Abstract
An optimal CD8+ T cell response requires signals from the T cell receptor (TCR), co-stimulatory molecules, and cytokines. In most cases, the relative contribution of these signals to CD8+ T cell proliferation, accumulation, effector function, and differentiation to memory is unknown. Recent work (Boyman, O., M. Kovar, M.P. Rubinstein, C.D. Surh, and J. Sprent. 2006. Science. 311:1924–1927; Kamimura, D., Y. Sawa, M. Sato, E. Agung, T. Hirano, and M. Murakami. 2006. J. Immunol. 177:306–314) has shown that anti–interleukin (IL) 2 monoclonal antibodies that are neutralizing in vitro enhance the potency of IL-2 in vivo. We investigated the role of IL-2 signals in driving CD8+ T cell proliferation in the absence of TCR stimulation by foreign antigen. IL-2 signals induced rapid activation of signal transducer and activator of transcription 5 in all CD8+ T cells, both naive and memory phenotype, and promoted the differentiation of naive CD8+ T cells into effector cells. IL-2–anti–IL-2 complexes induced proliferation of naive CD8+ T cells in an environment with limited access to self–major histocompatibility complex (MHC) and when competition for self-MHC ligands was severe. After transfer into wild-type animals, IL-2–activated CD8+ T cells attained and maintained a central memory phenotype and protected against lethal bacterial infection. IL-2–anti–IL-2 complex–driven memory-like CD8+ T cells had incomplete cellular fitness compared with antigen-driven memory cells regarding homeostatic turnover and cytokine production. These results suggest that intense IL-2 signals, with limited contribution from the TCR, program the differentiation of protective memory-like CD8+ cells but are insufficient to guarantee overall cellular fitness.
The CD8+ T cell response to an acute systemic infection entails vigorous expansion of antigen-specific cells, followed by a contraction phase in which 90–95% of the cells die by apoptosis. The cells remaining after the contraction phase become memory T cells, which possess properties distinct from the naive population, such as rapid acquisition of effector function on reencounter with pathogen. Accumulating evidence suggests that naive CD8+ T cells are programmed to become memory cells in the early phase of the CD8+ T cell response, when appropriate signals from the TCR, co-stimulatory molecules, and cytokines associated with inflammation are thought to be required (1, 2).
IL-2, which is produced by activated T cells, is one of the cytokines involved in the CD8+ T cell response. IL-2 induces intracellular signals through the IL-2 receptor complex, consisting of CD25 (IL-2Rα), CD122 (IL-2/IL-15Rβ), and CD132 (common γ chain). Stimulation of the receptor complex by IL-2 induces several signal transduction pathways, including the activation of STAT5 (3). Recent studies have demonstrated that IL-2 supports the maintenance of Foxp3+ CD4+ regulatory T cells, which bear the IL-2Rα chain (CD25) (4–6). The requirement for IL-2 signals to maintain regulatory T cells limits the use of cytokine or cytokine receptor knockout mice to study other in vivo roles of IL-2. Instead, the effect of IL-2 signals on CD8+ T cells during an immune response has been investigated by creating situations in which CD25, CD122, or IL-2 are selectively deficient in CD8+ T cells. These studies revealed a modest role for IL-2 in the primary expansion and differentiation of CTL (7–12), whereas IL-2 appears to support expansion of primary CTL in nonlymphoid organs (13). Very recently, another role for IL-2 signals in antigen-stimulated CD8+ T cells was revealed using mixed BM chimeric mice containing both wild-type and CD25−/− cells. In these mice a complete compartment of regulatory T cells is reconstituted, and the mice remain healthy (11, 12). Upon acute infection, effector and memory CD8+ T cells lacking CD25 were generated and normally maintained, but their secondary expansion after pathogen rechallenge was severely compromised compared with that of CD25-sufficient memory cells (11, 12). Intriguingly, replenishment of IL-2 signals to CD25−/− CD8+ T cells during the primary infection, but not during the secondary challenge, restored their ability to expand in a recall response (11). This result clearly indicates a programming effect of IL-2 signaling during the primary response in driving the complete differentiation of memory CD8+ T cells.
Recent data suggest that the anti–IL-2 mAb S4B6, which has been widely used as a neutralizing antibody in vitro, enhances the bioactivity of IL-2 in vivo (14, 15). Administration of rIL-2 mixed with the anti–IL-2 mAb (IL-2–anti–IL-2 complexes) or the concurrent treatment with plasmid DNA expressing mouse IL-2 and the antibody substantially and preferentially increased the proliferation of CD44hi CD122hi memory-phenotype CD8+ T cells and NK cells (14, 15). Injection of anti–IL-2 mAb alone had a similar effect because of the capture of endogenously secreted IL-2 by the mAb, although the efficacy of this treatment is much weaker than the cotreatment with IL-2–anti–IL-2 complexes (14, 15). The precise mechanism of the enhanced potency of the immune complex remains unclear, although the presentation of IL-2–anti–IL-2 complexes via the Fc portion of the mAb has been suggested (14).
We report that the administration of IL-2–anti–IL-2 complexes stimulated all CD8+ T cells, both naive and memory phenotype, in vivo. The naive CD8+ T cells proliferated, became effector cells, and differentiated to memory-phenotype cells capable of providing protection against pathogen challenge. Remarkably, proliferation of naive CD8+ T cells by treatment with IL-2–anti–IL-2 complexes was induced in the absence of foreign antigen and when competition for self-MHC class I ligands was intense. Because most experimental systems used for the study of CD8+ T cell responses are associated with strong TCR stimulation, our approach using IL-2–anti–IL-2 complexes allowed us to dissect and examine the relative contribution of IL-2 and antigen signals to CD8+ T cell responses, revealing that intense IL-2 signals coupled with weak TCR ligation have the potential to program the differen tiation of protective memory-like CD8+ T cells in vivo.
RESULTS
IL-2–anti–IL-2 complexes activate STAT5 in all CD8+ T cells
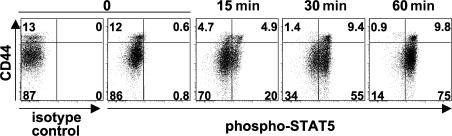
CD44hi CD122hi memory-phenotype CD8+ T cells and NK cells express high levels of CD122 (IL-2/IL-15Rβ), providing an explanation for the preferential expansion of these populations after treatment with complexes of rIL-2 and anti–IL-2 mAb (IL-2–anti–IL-2 complexes) (14, 15). However, CD44lo naive CD8+ T cells also express detectable levels of CD122, intermediate between those of memory-phenotype CD8+ T cells and naive CD4+ T cells (unpublished data) (16). In addition, after treatment with IL-2–anti–IL-2 complexes, almost the entire CD8+ population adopts a CD44hi CD122hi phenotype (unpublished data) (14). We therefore asked whether CD44lo naive CD8+ T cells could respond to IL-2–anti–IL-2 complexes injected in vivo. IL-2 signal transduction is known to involve the activation of STAT5, which is important for the biological activity of IL-2 (3, 4). Therefore, we used the phosphorylation status of STAT5 as a readout of induction of an IL-2 signal in vivo. C57BL/6 (B6) mice were given a single injection of IL-2–anti–IL-2 complexes, and at the indicated time points, splenocytes were fixed without restimulation in vitro, and activated (tyrosine-phosphorylated) STAT5 was detected by flow cytometry. As shown in Fig. 1, CD44hi memory-phenotype CD8+ T cells responded rapidly after the treatment: the phosphorylation of STAT5 was induced in ∼50% of the population at 15 min and increased to >90% by 60 min. Surprisingly, the rapid activation of STAT5 was also observed in CD44lo naive CD8+ T cells with slightly delayed kinetics. At 60 min, >80% of the CD44lo naive CD8+ population contained the activated form of STAT5. NK cells (NK1.1+ TCRβNEG) also showed STAT5 activation, whereas B cells (B220+ TCRβNEG) showed no detectable activation of STAT5 at 60 min (unpublished data). The rapid activation of STAT5 suggests that CD44lo naive CD8+ T cells, as well as memory-phenotype CD8+ T cells, receive signals directly from IL-2–anti–IL-2 complexes.
Figure 1.
IL-2–anti–IL-2 complex treatment induces rapid activation of STAT5 in naive CD8+ T cells. Mice were given a single injection of IL-2–anti–IL-2 complexes. At the indicated time points, splenocytes were fixed and permeabilized for intracellular staining of the tyrosine-phosphorylated form of STAT5. Mice for the zero time point received no injection. The data are gated on the CD8+ population, and the numbers represent the percentage of gated cells in each quadrant.
IL-2–anti–IL-2 complexes induce proliferation of naive CD8+ T cells
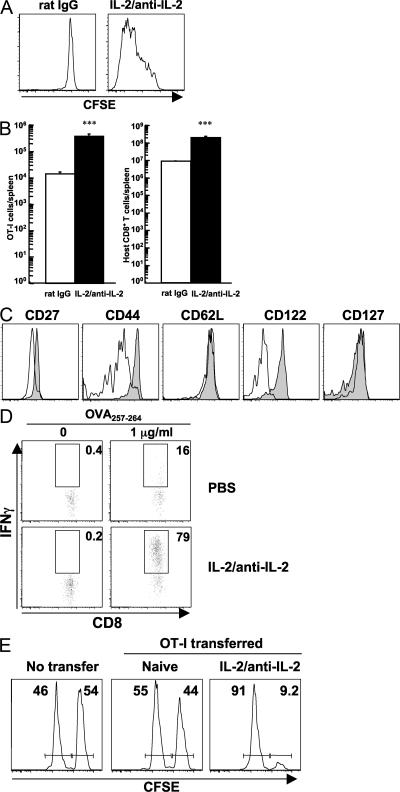
These results prompted us to examine the physiological consequence of treating naive CD8+ T cells with IL-2 signals in vivo. Congenically marked CD44lo CD122lo naive OT-I cells were isolated, labeled with CFSE, and transferred into nonirradiated syngeneic B6 mice (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20070543/DC1). After treatment of the host animals with IL-2–anti–IL-2 complexes, the naive OT-I cells divided extensively (Fig. 2 A) and accumulated (Fig. 2 B) in the absence of any other stimulation. The expansion of naive OT-I cells was almost equivalent to that of the host CD8+ T cell population (Fig. 2 B). Surface markers associated with activation/memory, including CD27, CD44, and CD122, were up-regulated on OT-I cells that received IL-2 signals, whereas CD62L and CD127 levels remained unchanged as compared with OT-I cells in animals receiving control injections (Fig. 2 C). This phenotype resembles a central memory phenotype (17). IL-2–anti–IL-2 complex–mediated proliferation in a lympho-replete environment was not unique to OT-I cells or mediated by a second TCR possibly expressed on TCR-transgenic cells, because other naive TCR-transgenic CD8+ T cells (from P14 or Vβ5 TCR-transgenic mice) and OT-I cells on a RAG-1−/− background also proliferated (Fig. S2). In contrast, proliferation of naive polyclonal CD8+ T cells was not as vigorous (unpublished data), as previously reported (14). Functionally, OT-I cells that had received IL-2 signals were able to produce IFN-γ after a 4-h stimulation with their cognate peptide in vitro (Fig. 2 D) and killed target cells in an antigen-specific manner in vivo (Fig. 2 E), both of which are typical characteristics of effector cells. These results indicate that signals provided by IL-2–anti–IL-2 complexes induce the proliferation and activation of naive antigen-specific CD8+ T cells in nonlymphopenic environments without cognate antigen stimulation.
Figure 2.
IL-2–anti–IL-2 complexes induce proliferation of naive antigen-specific CD8+ T cells. 0.5 × 106 purified CD44lo CD122lo naive OT-I cells were transferred into nonirradiated mice on day −1. The mice were given IL-2–anti–IL-2 complexes or control (rat IgG) on days 0 and 2, and the assays were performed on day 6. (A) Proliferation of donor cells was evaluated by CFSE dilution. (B) Absolute number of donor cells (left) and host CD8+ T cells (right) in the spleen. The data represent the mean + SEM (n = 3). ***, P < 0.001 versus rat IgG treatment. (C) Phenotypic changes of the donor cells. Rat IgG and IL-2–anti–IL-2 complex treatment are indicated with open and shaded histograms, respectively. (D) IFN-γ production by donor OT-I cells was assessed after 4 h of stimulation in vitro. Numbers represent the percentage of gated OT-I cells making IFN-γ. (E) Equal numbers (1.1 × 106 cells) of naive OT-I cells and IL-2–anti–IL-2 complex–activated OT-I cells were transferred to naive animals. An in vivo CTL assay with OVA peptide–pulsed (CFSEhi) and control (CFSElo) targets was performed the next day. The killing activity was examined 20 h later. Numbers represent the percentage of CFSEhi and CFSElo targets remaining. The donor cells (A–D) and target cells (E) were distinguished from host populations by congenic markers.
Fcγ receptors are dispensable for the action of IL-2–anti–IL-2 complexes
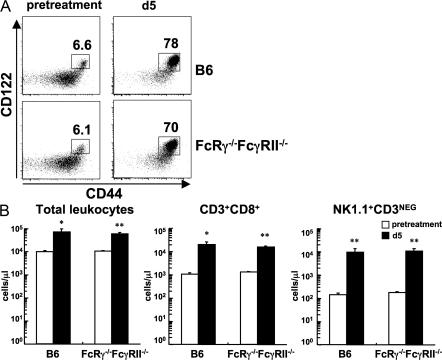
Why IL-2–anti–IL-2 complexes are so potent in vivo is still unclear. One possible mechanism that has been proposed is the presentation of IL-2–anti–IL-2 complexes by Fcγ receptor–bearing cells to cells expressing CD122/CD132 (14). Thus, IL-2 complexed to F(ab′)2 fragments of the anti–IL-2 mAb had only a modest effect in vivo (14). To investigate the role of Fcγ receptors, mice deficient in both FcRγ chain and FcγRII (FcRγ−/−FcγRII−/−) were used. Because the FcRγ chain is necessary for surface expression of the activating IgG Fc receptors, including FcγRI, III, and IV, whereas the inhibitory receptor FcγRII does not share the FcRγ chain (18, 19), FcRγ−/−FcγRII−/− mice should be devoid of known functional Fcγ receptors on hematopoietic cells. In fact, FcγRII and FcγRIII expression was undetectable on B cells from FcRγ−/−FcγRII−/− mice by staining with anti-CD16/32 (FcγRIII/II) mAb (Fig. S3 A, available at http://www.jem.org/cgi/content/full/jem.20070543/DC1). In addition, anti-CD3–induced cytokine release in vivo, which requires presentation and cross-linking via Fcγ receptors (20, 21), was severely impaired in FcRγ−/−FcγRII−/− mice, indicating a gross functional defect of Fcγ receptors in these mice (Fig. S3 B). Because FcRγ−/−FcγRII−/− mice are on a mixed genetic background, we tested the efficacy of IL-2–anti–IL-2 complexes by sampling PBLs before and after treatment rather than using an adoptive transfer system. Both control and FcRγ−/−FcγRII−/− mice displayed a similar small population of CD44hi CD122hi memory-phenotype CD8+ T cells before treatment (Fig. 3 A). By day 5 after treatment with IL-2–anti–IL-2 complexes, CD44hi CD122hi memory-phenotype CD8+ T cells increased dramatically in control and FcRγ−/−FcγRII−/− mice (Fig. 3 A). Total numbers of PBLs increased significantly after treatment in both strains and correlated with vigorous expansion of CD8+ T cells and NK cells (Fig. 3 B). These results suggest that Fcγ receptor–mediated presentation is dispensable for the action of IL-2–anti–IL-2 complexes in vivo.
Figure 3.
Fcγ receptors are dispensable for the action of IL-2–anti–IL-2 complexes. B6 mice and FcRγ−/−FcγRII−/− mice were treated with IL-2–anti–IL-2 complexes on days 0 and 2. The mice were bled before starting the treatment (pretreatment) and on day 5. (A) Phenotype of the CD8+ T cell population of the same animal in each group is shown. The data are gated on CD3+ CD8+ cells. Numbers represent the percentage of gated cells within the box. (B) Absolute numbers of total leukocytes, CD8+ T cells (CD3+ CD8+), and NK cells (NK1.1+ CD3NEG) in the peripheral blood before and after (day 5) the treatment. Each column represents the mean + SEM (n = 3). *, P < 0.05; or **, P < 0.01 versus pretreatment.
IL-2–anti–IL-2 complexes stimulate naive CD8+ T cells with limited TCR stimulation by self-ligands
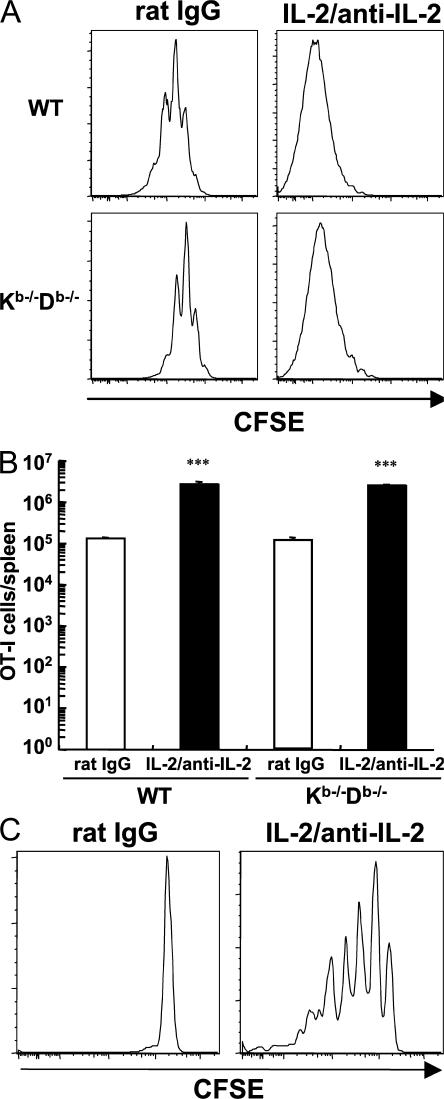
IL-2 signals induced the proliferation of naive TCR-transgenic CD8+ T cells in a complete lymphoid compartment and in the absence of cognate antigen stimulation (Fig. 2 A and Fig. S2). However, it was possible that TCR stimulation by self-ligands had an important role, as is the case for homeostatic proliferation (HP) of naive CD8+ T cells in a lymphopenic environment (22–24). To explore the dependency on MHC class I ligands, we chose MHC class I Kb−/−Db−/− mice as host animals, because the homeostasis of IgG is impaired in β2 microglobulin–deficient mice (25), which might affect the half-life of IL-2–anti–IL-2 complexes. Because CD8+ T cells transferred into MHC class Ia–deficient mice were almost completely rejected after IL-2–anti–IL-2 complex treatment (unpublished data), MHC class Ia–deficient mice were irradiated with 10 Gy and transplanted with syngeneic BM cells to prevent rejection. Irradiated, BM-reconstituted mice were injected with CFSE-labeled naive OT-I cells and IL-2–anti–IL-2 complexes. As controls, B6 mice were also irradiated and reconstituted with syngeneic BM. As shown in Fig. 4 A, naive OT-I cells underwent three to four divisions in lethally irradiated WT hosts after rat IgG treatment, because of lymphopenia-induced HP. Proliferation was delayed in MHC class Ia–deficient hosts, confirming that HP of naive CD8+ T cells is MHC class I–dependent (22–24). In contrast, IL-2 signals provided by injected IL-2–anti–IL-2 complexes stimulated extensive division of naive OT-I cells in the MHC class Ia–deficient and –sufficient host animals. The recovery of donor OT-I cells in the spleen after the complex treatment was also similar in both groups (Fig. 4 B).
Figure 4.
IL-2–anti–IL-2 complexes stimulate naive CD8+ T cells with limited TCR stimulation. Host mice that received CFSE-labeled naive OT-I cells 1 d earlier were treated with IL-2–anti–IL-2 complexes on days 0 and 2. CFSE levels of donor cells from the spleen were examined on day 6. The data are gated on CD3+ CD8+ congenically marked donor cells. (A) WT and MHC class I Kb−/−Db−/− mice that were irradiated with 10 Gy and transplanted with syngeneic BM cells were used as host animals. (B) Absolute number of OT-I cells recovered from the spleen of animals in A is shown. The data represent the mean + SEM (n = 2). ***, P < 0.001 versus rat IgG treatment. (C) Nonirradiated OT-I/RAG-1−/− mice were used as hosts.
These results suggest a minimal requirement for TCR ligation by self-MHC for the proliferation of naive CD8+ T cells stimulated by IL-2–anti–IL-2 complexes, but it does not rule out the possibility of stimulation by MHC class I molecules on the donor cells themselves or by irradiation-induced cytokines. In this regard, a recent report has strongly suggested the requirement for TCR recognition of self-MHC ligands to observe the accumulation of IL-2–driven CD8+ cells (26). We took a second approach to examine the dependency on TCR stimulation based on the finding that adoptively transferred TCR-transgenic CD8+ T cells do not undergo HP in hosts with the identical TCR clonotype because of clonal competition for MHC class I/peptide ligands (27, 28). Accordingly, naive OT-I cells were labeled with CFSE and transferred into nonirradiated OT-I/RAG-1−/− hosts. As shown in Fig. 4 C, signals provided by IL-2–anti–IL-2 complexes induced several divisions of naive OT-I cells even in this environment, where competition for self-MHC ligands is intense. This result suggested that robust expansion of naive CD8+ T cells in response to potent IL-2 signals requires only minimal TCR stimulation.
IL-2–anti–IL-2 complexes generate memory cells with a central memory phenotype
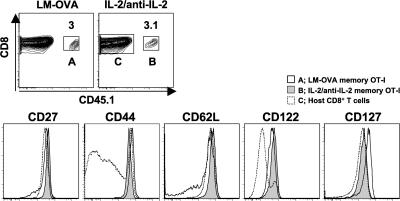
We next addressed whether naive CD8+ T cells activated by IL-2–anti–IL-2 complexes could differentiate to memory cells. OT-I mice were injected with IL-2–anti–IL-2 complexes, resulting in the conversion of almost all the CD8+ T cells to a CD44hi CD122hi activated phenotype (Fig. S1 B). 15 × 106 IL-2–activated OT-I cells were then transferred at day 12 to normal B6 hosts. At 1 d after transfer, the OT-I cells exhibited rapid IFN-γ production in response to peptide stimulation in vitro (unpublished data). For comparison, conventional, antigen-driven OT-I cells were generated by transfer of 104 naive OT-I cells into B6 recipients, followed by immunization with Listeria monocytogenes–expressing soluble OVA (LM-OVA; Fig. S1 C). More than 75 d after transfer of the IL-2–anti–IL-2 complex–driven or antigen-driven memory cells, the OT-I cells accounted for ∼3% of the CD8+ population in the spleen (Fig. 5). At this point, the antigen-driven memory OT-I cells were CD44hi CD122hi and contained CD62Lhi central and CD62Llo effector memory subsets. In contrast, all of the IL-2–anti–IL-2 complex–driven cells had the phenotype of central memory cells. These results suggest that naive CD8+ T cells activated by IL-2–anti–IL-2 complex treatment revert to stable central memory-phenotype cells.
Figure 5.
IL-2–anti–IL-2 complexes generate memory cells with a central memory phenotype. Memory OT-I cells generated by LM-OVA infection (75 d after infection; population A), IL-2–anti–IL-2 complex memory OT-I cells (12 d of treatment plus 76 d after transfer; population B), and host CD8+ T cells (population C) were examined for the expression of cell-surface markers. Contour plots are gated on CD8+ cells. Numbers represent the percentage of CD8+ cells within the box.
IL-2–anti–IL-2 complex memory CD8+ T cells protect against bacterial infection
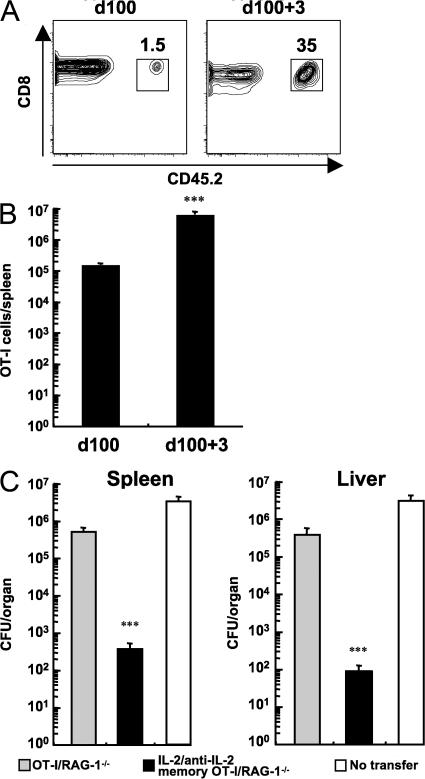
Functional memory CD8+ T cells rapidly expand in numbers and effectively control a challenge with pathogen (1, 2). We therefore assessed the ability of IL-2–anti–IL-2 complex memory OT-I cells to mount a protective recall response to a lethal dose of LM-OVA. Mice bearing IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells (91 d after transfer) were infected with a lethal dose of LM-OVA. As shown in Fig. 6 (A and B), IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells expanded over 30-fold within 3 d after the LM-OVA challenge. To examine the protective ability of IL-2–driven memory cells, a control group of mice received 1.5 × 106 untreated OT-I/RAG-1−/− cells to constitute a population of these cells at an equivalent level to that of mice containing IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells (132 d after transfer). Before challenge with LM-OVA, the OT-I donor cell populations in peripheral blood were 1.3 ± 0.11% and 1 ± 0.04% of CD8+ T cells in control and IL-2–anti–IL-2 complex memory groups, respectively. At 3 d after challenge, mice containing IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells were able to significantly decrease LM-OVA burden in both the spleen and liver compared with mice containing untreated OT-I/RAG-1−/− cells and mice without cell transfer (Fig. 6 C). Furthermore, adoptive transfer of IL-2–anti–IL-2 complex memory OT-I cells provided significant protection to naive animals, whereas an equal number of naive OT-I cells did not (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070543/DC1). These results demonstrate that IL-2–anti–IL-2 complex–driven memory CD8+ T cells are able to mount a rapid and robust response to antigen challenge and afford protection.
Figure 6.
IL-2–anti–IL-2 complex memory CD8+ T cells protect against bacterial infection. (A) Enumeration of IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells (9 d of treatment and 91 d after transfer; CD45.2+) in the spleen before and 3 d after challenge with 105 CFU of LM-OVA. The data are gated on CD8+ cells. Numbers represent the percentage of CD8+ cells within the box. (B) Absolute numbers of IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells in the spleen before and after LM-OVA challenge. Data represent the mean + SEM (n = 3–4). ***, P < 0.001. (c) CFU of LM-OVA in the spleen and liver at 3 d after challenge of mice containing equal numbers of naive OT-I/RAG-1−/− or IL-2–anti–IL-2 memory OT-I/RAG-1−/− cells. Data represent the mean + SEM (n = 4–6). ***, P < 0.001 versus the OT-I/RAG-1−/− group.
IL-2–anti–IL-2 complex memory CD8+ T cells display incomplete cellular fitness
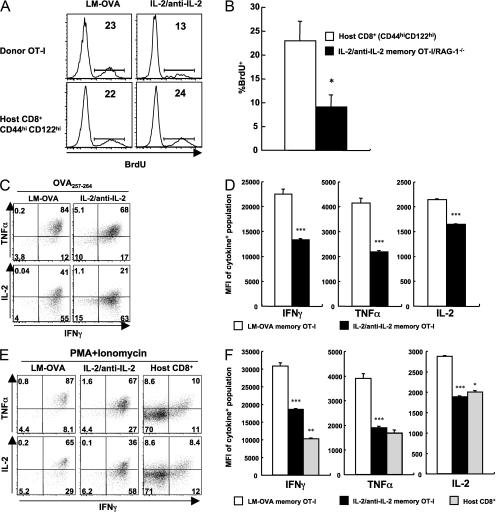
We further characterized IL-2–anti–IL-2 complex memory OT-I cells, and compared them to LM-OVA memory OT-I cells and with spontaneously arising memory-phenotype CD8+ T cells. Memory CD8+ T cells proliferate slowly to maintain a stable pool (6, 24), and this homeostatic turnover can be measured by the uptake of BrdU. LM-OVA antigen-driven memory OT-I cells displayed a rate of homeostatic turnover that was similar to that of memory-phenotype CD8+ T cells in the same mouse (Fig. 7 A). In contrast, although IL-2–anti–IL-2 complex memory OT-I cells also exhibited homeostatic turnover, the rate was significantly less than that of antigen-driven memory cells or CD44hi CD122hi memory-phenotype CD8+ T cells in the same mouse (Fig. 7, A and B).
Figure 7.
IL-2–anti–IL-2 complex memory CD8+ T cells display incomplete cellular fitness. (A) Mice bearing LM-OVA antigen-driven memory OT-I cells at 68 d after infection or IL-2–anti–IL-2 complex memory OT-I cells after 81 d (12 d of treatment plus 69 d after transfer) were given BrdU in drinking water for 7 d. BrdU incorporation by the indicated subsets of memory CD8+ T cells in the spleen is shown. Numbers represent the percentage of gated cells that are BrdU positive. (B) BrdU-positive population of host CD44hi CD122hi CD8+ T cells and IL-2–anti–IL-2 complex memory OT-I/RAG-1−/− cells after 61 d (9 d of treatment plus 52 d after transfer). The data are the mean + SEM (n = 3). *, P < 0.05. (C–F) Splenocytes containing memory OT-I cells generated by LM-OVA infection (62 d after infection) or IL-2 signals (12 d of treatment plus 63 d after transfer) were stimulated in vitro with OVA peptide (C and D) or PMA plus ionomycin (E and F). Intracellular cytokine production was examined 4 h later. Numbers in C and E represent the percentage of gated cells in each quadrant. (D and F) The mean fluorescent intensity (MFI) of cytokine staining is shown (mean + SEM; n = 3). The MFI was calculated within the population positive for the respective cytokine. * and **, P < 0.05 and 0.001, respectively, versus the complex-driven memory cells; ***, P < 0.001 versus LM-OVA memory cells.
Another cardinal feature of memory CD8+ T cells is the rapid production of cytokines after reencounter with antigen (1, 2). Splenocytes containing IL-2–anti–IL-2 complex memory OT-I cells or LM-OVA memory OT-I cells were stimulated with OVA peptide or PMA plus ionomycin for 4 h in vitro, and cytokine production was assessed by flow cytometry. Upon antigenic stimulation, almost all (>95%) antigen-driven memory cells produced IFN-γ; most of the IFN-γ–producing population also produced TNF-α, and ∼40% of them were IL-2 positive (Fig. 7 C). In comparison, a reduced fraction of memory cells generated by IL-2–anti–IL-2 complexes were positive for the cytokines tested (Fig. 7 C). In addition, the amounts of IFN-γ, TNF-α, and IL-2 produced on a per-cell basis, as judged by the mean fluorescence intensity, were also decreased in IL-2–anti–IL-2 complex memory OT-I cells compared with antigen-driven memory cells (Fig. 7 D). Differences in cytokine production were evident when IL-2 complex–driven or antigen-driven memory cells were stimulated with PMA plus ionomycin (Figs. 7, E and F), implying that alterations in signal transduction proximal to the TCR do not account for the changes. The cytokine production levels of IL-2–anti–IL-2 complex memory OT-I cells resembled those of the host memory-phenotype CD8+ T cells upon PMA/ionomycin stimulation, although a marked difference was observed in IFN-γ levels (Fig. 7 F). Similar results were obtained when activated OT-I cells were prepared from OT-I/RAG-1−/− mice that had been treated with IL-2–anti–IL-2 complexes (Fig. 7 B and not depicted). These results suggest that strong IL-2 signals coupled with weak TCR signals are not sufficient to guarantee the overall cellular fitness of memory CD8+ T cells.
DISCUSSION
The recent realization that complexing IL-2 with anti–IL-2 mAbs leads to a dramatic increase in the potency of signaling via the intermediate affinity CD122/CD132 receptor in vivo (14, 15) allowed us to examine the consequences of such signals to CD8+ T cells in normal, nonlymphopenic animals in the absence of foreign antigen stimulation. Injected IL-2–anti–IL-2 complexes caused the rapid activation of STAT5 in naive as well as memory-phenotype CD8+ T cells (Fig. 1). This was the case even though CD122 levels are much higher on memory than on naive CD8+ T cells (unpublished data) (16) and the IL-2–anti–IL-2 complex signaling depends on CD122 (29). Under such conditions, potent IL-2 signals stimulated naive CD8+ T cells to proliferate and differentiate into functional memory cells. Such IL-2–anti–IL-2 complex–driven memory cells had a conventional central memory phenotype (Fig. 5) and were able to control a bacterial challenge in both lymphoid and nonlymphoid organs (Fig. 6 C and Fig. S4). The downstream molecular basis for the IL-2–anti–IL-2 complex–driven programming of memory CD8+ T cells awaits further investigation. Target genes of STAT5, an important component of IL-2 signaling (3, 4), are likely to be a key component of this process, because overexpression of STAT5 in T cells results in the selective expansion of memory-phenotype CD8+ T cells (30, 31).
Although IL-2–anti–IL-2 complex–generated memory CD8+ T cells were able to expand, accumulate, and provide protection after challenge with a high dose Listeria infection (Fig. 6 and Fig. S4), we found that they differed from conventional antigen-driven memory cells. In comparison with conventional memory cells, they had a slower rate of homeostatic turnover and reduced cytokine production after short-term restimulation (Fig. 7). This slow turnover may result in dis appearance of the complex-driven memory cells in the long term, although a clear correlation between the reduced BrdU uptake and loss of donor cells was not consistently observed up to 80 d after transfer (unpublished data). The turnover of memory CD8+ T cells is regulated by cytokines such as IL-7 and IL-15 (6). The expression levels of the receptors for these cytokines, namely CD127 (IL-7Rα) and CD122 (IL-2/IL-15Rβ) and CD132, did not noticeably differ between complex-driven memory cells, antigen-driven memory cells, and memory-phenotype cells (Fig. 5 and not depicted). In addition, treatment with PMA plus ionomycin did not override the defect in cytokine production of IL-2–anti–IL-2 complex memory CD8+ T cells (Fig. 7, E and F), implying that the reduced fitness of IL-2–anti–IL-2 complex memory CD8+ T cells might be caused by intracellular differences, such as in the epigenetic remodeling of genes involved in hallmark characteristics of memory cells. We speculate that more potent signals via the TCR may be required to operate in conjunction with IL-2 signals to promote the complete programming of memory CD8+ T cell differentiation. In this context, it is interesting to note that naive CD8+ T cells that receive strong TCR signals after pathogen infection, but weak or no IL-2 signals (i.e., IL-2Rα−/− cells), develop into memory cells that show normal levels of homeostatic turnover and cytokine production but fail to accumulate in response to secondary antigenic challenge (11). Thus, predominant TCR signals without IL-2 give rise to memory CD8+ T cells that fail to accumulate in response to a secondary challenge, whereas predominant IL-2 signals with weak TCR stimulation result in memory cells with reduced turnover and cytokine production.
IL-2–anti–IL-2 complexes dramatically enhance IL-2 activity in vivo (14, 15), but the mechanism of this enhancement is still unclear. Because IL-2–anti–IL-2 complexes lacking the Fc region (i.e., IL-2 plus the F(ab′)2 portion of the anti–IL-2 mAb) had less activity than complexes with the whole IgG, it was suggested that Fcγ receptor–positive cells in lymphoid organs presented the IL-2 to T cells (14). However, we show that IL-2–anti–IL-2 complexes showed similar potent activity in FcRγ−/−FcγRII−/− mice as in B6 control mice (Fig. 3). It is possible that other nonclassical Fc receptor–like molecules (32) or leaky expression of FcγRI without the FcRγ subunit (33) may mediate the activity of IL-2–anti–IL-2 complexes in vivo. However, FcRγ−/−FcγRII−/− mice showed a gross functional defect in the stimulatory presentation of anti-CD3 IgG (Fig. S3 B), leading us to conclude that presentation of IL-2–anti–IL-2 complexes via Fcγ receptors may not be responsible for the enhancing effect. The half-life of IgG in vivo is dependent on the presence of the Fc region (25, 34), and this may explain the weak activity of IL-2–anti–IL-2 immune complexes lacking Fc. Moreover, there are many studies documenting the fact that the in vivo half-life of various cytokines is prolonged by the formation of immune complexes. These include human IL-2–anti–IL-2 (35), IL-4–anti–IL-4 (36), IL-6–anti–IL-6 (37), and soluble IL-15–IL-15Rα-Fc (38) complexes. Therefore, we favor the idea that IL-2–anti–IL-2 complexes extend the half-life of IL-2 in vivo by preventing renal excretion, the major pathway of IL-2 clearance in mice (39).
Other recent reports have demonstrated the development of memory CD8+ T cells from naive cells without cognate antigen stimulation. Stimulation of naive CD8+ T cells with soluble IL-15–IL-15Rα complexes, which act as superagonists (40, 41), induced proliferation and converted the cells to memory-phenotype CD8+ T cells (38). Because both soluble IL-15–IL-15Rα complexes and IL-2–anti–IL-2 immune complexes signal through the CD122/CD132 receptor complex on CD8+ T cells (6, 42), these two types of complex may result in similar consequences, although the function of the memory-phenotype CD8+ T cells generated by soluble IL-15–IL-15Rα complexes was not addressed in detail (38). In another report, Hamilton et al. demonstrated that lymphopenia-induced HP resulted in the generation of protective memory-like CD8+ T cells (43). Similar to IL-2–anti–IL-2 complex–mediated proliferation, HP is induced in the absence of cognate antigen (22–24), and similar phenotypic changes occur after proliferation induced by IL-2 signals (Fig. 2 C and Fig. 5) and by HP (43). HP of naive CD8+ T cells in a lymphopenic environment requires both TCR stimulation through MHC class I–peptide complexes, in addition to cytokines such as IL-7 (22–24). Proliferation of naive CD8+ cells driven by IL-2–anti–IL-2 complexes in irradiated mice may also require TCR signals from self-MHC ligands (26). IL-7–dependent HP is enhanced by IL-12, but no substantial involvement of IL-2 has been reported (44–46). Given that stimulation by foreign antigen plus cytokines (such as pathogen infections), self-antigen plus IL-7 (HP), or intense IL-2 signals (IL-2–anti–IL-2 complexes) all give rise to protective memory CD8+ T cells, key components of programming memory CD8+ T cell differentiation may reside within pathways common to these stimulations.
MATERIALS AND METHODS
Mice.
B6 mice were obtained from the Jackson Laboratory. B6.SJL, MHC class I Kb−/−Db−/−, OT-I/RAG-1−/−, and FcRγ−/−FcγRII−/− mice were purchased from Taconic. These animals were backcrossed to a B6 genetic background, except for FcRγ−/−FcγRII−/− mice, which were on a 129 × B6 mixed background. Animals were housed in specific pathogen-free conditions in the animal facilities at the University of Washington. OT-I TCR-transgenic mice (47) congenic for Thy1.1 and CD45.1 were bred and maintained in the same facilities. All experiments were performed in compliance with the University of Washington Institutional Animal Care and Use Committee regulations.
Preparation of IL-2–anti–IL-2 complexes.
Carrier-free recombinant mouse IL-2 was obtained from eBioscience. Hybridoma of anti–IL-2 mAb (S4B6) was grown in HB basal medium with HB101 supplement (Irvine Scientific). The anti–IL-2 mAb was purified from the culture medium with a protein G column. IL-2–anti–IL-2 complexes were prepared by mixing 1.5 μg rIL-2 and 50 μg anti–IL-2 mAb per mouse. Rat IgG (Sigma-Aldrich) plus PBS was used as a control treatment, as previously described (15, 48). The dose of IL-2–anti–IL-2 complexes has been shown to be effective in vivo in previous studies (11, 14).
Detection of phospho-STAT5 in vivo.
Mice were killed at the time points indicated in the figures after a single i.p. injection of IL-2–anti–IL-2 complexes. The intracellular phospho-STAT5 staining was performed as previously described (49, 50). In brief, splenocytes were fixed in 1.6% formaldehyde/PBS for 30 min at room temperature, followed by permeabilization with ice-cold methanol. The fixed and permeabilized cells were stained in 2% FCS/PBS with PECy7-labeled anti-CD44, PerCP-labeled anti-CD8α, and Alexa Fluor 647–labeled anti–phospho-STAT5 (Y694; Phosflow, BD Biosciences) mAbs. Alexa Fluor 647–labeled mouse IgG1 mAb (Phosflow; BD Biosciences) was used as the isotype control.
Adoptive transfer of donor CD8+ T cells.
CD44lo naive CD8+ T cells were purified by magnetic cell sorting using the CD8a+ T cell isolation kit (Miltenyi Biotec) supplemented with 0.003 μg per million cells of biotinylated anti-CD44 mAb (BD Biosciences) to deplete CD44hi CD8+ T cells (46). After sorting, >98% of CD8+ T cells were CD44lo CD122lo naive phenotype. 0.5–106 donor cells per mouse were transferred i.v. into nonirradiated hosts. In some experiments, the donor cells were labeled with 1.6 μM CFSE (Invitrogen) for 10 min at 37°C before transfer. Host animals in Fig. 4 (A and B) received 10 Gy total body irradiation and were reconstituted with syngeneic BM trans plantation. For assays of IL-2–anti–IL-2 complex memory cells, OT-I or OT-I/RAG-1−/− mice were injected with IL-2–anti–IL-2 complexes five to six times every other day until most OT-I CD8+ T cells acquired the CD44hi CD122hi phenotype. These cells were isolated by the magnetic cell sorting system without biotinylated anti-CD44 mAb. 7–17 × 106 of these activated OT-I cells per mouse were injected i.v. into host mice. The analysis of memory OT-I cells was performed at least 50 d after transfer. Engraftment of the donor cells at the time of assay was between 1 and 10% of total CD8+ T cells.
Intracellular cytokine staining.
Splenocytes were incubated with 1 μg/ml OVA257-264 (SIINFEKL) or PMA plus ionomycin in the presence of Brefeldin A (GolgiPlug; BD Biosciences) for 4 h at 37°C. After staining of cell-surface molecules, the cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences). The intracellular cytokine staining was performed with FITC-labeled anti–TNF-α, PE-labeled anti–IL-2, and PECy7-labeled IFN-γ mAbs (eBioscience).
In vivo CTL assay.
To prepare target cells, splenocytes were incubated with or without 10 μg/ml OVA257-264 peptide for 30 min at 37°C. These peptide-pulsed target and nontarget populations were labeled with 1.6 and 0.16 μM CFSE, respectively, mixed at a 1:1 ratio, and injected i.v. into mice. After 20 h, the target cell killing activity was evaluated using the ratio of CFSE/positive populations in the spleen. The CFSE-labeled populations were distinguished from host populations by congenic markers.
BrdU labeling in vivo.
Mice were given 0.8 mg/ml BrdU (Sigma-Aldrich) in their drinking water for 7 d. Intracellular BrdU staining was performed by following the instructions of the BrdU Flow Kit (BD Biosciences).
LM-OVA infection, generation of LM-OVA–induced memory OT-I cells, and protection assay.
An erythromycin-resistant recombinant strain of L. monocytogenes that expresses a secreted form of OVA (LM-OVA) was provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) (51). LM-OVA was grown in Brain-Heart Infusion broth (BD Biosciences). At a midlog growth phase, culture samples were measured by OD and diluted in PBS for the desired titers. For preparation of LM-OVA–induced OT-I memory cells, mice that received 104 CD44lo CD122lo naive OT-I cells per mouse 1 or 2 d earlier were infected i.v. with 3,000 CFU of LM-OVA. These mice were used at least 50 d later. Engraftment of the donor cells at the time of assay was between 1 and 10% of total CD8+ T cells. For lethal infection, mice were inoculated i.v. with 105 CFU. For protection assay, organ suspension was prepared in 5 ml PBS, and 10-fold serial dilutions were made in PBS containing 0.1% NP-40. These 100-μl dilutions were plated onto the Brain-Heart Infusion plates containing 5 μg/ml erythromycin. The limit of detection was 50 CFU per organ.
Statistical analysis.
Data with logarithmic presentation were transformed to log10 (43). Statistical differences between groups were examined by a paired, two-tailed (for Fig. 3 B) or an unpaired, two-tailed Student's t test using Prism software (GraphPad). P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows the experimental protocols used for studying IL-2–anti–IL-2 complex–induced proliferation and for the generation of IL-2–anti–IL-2 complex memory and antigen-driven memory cells. Fig. S2 shows the proliferation of different kinds of TCR-transgenic CD8+ T cells induced by IL-2–anti–IL-2 complexes. Fig. S3 shows the surface staining and the functional defect in FcRγ−/−FcγRII−/− mice. Fig. S4 shows the protection against bacterial challenge afforded by an equal number of naive OT-I, IL-2–anti–IL-2 complex memory OT-I, or LM-OVA–induced memory OT-I. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070543/DC1.
Supplemental Material
Acknowledgments
We thank Beverly Dere and Xiao-cun Pan for excellent technical support.
This work was supported by the Howard Hughes Medical Institute and National Institutes of Health grant AI19335 (to M.J. Bevan).
The authors have no conflicting financial interests.
Abbreviations used: C57BL/6, B6; HP, homeostatic pro liferation; LM-OVA, Listeria monocytogenes–expressing soluble OVA.
References
- 1.Haring, J.S., V.P. Badovinac, and J.T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity. 25:19–29. [DOI] [PubMed] [Google Scholar]
- 2.Williams, M.A., and M.J. Bevan. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. [DOI] [PubMed] [Google Scholar]
- 3.Lin, J.X., and W.J. Leonard. 2000. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 19:2566–2576. [DOI] [PubMed] [Google Scholar]
- 4.Malek, T.R., and A.L. Bayer. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 4:665–674. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi, S., M. Ono, R. Setoguchi, H. Yagi, S. Hori, Z. Fehervari, J. Shimizu, T. Takahashi, and T. Nomura. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8–27. [DOI] [PubMed] [Google Scholar]
- 6.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657–679. [DOI] [PubMed] [Google Scholar]
- 7.D'Souza, W.N., and L. Lefrancois. 2003. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 171:5727–5735. [DOI] [PubMed] [Google Scholar]
- 8.Yu, A., J. Zhou, N. Marten, C.C. Bergmann, M. Mammolenti, R.B. Levy, and T.R. Malek. 2003. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J. Immunol. 170:236–242. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza, W.N., and L. Lefrancois. 2004. Frontline: An in-depth evaluation of the production of IL-2 by antigen-specific CD8 T cells in vivo. Eur. J. Immunol. 34:2977–2985. [DOI] [PubMed] [Google Scholar]
- 10.Teague, R.M., R.M. Tempero, S. Thomas, K. Murali-Krishna, and B.H. Nelson. 2004. Proliferation and differentiation of CD8+ T cells in the absence of IL-2/15 receptor beta-chain expression or STAT5 activation. J. Immunol. 173:3131–3139. [DOI] [PubMed] [Google Scholar]
- 11.Williams, M.A., A.J. Tyznik, and M.J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmann, M.F., P. Wolint, S. Walton, K. Schwarz, and A. Oxenius. 2007. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 37:1502–1512. [DOI] [PubMed] [Google Scholar]
- 13.D'Souza, W.N., K.S. Schluns, D. Masopust, and L. Lefrancois. 2002. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 168:5566–5572. [DOI] [PubMed] [Google Scholar]
- 14.Boyman, O., M. Kovar, M.P. Rubinstein, C.D. Surh, and J. Sprent. 2006. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 311:1924–1927. [DOI] [PubMed] [Google Scholar]
- 15.Kamimura, D., Y. Sawa, M. Sato, E. Agung, T. Hirano, and M. Murakami. 2006. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J. Immunol. 177:306–314. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 17.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 18.Ravetch, J.V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275–290. [DOI] [PubMed] [Google Scholar]
- 19.Nimmerjahn, F., P. Bruhns, K. Horiuchi, and J.V. Ravetch. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 23:41–51. [DOI] [PubMed] [Google Scholar]
- 20.Alegre, M.L., J.Y. Tso, H.A. Sattar, J. Smith, F. Desalle, M. Cole, and J.A. Bluestone. 1995. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J. Immunol. 155:1544–1555. [PubMed] [Google Scholar]
- 21.Vossen, A.C., G.J. Tibbe, M.J. Kroos, J.G. van de Winkel, R. Benner, and H.F. Savelkoul. 1995. Fc receptor binding of anti-CD3 monoclonal antibodies is not essential for immunosuppression, but triggers cytokine-related side effects. Eur. J. Immunol. 25:1492–1496. [DOI] [PubMed] [Google Scholar]
- 22.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prlic, M., and S.C. Jameson. 2002. Homeostatic expansion versus antigen-driven proliferation: common ends by different means? Microbes Infect. 4:531–537. [DOI] [PubMed] [Google Scholar]
- 24.Surh, C.D., and J. Sprent. 2005. Regulation of mature T cell homeostasis. Semin. Immunol. 17:183–191. [DOI] [PubMed] [Google Scholar]
- 25.Junghans, R.P., and C.L. Anderson. 1996. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA. 93:5512–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho, J.H., O. Boyman, H.-O. Kim, B. Hahm, M.P. Rubinstein, C.D. Surh, and J. Sprent. 2007. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J. Exp. Med. 204:1787–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troy, A.E., and H. Shen. 2003. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J. Immunol. 170:672–676. [DOI] [PubMed] [Google Scholar]
- 28.Kieper, W.C., J.T. Burghardt, and C.D. Surh. 2004. A role for TCR affinity in regulating naive T cell homeostasis. J. Immunol. 172:40–44. [DOI] [PubMed] [Google Scholar]
- 29.Kamimura, D., N. Ueda, Y. Sawa, S. Hachida, T. Atsumi, T. Nakagawa, S. Sawa, G.H. Jin, H. Suzuki, K. Ishihara, et al. 2004. Evidence of a novel IL-2/15R beta-targeted cytokine involved in homeostatic proliferation of memory CD8+ T cells. J. Immunol. 173:6041–6049. [DOI] [PubMed] [Google Scholar]
- 30.Kelly, J., R. Spolski, K. Imada, J. Bollenbacher, S. Lee, and W.J. Leonard. 2003. A role for Stat5 in CD8+ T cell homeostasis. J. Immunol. 170:210–217. [DOI] [PubMed] [Google Scholar]
- 31.Burchill, M.A., C.A. Goetz, M. Prlic, J.J. O'Neil, I.R. Harmon, S.J. Bensinger, L.A. Turka, P. Brennan, S.C. Jameson, and M.A. Farrar. 2003. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 171:5853–5864. [DOI] [PubMed] [Google Scholar]
- 32.Davis, R.S. 2007. Fc receptor-like molecules. Annu. Rev. Immunol. 25:525–560. [DOI] [PubMed] [Google Scholar]
- 33.Barnes, N., A.L. Gavin, P.S. Tan, P. Mottram, F. Koentgen, and P.M. Hogarth. 2002. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 16:379–389. [DOI] [PubMed] [Google Scholar]
- 34.Ghetie, V., and E.S. Ward. 2000. Multiple roles for the major histo compatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 18:739–766. [DOI] [PubMed] [Google Scholar]
- 35.Courtney, L.P., J.L. Phelps, and L.M. Karavodin. 1994. An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology. 28:223–232. [DOI] [PubMed] [Google Scholar]
- 36.Finkelman, F.D., K.B. Madden, S.C. Morris, J.M. Holmes, N. Boiani, I.M. Katona, and C.R. Maliszewski. 1993. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151:1235–1244. [PubMed] [Google Scholar]
- 37.May, L.T., R. Neta, L.L. Moldawer, J.S. Kenney, K. Patel, and P.B. Sehgal. 1993. Antibodies chaperone circulating IL-6. Paradoxical effects of anti-IL-6 “neutralizing” antibodies in vivo. J. Immunol. 151:3225–3236. [PubMed] [Google Scholar]
- 38.Stoklasek, T.A., K.S. Schluns, and L. Lefrancois. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donohue, J.H., and S.A. Rosenberg. 1983. The fate of interleukin-2 after in vivo administration. J. Immunol. 130:2203–2208. [PubMed] [Google Scholar]
- 40.Rubinstein, M.P., M. Kovar, J.F. Purton, J.H. Cho, O. Boyman, C.D. Surh, and J. Sprent. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha }. Proc. Natl. Acad. Sci. USA. 103:9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortier, E., A. Quemener, P. Vusio, I. Lorenzen, Y. Boublik, J. Grotzinger, A. Plet, and Y. Jacques. 2006. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J. Biol. Chem. 281:1612–1619. [DOI] [PubMed] [Google Scholar]
- 42.Waldmann, T.A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6:595–601. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton, S.E., M.C. Wolkers, S.P. Schoenberger, and S.C. Jameson. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7:475–481. [DOI] [PubMed] [Google Scholar]
- 44.Rocha, B., M.P. Lembezat, A. Freitas, and A. Bandeira. 1989. Interleukin 2 receptor expression and interleukin 2 production in exponentially growing T cells: major differences between in vivo and in vitro proliferating T lymphocytes. Eur. J. Immunol. 19:1137–1145. [DOI] [PubMed] [Google Scholar]
- 45.Cho, B.K., V.P. Rao, Q. Ge, H.N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kieper, W.C., M. Prlic, C.S. Schmidt, M.F. Mescher, and S.C. Jameson. 2001. IL-12 enhances CD8 T cell homeostatic expansion. J. Immunol. 166:5515–5521. [DOI] [PubMed] [Google Scholar]
- 47.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 48.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 49.Krutzik, P.O., M.R. Clutter, and G.P. Nolan. 2005. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J. Immunol. 175:2357–2365. [DOI] [PubMed] [Google Scholar]
- 50.Long, M., and A.J. Adler. 2006. Cutting edge: Paracrine, but not autocrine, IL-2 signaling is sustained during early antiviral CD4 T cell response. J. Immunol. 177:4257–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pope, C., S.K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.