolated resting or activated Th2 memory cells expanded by TSLP-DCs for 7 d were collected, washed, and stimulated directly with IL-25 or cultured in medium only for 24 h in the presence or absence of neutralizing soluble receptor IL-17RB-FC or anti-IL-4 mAbs. Cultured supernatants were collected for ELISA analyses. Data represent the mean ± SD of five experiments.
IL-25 induces sustained expression of GATA-3, c-maf, and JunB by activated Th2 memory cells
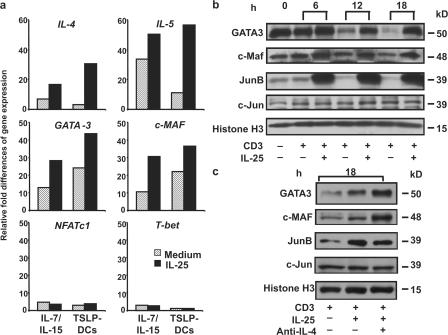
To address the molecular mechanism by which IL-25 strengthens the Th2 properties of Th2 memory cells, we tested whether IL-25 regulates the expression of genes involved in Th2 polarization during their expansions driven by TSLP-DCs or IL-15 plus IL-7. Quantitative PCR analyses revealed that IL-25 up-regulated the expression of IL-4 and IL-5, as well as GATA3 and c-Maf, the master regulators for Th2 cell differentiation, but not NFATc1 or T-bet transcripts, in the proliferating Th2 memory cells driven by TSLP-DCs or IL-15 plus IL-7 (Fig. 4 a). To investigate why IL-25 can directly stimulate activated Th2 memory cells to produce augmented Th2 cytokines, the effect of IL-25 on the expression of GATA3, c-MAF, and JunB was examined. Before restimulations with anti-CD3 mAbs, Th2 memory cells cultured with TSLP-DCs for 7 d were found to express substantial levels of GATA3, c-MAF, and JunB (Fig. 4 b). However, restimulation of TSLP-DC–activated Th2 memory cells by prolonged TCR triggering resulted in a gradual loss of these transcription factors after 12 h (Fig. 4 b). The addition of IL-25 sustained GATA3 expression and further promoted the expression of c-MAF and JunB, but not c-Jun, after 12 h of TCR triggering in culture (Fig. 4 b). Although IL-4 was known to induce GATA3 expression, anti–IL-4 mAbs could not abrogate the sustained expression of Th2 transcription factors induced by IL-25 during 18 h of culture with anti-CD3 mAb (Fig. 4 c). These results suggest that IL-25 may enhance the polarization and cytokine production of Th2 memory cells by sustaining the expression of Th2 transcription factors in an IL-4–independent manner.
Figure 4.
IL-25 up-regulates the expression of GATA3, c-MAF, and JunB. Sorted Th2 memory T cells were cultured with the cytokine IL-15 plus IL-7 or TSLP-DCs for 7 d in the presence or absence of IL-25. Expression levels of the indicated genes that are involved in Th2 cell differentiation were measured by real-time PCR, as described in Materials and methods. (a) Fold differences in the changes of expression of each gene between samples are marked on the y axes. Th2 memory cells expanded by TSLP-DCs for 7 d were collected (0 h; b) and restimulated with anti-CD3 mAb for different time courses (b) or 18 h (c) in the presence or absence of IL-25 (b and c) or anti-IL-4 mAbs (c) before collection and were subjected to Western blot analysis. Data are from one of three independent experiments.
Eosinophils and basophils secrete IL-25 upon activation
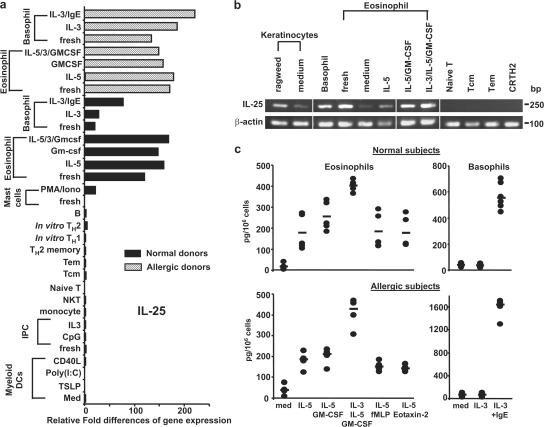
To search for IL-25–producing cells in humans, the expression of IL-25 transcript by hematopoietic cell lineages was examined. We found that resting and activated peripheral blood eosinophils and basophils from normal or allergic subjects expressed substantial levels of IL-25 transcripts by quantitative real-time PCR and RT-PCR analyses (Fig. 5, a and b). Although in vitro–derived mast cells (11) expressed low levels of IL-25 transcripts after activation with PMA and ionomycin, other cell lineages, including T cells, B cells, NK cells, monocytes, plasmacytoid DCs, and myeloid DCs do not express detectable levels of IL-25 mRNA (Fig. 5 a). In addition, keratinocytes cultured with medium only or allergen ragweed extracts were also found to express IL-25 transcripts (Fig. 5 b). These molecular analyses suggest that eosinophils and basophils may have the potential to secrete IL-25 proteins. To confirm these observations at the protein level and further investigate the regulation of IL-25 production by eosinophils and basophils, isolated eosinophils or basophils were cultured in medium only or the conditions known to activate eosinophils or basophils for 3d, and cultured supernatants were collected for ELISA analyses. We found that IL-5 alone induced IL-25 production by eosinophils and the combinations of IL-5 with GM-CSF or GM-CSF plus IL-3, but not FMLP or chemokine eotaxin-2, could further up-regulate IL-25 production (Fig. 5 c). Moreover, eosinophils isolated from normal or allergic subjects produced similar levels of IL-25 in these culture conditions (Fig. 5 c). Although basophils cultured in IL-3 alone did not produce IL-25, IgE cross-linking could trigger strong IL-25 production by basophils (Fig. 5 c). Interestingly, activated basophils from allergic subjects appeared to produce twofold more IL-25 than that produced by basophils from nonallergic subjects after IgE cross-linking (Fig. 5c). In addition, we could not detect secreted IL-25 proteins by cultured keratinocytes (unpublished data). These results suggest that activated innate effectors, eosinophils, and basophils may represent the major cell types that produce IL-25 in humans.
Figure 5.
Activated eosinophils and basophils are the producers of IL-25. Expression of IL-25 transcript was measured by quantitative PCR on a panel of human cDNA libraries made from various cell types (a) or examined by RT-PCR analysis (b), as described in Materials and methods. Purified eosinophils and basophils obtained from normal or atopic subjects were cultured for 3 d with the indicated cytokines or stimuli before the RNA isolation for gene expression analysis (a) or harvesting of supernatants for the measurement of IL-25 using ELISA analyses (b). Relative fold differences in IL-25 gene expression between samples listed in panel a are indicated on the x axis. Data in panel a are from one of three independent experiments. Data in panel c are from five normal or four atopic subjects, respectively, and represent the mean ± SD of tested samples (c). White lines indicate that intervening lanes have been spliced out.
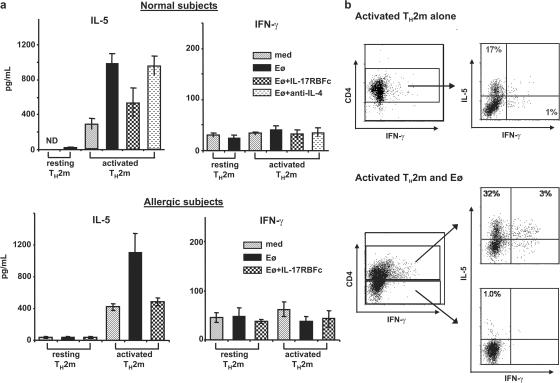
Eosinophil-derived IL-25 induces elevated IL-5 production
The reciprocal expression of IL-25 and its cognate receptor by eosinophils and Th2 memory cells, respectively, prompted us to investigate whether eosinophils can regulate the functions of Th2 memory cells through their production of IL-25. Resting Th2 memory cells or Th2 memory cells stimulated by TSLP-DCs for 7 d were collected and cultured in medium alone or with freshly isolated eosinophils in the presence or absence of IL-17RB-FC or anti–IL-4 mAbs. After 3 d of co-culture, the supernatants were collected, and cytokine production by cultured Th2 memory cells was analyzed by ELISA. Although eosinophils did not modulate cytokine production by the resting Th2 memory cells, eosinophils strongly promoted IL-5 production by TSLP-DC–activated Th2 memory cells (Fig. 6 a). The eosinophil-derived IL-25 induced a moderate increase of IL-13 and IL-4, but not IFN-γ, production by activated Th2 memory cells (Fig. 6 a; and not depicted). The ability of eosinophils to enhance IL-5 production by TSLP-DC–activated Th2 memory cells was largely abrogated by soluble IL-25R but not anti–IL-4 mAb (Fig. 6 a). Similarly, eosinophils from allergic subjects were found to enhance IL-5 production by autologous TSLP-DC–activated Th2 memory cells, but not resting Th2 memory cells, through their production of IL-25 (Fig. 6 a). Because eosinophils have been shown to express Th2 cytokine transcripts (21), we addressed whether co-cultured eosinophils themselves may contribute to the elevated IL-5 production. Although TSLP-DC–activated CD4+ Th2 memory cells cultured in medium only were capable of producing IL-5 after PMA and ionomycin stimulations, co-cultured eosinophils induced a substantial increase in IL-5 production by CD4+-activated Th2 memory cells (Fig. 6 b), In contrast, we failed to detect evident IL-5 production by CD4− co-cultured eosinophils using an intracellular cytokine analysis (Fig. 6 b). To test whether eosinophils can respond to IL-25, eosinophils activated by IL-5, GM-CSF, and IL-3 for 3 d were washed and cultured in medium with or without IL-25 treatment for 24 h We found that activated eosinophils did not produce detectable levels of IL-4 or IL-5, but low levels of IL-13, and IL-25 treatment did not enhance their Th2 cytokine production by ELISA analyses (unpublished data). These results suggest that activated, but not resting, Th2 memory cells can continuously produce low levels of IL-5, which promotes the longevity and activation of co-cultured eosinophils. In turn, the activated eosinophils produce IL-25 and induce the augmented Th2 cytokine production, particularly of IL-5, by TSLP-DC–activated Th2 memory cells but not co-cultured eosinophils themselves.
Figure 6.
Eosinophil-secreted IL-25 promotes cytokine production by TSLP-DC–activated Th2 memory cells. Resting or TSLP-DC–activated Th2 memory cells purified from normal (a and b) or atopic subjects (a) were cultured in medium only or co-cultured with autologous eosinophils for 3 d in the presence or absence of anti–IL-4 Abs or IL-17RB-Fc recombinant proteins (a). Co-cultured supernatants were harvested for the measurement of Th2 cytokine production using ELISA analyses (a). Activated Th2 memory cells cultured in medium alone or co-cultured with eosinophils for 3 d were collected and restimulated with PMA plus ionomycin before being stained with CD4 and the indicated intracellular cytokines. Numbers within the quadrants indicate the percentage of cells that stained positive for each respective cytokine. Data are from five normal or four atopic subjects and represent the mean ± SD of tested samples.
Expression of IL-25/IL-25R transcripts is associated with chronic allergic diseases
To search for the relevance of IL-25 and IL-25R expression in human allergic diseases, we performed extensive quantitative real-time PCR analyses to examine the expression of both IL-25 and IL25R transcripts by chronic asthmatic and normal lung tissues and lesions obtained from varieties of Th1 cell– and Th2 cell–mediated skin diseases. As shown in Fig. 7, the expression of both IL-25 and IL25R transcripts was found to be considerably elevated in chronic asthmatic lungs, as well as in atopic dermatitis skin lesions, but not in those from Th1 cell–mediated skin diseases, implying their potential roles during allergic inflammation.
Figure 7.
Expression of IL-25 and IL-25R transcripts by asthmatic and atopic subjects. Expression of IL-25 and IL-25R transcripts were measured by quantitative PCR on cDNA templates obtained from a bronchial biopsy of asthmatic or normal subjects, and a skin biopsy, including psoriasis vulgaris, cutaneous lupus erythematosus, prurigo nodularis, atopic dermatitis, and normal subjects. Relative fold differences in gene expression between samples are indicated on the x axis.
DISCUSSION
Distinct from other IL-17 cytokine family members, IL-25 (IL-17E) has been shown to be capable of amplifying Th2 cell–mediated inflammatory responses in animal studies (5–10). Systemic administration of IL-25 protein (8, 9) or overexpression of IL-25 (5, 7) induces elevated Th2 cytokine and eotaxin production, which results in eosinophilia, increased serum IgE, and pathological changes in many tissues. Although studies in animal models implicate the possible functions of IL-25 in allergic diseases, the mechanism by which IL-25 is involved in allergic immune responses and the cell types responding to this cytokine remain elusive. The effect of exogenous IL-25 protein in inducing acute lung inflammation was shown to be dependent on CD4+ T cells (22) or mediated by unidentified IL-5–producing cells (8). Other recent animal studies demonstrated that the function of IL-25 in inducing protective type 2 immunity against Trichuris muris infection is lymphocyte dependent (6), whereas the non–B or –T c-kit+ cells were responsible for the rapid clearance of Nippostrongylus brasiliensis infection (10). These findings using different animal models have perplexed our understanding of the cellular and molecular mechanisms by which IL-25 regulates type 2 immunity.
The identification of a circulating CD4+ T cell subset expressing the prostaglandin D2 receptor (CRTH2) as human Th2 central memory T cells and linking the function of TSLP-DCs on the maintenance and commitment of Th2 memory cells has led us to uncover many unique features of naturally occurring Th2 memory cells. One of the distinctive characteristics of Th2 memory cells is their selective IL-25R expression (20). Although the counterpart of mouse IL-25–responding non–B or –T c-kit+ cells in humans remains elusive, we demonstrate in this study that, in addition to lung fibroblasts (23), human CD4+CRTH2+ Th2 memory T cells expressing high levels of functional IL-25R may represent one of the major cell types responding to IL-25 during allergic inflammation. Selective expression of IL-25R on Th2 memory cells can be divided into two stages. During the resting stage, the expression of IL-25R transcript by circulating Th2 central memory cells is 1,000-fold higher than that by naive or other T cell subsets (20). However, the circulating Th2 memory T cells express low levels of surface IL-25R expression and do not respond to either recombinant IL-25 or IL-25 secreted by activated eosinophils. After stimulation by TSLP-DCs, activated Th2 memory cells further up-regulate the expression of IL-25R transcript and surface protein and become responsive to IL-25 stimulation. IL-25 co-stimulates the proliferation of the Th2 memory cells and enhances their Th2 polarization and cytokine production by up-regulating the gene expression of the transcription factors GATA-3, c-MAF, and junB during expansion driven by TSLP-DCs. Moreover, IL-25 can up-regulate surface CCR4, IL-4Rα, and IL-7Rα expression on Th2 central memory cells, while maintaining CRTH2 and CD62L, but down-regulating CCR7 and CD27 expression. These results imply that IL-25, together with TSLP-DCs, can induce infiltrated Th2 central memory cells at local inflammation sites to undergo further Th2 differentiation and expansion, leading to augmented Th2 functions and acquired effector–memory phenotype. Moreover, IL-25 may directly stimulate activated Th2 memory cells to produce elevated IL-5 and IL-13 by sustaining the expression of the transcription factors GATA-3, c-MAF, and junB in an IL-4–independent manner. Collectively, these results suggest that IL-25 may regulate Th2 memory cells in two phases: (a) the enhancement of expansion and Th2 polarization and (b) sustaining their Th2 cell activation status, thereby resulting in an amplified Th2 immune response.
IL-25 was first described as a Th2 cell–derived cytokine (9) and was also found to be expressed by mast cells (11) in mouse studies. However, it remains unclear whether these cells can secrete bioactive IL-25 protein; in addition, the cellular source of human IL-25 has never been addressed. In this paper, we show that human eosinophils, basophils, mast cells, and keratinocytes, but not other immune cell types, have the ability to express IL-25 transcript. More importantly, we demonstrate that human eosinophils and basophils secrete bioactive IL-25 protein upon activation. Co-cultured eosinophils can induce enhanced productions of IL-5 and GM-CSF by autologous TSLP-DC–activated Th2 memory cells in an IL-25– but not IL-4–dependent fashion. Traditionally, eosinophils are thought to function as the terminal effectors of allergic and antiparasitic responses mainly through the release of proinflammatory mediators (3). Recently, accumulating evidence suggests that eosinophils may be capable of regulating the functions of CD4+ Th2 cells (24, 25). Tissue-dwelling, but not circulating, eosinophils in allergic individuals express HLA-DR (24), suggesting a functional role of recruited eosinophils in regulating local Th2 memory cells. We show that, in addition to HLA-DR, eosinophils may regulate the functions of activated Th2 memory cells through the production of IL-25. Conversely, the elevated cytokine productions by Th2 memory cells promote the prolonged viability and enhanced activation of local eosinophils. The findings of elevated expression of IL-25/IL-25R transcripts associated with chronic allergic disease tissues further suggest that IL25/IL-25R may represent a key molecular pair in the maintenance of allergic inflammation in humans and, therefore, can be potential targets for the development of novel therapy for human allergic diseases. Our findings vindicate a novel concept: namely, that IL-25–mediated collaborative interactions between eosinophils/basophils and Th2 memory cells may occur and, thus, propagate a positive feedback loop between innate effector and adaptive immunity, leading to the amplification of allergic inflammation. Cross talk between these effectors may represent a key regulatory mechanism of allergic inflammation, and further understanding this process may point to novel approaches to therapy.
MATERIALS AND METHODS
Purification of myeloid DCs, Th2 memory cells, eosinophils, and other cell lineages.
This study was approved by the institutional review boards for human research at the University of Texas M.D. Anderson Cancer Center or the Baylor College of Medicine. CD11c+lineage− DCs were isolated from the blood of healthy donors (at the Gulf Coast Regional Blood Center) or allergic subjects (at Baylor College of Medicine) using a FACSAria (BD Biosciences), as previously described (15). Sorted CD11c+ DCs with a purity >99% were cultured in Yssel's medium containing 2% human AB serum (Gemini Bio-Products) or stimulated with 15 ng/ml TSLP, 10 μg/ml poly(I:C) for 24 h. For Th2 memory cell isolation, autologous CD4+ T cells were enriched using the CD4 T cell isolation kit (Miltenyi Biotec), according to manufacturer's instructions. Enriched CD4+ T cells were stained and sorted as CD4+CD45RO+CRTH2+ Th2 memory cells with a purity >99%, as previously described (20). For eosinophil isolation, peripheral blood samples were centrifuged over a Ficoll-Paque Plus gradient to separate mononuclear cells from granulocytes. After removal of the leukocyte layer, RBCs were lysed by incubation in sterile, cold water for 30 s. Eosinophils were further purified from the remaining white blood cells using an eosinophil isolation kit (Miltenyi Biotec), according to the manufacturer's instructions. Isolated eosinophils were cytospun, fixed, and stained with Giemsa to examine for purity (> 95%). Basophils were sorted based on their expression of CRTH2hiCD4−lineage− with a purity >99%. NKT cells, B cells, monocytes, and plasmacytoid DCs were isolated as previously described (20, 26–28). Human naive mast cell were generated in vitro as previously described (29). Primary human epidermal keratinocytes (Cambrex/Bio Whitakker) were maintained in keratinocyte growth medium (Cambrex/Bio Whitakker) or were stimulated with 10 ng/ml of ragweed extract overnight before RNA isolation.
DC–T cell and eosinophil–Th2 memory cell co-culture.
After 24 h of culture, stimulated CD11c+ DCs were collected, washed, and co-cultured with 2 × 104 autologous CD4+CRTH2+ Th2 memory cells in triplicate at a DC/T cell ratio of 1:2. In some experiments, CD4+CRTH2+ Th2 memory cells were cultured with 10 μg/ml of immobilized anti-CD3 (OKT3) and 1 μg/ml of soluble anti-CD28 (L293.1), or 20 ng/ml IL-7, and 10 ng/ml IL-15, or 100 ng/ml IL-25 (R&D Systems). For eosinophil–Th2 memory cell co-culture, sorted CD4+CRTH2+ Th2 memory cells were activated and expanded by TSLP-DCs for 7 d. 105 activated CD4+CRTH2+ Th2 memory cells were collected and washed before co-culture with 2.5 × 105 isolated eosinophils for 3 d. In some experiments, freshly isolated CD4+CRTH2+ Th2 memory cells were directly co- cultured with purified eosinophils for 3 d. To examine the amount of T cell proliferation, collected cells were counted with trypan blue to exclude dead cells, using a hemacytometer (Bright-Line/1492; Hausser Scientific). The following reagents were used in some assays: 1 μg/ml anti–IL-4 or 1 μg/ml IL-17RB-Fc (R&D Systems).
Analysis of cytokine production.
In primary DC–T cell culture, sorted Th2 memory cells were co-cultured with autologous TSLP-DCs in the presence or absence of IL-25 for 7 d. Expanded T cells were washed and restimulated with 5 μg/ml of immobilized anti-CD3 (OKT3) and 1 μg/ml of soluble anti-CD28 (L293.1) for 24 h. Collected supernatants were assessed by ELISA for IL-4, IL-5, IL-13, GM-CSF, IFN-γ, and TNF-α production (R&D Systems). Intracellular cytokine staining was performed using PE-labeled IL-4, IL-5, and IL-13; FITC–TNF-α; and allophycocyanin–IFN-γ (BD Bioscience), as previously described (13). For the measurement of secreted IL-25, supernatants collected from eosinophils and basophils, stimulated with cytokine combinations (10 ng/ml IL-5, 5 ng/ml GM-CSF, and 5 ng/ml IL-3) or 1 μg/ml IgE cross-linking (Sigma-Aldrich) were assessed by ELISA (PeproTech).
Analysis of cell-surface markers.
Freshly isolated or activated CRTH2+CD4+ Th2 memory cells co-cultured with TSLP-DCs for 7 d in the presence or absence of IL-25 were washed, blocked with FcR-blocking reagent (Miltenyi Biotec), and stained with PE-labeled anti-CRTH2, CCR7, CD62L, CD27, and CCR4 (all obtained from BD Biosciences) before analysis with a FACSCalibur (BD Biosciences). In some experiments, sorted resting T cell subsets or activated CRTH2+CD4+ Th2 memory cells were stained with biotinylated goat anti–human IL-17RB polyclonal antibody (R&D Systems) and revealed with streptavidin-PE.
RNA isolation and real-time quantitative RT-PCR.
Total RNA samples from sorted cells were isolated by an RNeasy kit (QIAGEN). The cDNA templates were synthesized using SuperScript II (Life Technologies). Oligonucleotide primers were selected using Primer Express (version 2.0; Applied Biosystems). Real-time quantitative PCR was performed with a detection system (ABI Prism 7900; Applied Biosystems). Real-time PCR primers were as follows: IL-25R, 5′-GAGAGCCGACCGTTCAATGT-3′ and 5′-TCCACTCTGGAGATGGCCC-3′; and IL-25, 5′-CCAGGTGGTTGCATTCTTGG-3′ and 5′-TGGCTGTAGGTGTGGGTTCC-3′. For the analysis of GATA3, c-maf, T-bet, NFATc1, IL-4, and IL-5 gene expression, real-time PCR probes were purchased directly from the manufacturer (Applied Biosystems). Equal amounts of RNA from samples were used as PCR templates in reactions to obtain the threshold cycle (Ct), and the Ct was normalized using the known Ct from 18S RNAs to obtain ΔCt. To examine the relative levels of gene expression in different cells, ΔΔCt values were calculated by using the ΔCt values associated with the lowest expression levels as the baseline. The ΔΔCt was then transformed to the real value of increase in expression by 2ΔΔCt. For RT-PCR analysis, IL-25R transcripts were amplified using the following primers: 5′-ATGTCGCTCGTGCTGCTAAGCCT-3′ and 5′-TAACTGGTGTCTGCTGGCAGAAGTGCATT-3′, with an annealing temperature at 65°C. IL-25 was amplified using the following primers: 5′-CCAGGTGGTTGCATTCTTGG-3′ and 5′-TGGCTGTAGGTGTGGGTTCC-3′, with an annealing temperature at 62°C.
Immunoblot analysis.
Sorted CRTH2+CD4+ Th2 memory cells were expanded and maintained with TSLP-DCs. Activated CRTH2+CD4+ Th2 memory cells were collected, washed, and restimulated with 1 μg/ml of immobilized anti-CD3 mAbs for various times before harvesting. Cell nuclear lysates prepared according to manufacturer's instructions (Pierce Chemical Co.) were subjected to Western blot analysis with antibodies against GATA-3, c-MAF, JunB, c-Jun (Santa Cruz Biotechnology, Inc.), and histone H3 (Cell Signaling).
Skin and bronchial biopsy samples.
After obtaining informed consent from patients, 3–6-mm punch biopsies were taken from either lesional or nonlesional skin from individuals with atopic dermatitis, psoriasis vulgaris, lupus erythematosus (tumidus and discoid), or lichen rubber, or from normal healthy individuals. Skin samples were immediately frozen in liquid nitrogen and stored at −80°C. The study was approved by the local ethics committees of the Department of Dermatology of the Heinrich-Heine-University. Bronchial biopsies were obtained from atopic asthmatics and normal subjects. The characteristics of these subjects were previously described (16). The study was approved by the Ethics Committee of King's College Hospital, London.
Acknowledgments
We thank Karen Ramirez, Zhiwei He, and Eric Wieder for cell sorting support.
Y.-H. Wang received a postdoctoral fellowship sponsored by Tanox, Inc. This work was supported by National Institute of Allergy and Infectious Diseases grant U19 AI071130-0 (to Y.-J. Liu) and funds from the Sandler Program for Asthma Research (to Y.-J. Liu).
The authors have no conflicting financial interests.
Abbreviations used: CCR, CC chemokine receptor; med-DC, CD11c+ DC in medium; TSLP, thymic stromal lymphopoietin; TSLP-DC, DC activated by TSLP.
References
- 1.Cohn, L., J.A. Elias, and G.L. Chupp. 2004. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 22:789–815. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp, M. 1999. Immunologic basis of antigen-induced airway hyper responsiveness. Annu. Rev. Immunol. 17:255–281. [DOI] [PubMed] [Google Scholar]
- 3.Gleich, G.J., C.R. Adolphson, and K.M. Leiferman. 1993. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 44:85–101. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberg, M.E. 1998. Eosinophilia. N. Engl. J. Med. 338:1592–1600. [DOI] [PubMed] [Google Scholar]
- 5.Pan, G., D. French, W. Mao, M. Maruoka, P. Risser, J. Lee, J. Foster, S. Aggarwal, K. Nicholes, S. Guillet, et al. 2001. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 167:6559–6567. [DOI] [PubMed] [Google Scholar]
- 6.Owyang, A.M., C. Zaph, E.H. Wilson, K.J. Guild, T. McClanahan, H.R. Miller, D.J. Cua, M. Goldschmidt, C.A. Hunter, R.A. Kastelein, and D. Artis. 2006. Interleukin 25 regulates type 2 cytokine–dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, M.R., R. Manoukian, R. Yeh, S.M. Silbiger, D.M. Danilenko, S. Scully, J. Sun, M.L. DeRose, M. Stolina, D. Chang, et al. 2002. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 100:2330–2340. [DOI] [PubMed] [Google Scholar]
- 8.Hurst, S.D., T. Muchamuel, D.M. Gorman, J.M. Gilbert, T. Clifford, S. Kwan, S. Menon, B. Seymour, C. Jackson, T.T. Kung, et al. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443–453. [DOI] [PubMed] [Google Scholar]
- 9.Fort, M.M., J. Cheung, D. Yen, J. Li, S.M. Zurawski, S. Lo, S. Menon, T. Clifford, B. Hunte, R. Lesley, et al. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 15:985–995. [DOI] [PubMed] [Google Scholar]
- 10.Fallon, P.G., S.J. Ballantyne, N.E. Mangan, J.L. Barlow, A. Dasvarma, D.R. Hewett, A. McIlgorm, H.E. Jolin, and A.N. McKenzie. 2006. Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda, K., H. Nakajima, K. Suzuki, S. Kagami, K. Hirose, A. Suto, Y. Saito, and I. Iwamoto. 2003. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 101:3594–3596. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Y.J. 2006. Thymic stromal lymphopoietin: master switch for allergic inflammation. J. Exp. Med. 203:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, T., Y.H. Wang, O. Duramad, T. Hori, G.J. Delespesse, N. Watanabe, F.X. Qin, Z. Yao, W. Cao, and Y.J. Liu. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Shami, A., R. Spolski, J. Kelly, A. Keane-Myers, and W.J. Leonard. 2005. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soumelis, V., P.A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673–680. [DOI] [PubMed] [Google Scholar]
- 16.Ying, S., B. O'Connor, J. Ratoff, Q. Meng, K. Mallett, D. Cousins, D. Robinson, G. Zhang, J. Zhao, T.H. Lee, and C. Corrigan. 2005. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 174:8183–8190. [DOI] [PubMed] [Google Scholar]
- 17.Yoo, J., M. Omori, D. Gyarmati, B. Zhou, T. Aye, A. Brewer, M.R. Comeau, D.J. Campbell, and S.F. Ziegler. 2005. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 202:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou, B., M.R. Comeau, T. De Smedt, H.D. Liggitt, M.E. Dahl, D.B. Lewis, D. Gyarmati, T. Aye, D.J. Campbell, and S.F. Ziegler. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047–1053. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe, N., S. Hanabuchi, V. Soumelis, W. Yuan, S. Ho, R. de Waal Malefyt, and Y.-J. Liu. 2004. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5:426–434. [DOI] [PubMed] [Google Scholar]
- 20.Wang, Y.H., T. Ito, Y.H. Wang, B. Homey, N. Watanabe, R. Martin, C.J. Barnes, B.W. McIntyre, M. Gilliet, R. Kumar, et al. 2006. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 24:827–838. [DOI] [PubMed] [Google Scholar]
- 21.Moqbel, R., F. Levi-Schaffer, and A.B. Kay. 1994. Cytokine generation by eosinophils. J. Allergy Clin. Immunol. 94:1183–1188. [DOI] [PubMed] [Google Scholar]
- 22.Tamachi, T., Y. Maezawa, K. Ikeda, S. Kagami, M. Hatano, Y. Seto, A. Suto, K. Suzuki, N. Watanabe, Y. Saito, et al. 2006. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 118:606–614. [DOI] [PubMed] [Google Scholar]
- 23.Letuve, S., S. Lajoie-Kadoch, S. Audusseau, M.E. Rothenberg, P.O. Fiset, M.S. Ludwig, and Q. Hamid. 2006. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J. Allergy Clin. Immunol. 117:590–596. [DOI] [PubMed] [Google Scholar]
- 24.MacKenzie, J.R., J. Mattes, L.A. Dent, and P.S. Foster. 2001. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J. Immunol. 167:3146–3155. [DOI] [PubMed] [Google Scholar]
- 25.Odemuyiwa, S.O., A. Ghahary, Y. Li, L. Puttagunta, J.E. Lee, S. Musat-Marcu, A. Ghahary, and R. Moqbel. 2004. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J. Immunol. 173:5909–5913. [DOI] [PubMed] [Google Scholar]
- 26.Cao, W., D.B. Rosen, T. Ito, L. Bover, M. Bao, G. Watanabe, Z. Yao, L. Zhang, L.L. Lanier, and Y.J. Liu. 2006. Plasmacytoid dendritic cell–specific receptor ILT7-FcεRIγ inhibits Toll-like receptor–induced interferon production. J. Exp. Med. 203:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadowaki, N., S. Antonenko, S. Ho, M.C. Rissoan, V. Soumelis, S.A. Porcelli, L.L. Lanier, and Y.J. Liu. 2001. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J. Exp. Med. 193:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reche, P.A., V. Soumelis, D.M. Gorman, T. Clifford, M. Liu, M. Travis, S.M. Zurawski, J. Johnston, Y.J. Liu, H. Spits, et al. 2001. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167:336–343. [DOI] [PubMed] [Google Scholar]
- 29.Bressler, R.B., J. Lesko, M.L. Jones, M. Wasserman, R.R. Dickason, M.M. Huston, S.W. Cook, and D.P. Huston. 1997. Production of IL-5 and granulocyte-macrophage colony-stimulating factor by naive human mast cells activated by high-affinity IgE receptor ligation. J. Allergy Clin. Immunol. 99:508–514. [DOI] [PubMed] [Google Scholar]