Abstract
Neutrophil serine proteases (NSPs; elastase, cathepsin G, and proteinase-3) directly kill invading microbes. However, excess NSPs in the lungs play a central role in the pathology of inflammatory pulmonary disease. We show that serpinb1, an efficient inhibitor of the three NSPs, preserves cell and molecular components responsible for host defense against Pseudomonas aeruginosa. On infection, wild-type (WT) and serpinb1-deficient mice mount similar early responses, including robust production of cytokines and chemokines, recruitment of neutrophils, and initial containment of bacteria. However, serpinb1−/− mice have considerably increased mortality relative to WT mice in association with late-onset failed bacterial clearance. We found that serpinb1-deficient neutrophils recruited to the lungs have an intrinsic defect in survival accompanied by release of neutrophil protease activity, sustained inflammatory cytokine production, and proteolysis of the collectin surfactant protein–D (SP-D). Coadministration of recombinant SERPINB1 with the P. aeruginosa inoculum normalized bacterial clearance in serpinb1−/− mice. Thus, regulation of pulmonary innate immunity by serpinb1 is nonredundant and is required to protect two key components, the neutrophil and SP-D, from NSP damage during the host response to infection.
Neutrophils are the first and most abundant phagocytes mobilized to clear pathogenic bacteria during acute lung infection. Prominent among their antimicrobial weapons, neutrophils carry high concentrations of a unique set of serine proteases in their granules, including neu trophil elastase (NE), cathepsin G (CG), and proteinase-3. These neutrophil serine proteases (NSPs) are required to kill phagocytosed bacteria and fungi (1, 2). Indeed, neutrophils lacking NE fail to kill phagocytosed pathogens, and mice deficient for NE and/or CG have increased mortality after infection with pulmonary pathogens (3, 4). However, NSPs in the lung airspace can have a detrimental effect in severe inflammatory lung disease through degradation of host defense and matrix proteins (5–7). Thus, understanding of the mechanisms that regulate NSP actions during lung infections associated with neutrophilia will help identify strategies to balance host defense and prevent infection-induced tissue injury.
SERPINB1, also known as monocyte NE inhibitor (8), is an ancestral serpin super-family protein and one of the most efficient inhibitors of NE, CG, and proteinase-3 (9, 10). SERPINB1 is broadly expressed and is at particularly high levels in the cytoplasm of neutrophils (11, 12). SERPINB1 has been found complexed to neutro phil proteases in lung fluids of cystic fibrosis patients and in a baboon model of bronchopulmonary dysplasia (13, 14). Although these studies suggest a role for SERPINB1 in regulating NSP activity, it is unclear whether these complexes reflect an important physiological role for SERPINB1 in the lung air space.
RESULTS
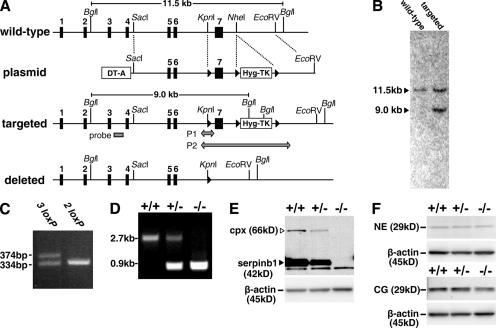
To define the physiological importance of SERPINB1 in shaping the outcome of bacterial lung infection, we generated mice deficient for serpinb1 (serpinb1−/−) by targeted mutagenesis in embryonic stem (ES) cells (Fig. 1, A–C). Crossings of heterozygous mice produced WT (+/+), heterozygous (+/−), and KO (−/−) mice for serpinb1 at expected Mendelian ratios (25% +/+, 51% +/−, and 24% −/−; n = 225; Fig. 1 D), indicating no embryonic lethality. Bone marrow neutrophils of serpinb1−/− mice lacked expression of the protein, whereas heterozygous serpinb1+/− mice had reduced levels compared with WT mice (Fig. 1 E). Importantly, levels of the cognate neutrophil proteases NE and CG, measured as antigenic units, were not altered by deletion of serpinb1 (Fig. 1 F). When maintained in a specific pathogen-free environment, serpinb1−/− mice did not differ from WT littermates in growth, litter size, or life span (followed up to 12 mo), and no gross or histopathological defects were observed at necropsy in 8-wk-old mice.
Figure 1.
Generation of serpinb1−/− mice. (A) The WT serpinb1 gene locus comprises seven exons (black rectangles). The targeting plasmid featured a negative selection cassette (DT-A) and a positive/negative selection cassette (Hyg-TK) flanked by two loxP sites (black triangles). The long homology arm also included a third loxP site in intron 6. Targeted ES clones selected for hygromycin resistance but not expressing DT-A were selected for homologous recombination by Southern blotting using an external probe in intron 3 (gray rectangle) after Bgl I restriction. Selected clones were further tested by PCR for the presence of the third loxP site in intron 6 (P1 arrow). ES clones with the deleted locus were identified by PCR (P2 arrow) after transient Cre expression and gancyclovir selection of targeted ES clones. (B) Southern blot of ES clones with WT or targeted loci. (C) PCR P1 was used to select homo logous recombination ES clones that contained the loxP site in intron 6 (denoted as 3 loxP). (D) PCR P2 of mouse tail genomic DNA was used to genotype WT (+/+), heterozygous (+/−), and homozygous (−/−) deleted serpinb1 mice. (E and F) Analysis of bone-marrow neutrophils. (E) Western blot analysis shows the native 42-kD serpinb1 (arrowhead) and the 66-kD serpinb1 complexed with neutrophil proteases (cpx). Protein molecular mass markers are indicated. Lower molecular mass nonspecific bands are also visible. (F) Western blot analysis show 29-kD NE (top) and CG (bottom). Blots were restained for β-actin to indicate equal protein loading.
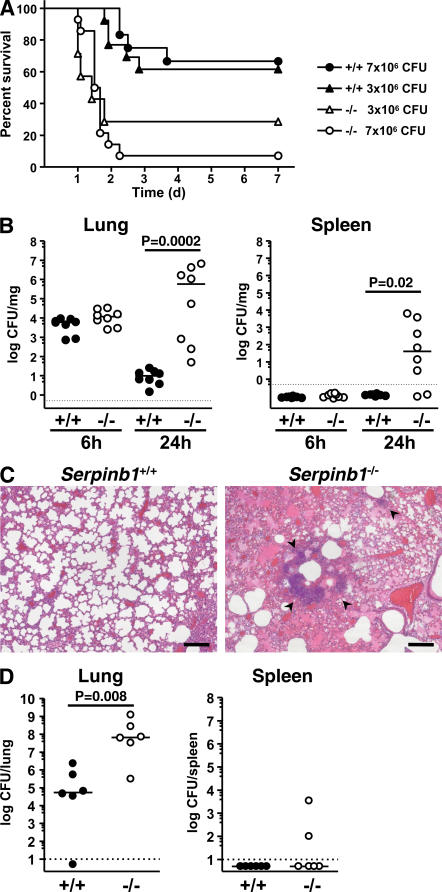
6–8-wk-old animals were intranasally inoculated with the nonmucoid Pseudomonas aeruginosa strain PAO1. Using two infection doses (3 × 106 and 7 × 106 CFU/mouse), serpinb1−/− mice had a significantly lower survival probability and a shorter median survival time compared with WT mice (Fig. 2 A). Further groups of infected mice were used to evaluate bacterial clearance. At 6 h after infection, the bacteria were similarly restricted in mice of the two genotypes, suggesting that the serpinb1−/− mice have a normal initial response to infection. At 24 h, the median bacterial count in the lungs of serpinb1−/− mice was five logs higher than that of the WT mice (P < 0.001), and the infection had spread systemically in serpinb1−/− mice but not in WT mice, as shown by high median CFU counts in the spleen (Fig. 2 B). Histological examination at 24 h after infection revealed abundant neutrophil infiltration in the lungs of both WT and serpinb1−/− mice, and consistent with the bacteriological findings, numerous foci of bacterial colonies and large areas of alveolar exudates were found in serpinb1−/− mice only (Fig. 2 C). When challenged with the mucoid P. aeruginosa clinical strain PA M57-15 isolated from a cystic fibrosis patient, WT mice cleared >99.9% of the inoculum within 24 h, whereas serpinb1-deficient mice failed to clear the infection (Fig. 2 D). Thus, the NSP inhibitor serpinb1 is essential for maximal protection against pneumonia induced by mucoid and nonmucoid strains of P. aeruginosa.
Figure 2.
Serpinb1−/− mice fail to clear P. aeruginosa lung infection. (A) Kaplan-Meier survival curves of WT (+/+) and serpinb1-deficient (−/−) mice intranasally inoculated with nonmucoid P. aeruginosa strain PAO1. Increased mortality of serpinb1−/− mice was statistically significant (P = 0.03 at 3 × 106 CFU/mouse; P < 0.0001 at 7 × 106 CFU/mouse). (B) CFUs per milligram of lung (left) and splenic (right) tissue determined 6 and 24 h after inoculation with 3 × 106 CFU P. aeruginosa PAO1 in WT (+/+, filled circles) and serpinb1−/− (−/−, open circles) mice. Each symbol represents a value for an individual mouse. Differences between median values (horizontal lines) were analyzed by the Mann-Whitney U test. Data below the limit of detection (dotted line) are plotted as 0.5 CFU × dilution factor. (C) Lung sections stained with hematoxylin and eosin show bacterial colonies (arrowheads) and alveolar exudate in lungs of serpinb1−/− mice 24 h after infection with P. aeruginosa PAO1. Bars, 50 μm. (D) Total CFUs in the lung and spleen 24 h after inoculation with 2 × 108 CFU of the mucoid P. aeruginosa strain PA M57-15 in WT (+/+, filled circles) and serpinb1−/− (−/−, open circles) mice. Differences between median values (horizontal lines) were analyzed by the Mann-Whitney U test.
To verify specificity of the gene deletion, we tested whether delivering rSERPINB1 would correct the defective phenotype. Indeed, intranasal instillation of rSERPINB1 to serpinb1−/− mice at the time of inoculation significantly improved clearance of P. aeruginosa PAO1 from the lungs assessed at 24 h and reduced bacteremia compared with infected serpinb1−/− mice that received PBS instead of the recombinant protein (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20070494/DC1). We have previously demonstrated that rSERPINB1 has no effect on the growth of P. aeruginosa in vitro (15) and does not induce bacterial aggrega tion (16). Also, rSERPINB1 mixed with PAO1 had no effect on adherence of the bacteria to human bronchial epithelial and corneal epithelial cell lines (unpublished data). Therefore, the improved bacterial clearance in treated serpinb1−/− mice is not related to a direct antibacterial role for rSERPINB1 but rather to reducing injury induced by excess neutrophil proteases. In addition, previous in vivo studies in WT rats showed that rSERPINB1 can protect against elastase-induced lung injury (17) and accelerate bacterial clearance two- to threefold in the Pseudomonas agar bead model (15).
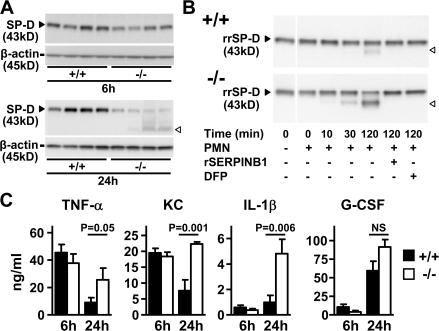
Evidence of excess NSP action was examined in the lungs of infected serpinb1−/− mice by measuring surfactant protein–D (SP-D). SP-D, a multimeric collagenous C-type lectin produced by alveolar epithelial cells, is highly relevant as a host defense molecule, because it functions as an opsonin in microbial clearance (18) and acts on alveolar macrophages to regulate pro- and antiinflammatory cytokine production (19). SP-D is also relevant as an NSP target because it is degraded in vitro by trace levels of each of the NSPs (16, 20). SP-D levels in lung homogenates of WT and serpinb1−/− mice were similar 6 h after P. aeruginosa infection. At 24 h, SP-D levels were reduced in the lungs of serpinb1−/− mice compared with WT mice, as indicated by immunoblots. A lower molecular mass band indicative of proteolytic degradation is also apparent (Fig. 3 A). Densitometry analysis of the 43-kD SP-D band relative to β-actin indicated that the reduction of SP-D level was statistically significant (+/+, 45 ± 6 [n = 8]; −/−, 10 ± 2 [n = 8]; P < 0.0001 according to the Student's t test). Furthermore, rSERPINB1 treatment of P. aeruginosa–infected serpinb1−/− mice partly prevented the degradation of SP-D in lung homogenates compared with nontreated mice (Fig. S1 B). As a further test of the impact of serpinb1 deletion on NSP activity, isolated neutrophils of serpinb1−/− mice were treated with LPS and FMLP and tested for their ability to cleave recombinant rat SP-D (rrSP-D) in vitro. The extent of rrSP-D cleavage by serpinb1−/− neutrophils was fourfold greater than by WT neutrophils, as determined by densitometry. The cleavage was specific for NSPs because it was abrogated by rSERPINB1 and diisopropyl fluorophosphate (Fig. 3 B). Collectively, these findings indicate a direct role for serpinb1 in regulating NSP activity released by neutrophils and in preserving SP-D, an important-host defense molecule.
Figure 3.
Proteolysis of SP-D and cytokine production in lungs of P. aeruginosa PAO1–infected mice. (A) Western blot analysis of SP-D (43 kD, black arrowheads) in disulfide-reduced lung homogenate samples at 6 and 24 h after infection. White arrowheads show the SP-D proteolytic cleavage product. Blots were restained for β-actin. (B) Increased in vitro proteolysis of rrSP-D by NSPs of serpinb1−/− PMNs compared with WT PMNs. (C) TNF-α, KC, IL-1β, and G-CSF were measured in BAL fluid of WT (+/+) and serpinb1-deficient (−/−) mice at 6 and 24 h after infection with P. aeruginosa (6 × 106 CFU/mouse; n = 6 per group). Data represent means ± SEM. Representative data from two or more independent experiments are shown (A–C). White lines indicate that intervening lanes have been spliced out.
Efficient clearance of P. aeruginosa infection requires an early cytokine and chemokine response coordinated by both resident alveolar macrophages and lung parenchymal cells (21, 22). The IL-8 homologue keratinocyte-derived chemokine (KC) and the cytokines TNF-α, IL-1β, and G-CSF were measured in cell-free bronchoalveolar (BAL) samples. Although the tested cytokines were undetectable in sham-infected mice of both genotypes (unpublished data), comparable induc tion of these cytokines was observed in BAL of WT and serpinb1−/− mice at 6 h after infection, demonstrating that there is no early defect in cytokine production in serpinb1−/− mice. At 24 h, levels of TNF-α, KC, and IL-1β were sustained or increased in serpinb1−/− mice and significantly higher than cytokine levels in WT mice. G-CSF levels at 24 h were elevated to a similar extent in BAL of WT and KO mice (Fig. 3 C). However, G-CSF levels were significantly higher in the serum of serpinb1−/− mice (WT, 336 ± 80 ng/ml; KO, 601 ± 13 ng/ml; n = 6 of each genotype; P < 0.01). In addition, serpinb1−/− mice that were treated at the time of infection with rSERPINB1 had cytokine levels in 24-h lung homogenates that were indistinguishable from those of infected WT mice (Fig. S1 C). The increased cytokine production in the lungs of infected serpinb1−/− mice may be caused by failed bacterial clearance but also by excess NSPs, which directly induce cytokine and neutrophil chemokine production in pulmonary parenchymal cells and alveolar macrophages (23, 24).
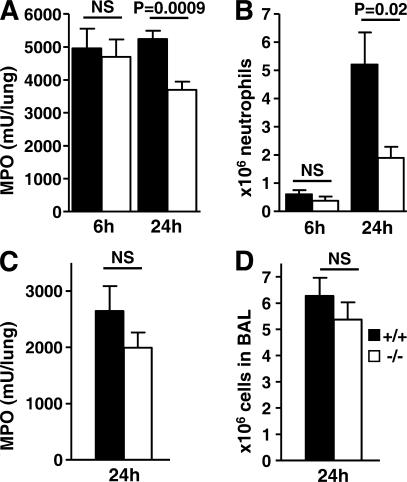
Neutrophil recruitment to the lungs was next examined as a pivotal event of the response to P. aeruginosa infection (25). Lung homogenates were assayed for the neutrophil-specific enzyme myeloperoxidase (MPO) to quantify marginating, interstitial, and alveolar neutrophils. Neutrophils in BAL fluid were directly counted as a measure of neutrophil accumulation in the alveolar and airway lumen. MPO in lung homo genates was undetectable in uninfected mice and was comparably increased in mice of both genotypes at 6 h, suggesting normal early serpinb1−/− neutrophil margination and migration into the interstitium. However, by 24 h after infection, MPO levels in lung homogenates remained high in WT mice but were significantly decreased in serpinb1−/− mice (Fig. 4 A). Importantly, the content of MPO per cell was the same for isolated neutrophils of WT and serpinb1−/− mice (+/+, 369 ± 33 mU/106 cells; −/−, 396 ± 27 mU/106 cells). The numbers of neutrophils in BAL were negligible in uninfected mice and were similarly increased in WT and serpinb1−/− mice at 6 h after infection. Neutrophil counts in BAL further increased at 24 h, but the mean BAL neutrophil numbers were significantly lower in serpinb1−/− mice compared with WT mice (Fig. 4 B). The evidence from the 6-h quantitation of MPO in homogenates and neutrophils in BAL strongly suggests that neutrophil recruitment is not defective in infected serpinb1−/− mice. Moreover, the high levels of cytokines and neutrophil chemoattractant KC in serpinb1−/− mice at 24 h (Fig. 3 C) also suggest that, potentially, more neutrophils should be recruited. Therefore, to examine neutrophil recruitment in serpinb1−/− mice, we used a noninfectious model in which neutrophils are mobilized to migrate to the lung after intranasal delivery of P. aeruginosa LPS. MPO levels in lung homogenate and neutrophil numbers in BAL were not statistically different in WT and serpinb1−/− mice 24 h after LPS instillation (Fig. 4, C and D). Furthermore, the number of circulating blood neutrophils and recruited peritoneal neutrophils after injection of sterile irritants glycogen and thioglycollate did not differ in WT and serpinb1−/− mice (unpublished data). Alveolar macrophage numbers were similar in uninfected mice of both genotypes (∼5 × 105 cells/mouse) and did not substantially change upon infection. Collectively, these findings show that neutrophil recruitment to the lungs in response to P. aeruginosa infection is not defective in serpinb1−/− mice, and therefore, the recovery of lower numbers of serpinb1−/− neutrophils at 24 h after infection suggests their decreased survival.
Figure 4.
Neutrophil recruitment to the lungs upon challenge with P. aeruginosa and LPS. (A) Neutrophil (PMN) sequestration in lungs assessed by MPO activity in total-lung homogenate and (B) PMN counts in BAL were determined at 6 and 24 h after infection with P. aeruginosa PAO1 (n = 6–8). (C) PMN sequestration and (D) BAL PMN 24 h after intranasal instillation of 10 μg LPS (n = 4–9). Means ± SEM are shown, and data were analyzed by the unpaired t test.
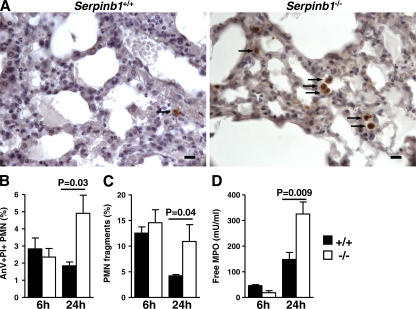
To examine the putative increased death of serpinb1−/− neutrophils in the lungs after P. aeruginosa infection, lung sections were analyzed by immunohistochemistry. Caspase-3–positive leukocytes were more relevant in the alveolar space of serpinb1−/− mice compared with WT mice at 24 h after infection, suggesting increased neutrophil apoptosis (Fig. 5 A). The positive cells were counted in 50 high power fields (hpf's), and mean numbers of caspase-3–stained cells were increased in the lungs of serpinb1−/− mice (1.8 ± 0.2 cells/hpf) compared with WT mice (0.4 ± 0.1 cells/hpf; P < 0.0001). To characterize neutrophils in the alveoli and airways, neutrophils in BAL were identified in flow cytometry by forward scatter (FSC) and side scatter and were stained with annexin V (AnV) and propidium iodide (PI). At 24 h after infection, the proportion of late apoptotic/necrotic neutrophils (AnV+PI+) was increased at the expense of viable neutrophils (AnV−PI−) in the BAL of serpinb1−/− mice compared with WT mice (Fig. 5 B). Neutrophil fragments in BAL were also identified in flow cytometry by low FSC (FSClow) within the neutrophil population defined by the neutrophil marker Gr-1. The number of neutrophil fragments (FSClow, Gr-1+) relative to intact neutrophils was increased two- to threefold at 24 h after infection for serpinb1−/− compared with WT mice (Fig. 5 C). Moreover, free MPO in BAL supernatants was increased in serpinb1−/− mice compared with WT mice at 24 h after infection, indicating increased PMN lysis or degranulation (Fig. 5 D).
Figure 5.
Increased death of recruited lung serpinb1−/− neutrophils in vivo. (A) Active caspase-3 staining of leukocytes (arrows) in alveolar space detected by immunohistochemistry at 24 h after infection with P. aeruginosa PAO1. Bars, 20 μm. (B) The percentage of late apoptotic neutrophils (PMNs; AnV+PI+) in BAL of P. aeruginosa–infected mice. (C) PMN fragments defined in flow cytometry by low FSC within the Gr1+ population (Gr1+FSClow). (D) MPO in cell-free BAL supernatant. Means ± SEM are shown (n = 4–6), and the data were analyzed by the unpaired t test.
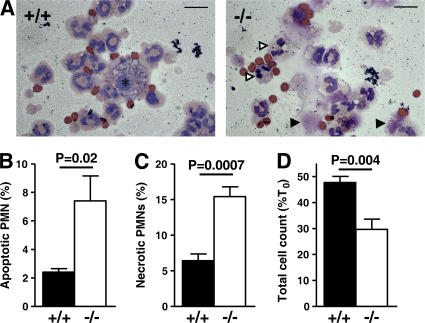
Finally, we questioned whether the enhanced death of serpinb1−/− pulmonary neutrophils was a primary effect of gene deletion or a secondary effect caused by, for example, bacteria or components of inflammation. To address this, neutrophils were collected using the noninfectious LPS recruitment model and were cultured in vitro to allow for spontaneous cell death. After 24 h, the percentages of apoptotic and necrotic neutrophils evaluated by microscopy were increased in serpinb1−/− neutrophils compared with WT neutrophils (Fig. 6, A–C). A similar increase in apoptotic cells was observed using AnV/PI staining and measurements of hypodiploid DNA (unpublished data). Moreover, live cell numbers from serpinb1−/− mice remaining in culture after 24 h were significantly decreased compared with WT mice (Fig. 6 D). The in vitro findings indicate that enhanced death of pulmonary neutrophils of infected serpinb1−/− mice is at least in part a cell-autonomous defect likely mediated by unchecked NSP actions.
Figure 6.
Increased apoptosis and necrosis of serpinb1−/− neutrophils in vitro. (A) Representative cytospins of LPS-recruited BAL cells after 24 h of in vitro culture. Macrophages are indicated by an asterisk, and apoptotic and lytic PMNs are indicated by white and black arrowheads, respectively. Bars, 10 μm. The percentage of PMNs with characteristic apoptotic (B) or lytic (C) morphologies relative to total PMNs after 24 h of in vitro culture. (D) The percentage of cells remaining after 24 h in culture. The means ± SEM of five experiments are shown, and data were analyzed by the unpaired t test (B–D).
DISCUSSION
In this paper, we have demonstrated that serpinb1, an intracellular serpin family member, regulates the innate immune response and protects the host during lung bacterial infection. Serpinb1 is among the most potent inhibitors of NSPs and is carried at high levels within neutrophils. Serpinb1-deficient mice fail to clear P. aeruginosa PAO1 lung infection and succumb from systemic bacterial spreading. The defective immune function in serpinb1−/− mice stems at least in part from an increased rate of neutrophil necrosis, reducing the number of phagocytes and leading to increased NSP activity in the lungs with proteolysis of SP-D. In addition, serpinb1-deficient mice also have impaired clearance of the mucoid clinical strain PA M57-15. Interestingly, mucoid strains of P. aeruginosa are cleared with a very high efficiency from the lungs of WT and cystic fibrosis transmembrane conductance regulator–deficient mice (26). The phenotype of serpinb1−/− mice reproduces major pathologic features of human pulmonary diseases characterized by excessive inflammation, massive neutrophil recruitment to the air space, and destruction of cellular and molecular protective mechanisms. Importantly, serpinb1 deficiency may be helpful as an alternative or additional model of the inflammatory lung pathology of cystic fibrosis.
The present study documents a key protective role for serpinb1 in regulating NSP actions in the lung. This role has previously been attributed to the NSP inhibitors α1-antitrypsin and secretory leukocyte protease inhibitor, which are found in the airway and alveolar lining fluid (27, 28). However, patients with α1-antitrypsin deficiency do not present with pulmonary infection secondary to innate immune defects despite increased NSP activity that leads to reduced lung elasticity and emphysema. Moreover, there is so far no evidence that deficiency in secretory leukocyte protease inhibitor results in failure to clear pulmonary infection. Because synthesis and storage of NSPs in granules is an event that exclusively takes place in bone marrow promyelocytes (29), the regulation of NSPs in the lung relies entirely on NSP inhibitors. Thus, the extent of the innate immune defect in serpinb1−/− mice and the normalization of bacterial clearance with topical rSERPINB1 treatment indicate that serpinb1 is required to regulate NSP activity in the airway fluids and that, during acute lung infection associated with high neutrophilic recruitment, there is insufficient compensation by other NSP inhibitors. The devastating effects of NSPs when released in the lungs by degranulating and necrotic neutrophils are well documented in human pulmonary diseases (5, 6, 30). Therefore, our findings clearly establish a physiological and nonredundant role for serpinb1 in regulating NSPs during pulmonary infection.
NSPs also cleave molecules involved in apoptotic cell clearance, including the surfactant protein SP-D and the phosphatidylserine receptor on macrophages (31, 32), thereby tipping the balance further toward a detrimental outcome. The increased numbers of leukocytes with active caspase-3 in the alveolar space of P. aeruginosa–infected serpinb1−/− mice suggest that the removal of apoptotic cells may be inadequate during infection. SP-D has been shown to stimulate phagocytosis of P. aeruginosa by alveolar macrophages in vitro (33), and SP-D–deficient mice were found to have defective early (6-h) clearance of P. aeruginosa from the lung (34). Although the destruction of SP-D alone may not entirely account for the defective phenotype of serpinb1−/− mice, loss of SP-D likely diminishes bacterial clearance and removal of apop totic neutrophils.
Given that NSPs also mediate bacterial killing, why would NSP excess lead to a failed bacterial clearance? In the NE KO mice, the decreased killing activity of neutrophils is a direct consequence of the loss of the bactericidal activity of NE. The absence of an early bacterial clearance defect at 6 h after infection in serpinb1−/− mice suggests that there is initially normal bacterial killing. The current understanding is that the compartmentalization of the NSPs is crucial to the outcome of their actions: on the one hand, NSPs are protective when killing microbes within phagosomes, and on the other hand, extracellular NSPs destroy innate immune defense molecules such as lung collectins, immunoglobulins, and complement receptors. We have shown that the regulation of NSP activity is essential and that cytoplasmic serpinb1 provides this crucial shield. Neutrophils undergoing cell death gradually transition from apoptosis, characterized by a nonpermeable plasma mem brane, to necrosis and lysis, where cellular and granule contents, including NSPs, are released. The increased pace of serpinb1−/− neutrophil cell death strongly suggests that unopposed NSPs may precipitate neutrophil demise and, therefore, reduce the neutrophil numbers leading to a late-onset innate immune defect. High levels of G-CSF, a prosurvival cytokine for neutrophils, also indicate that increased cell death is likely independent or downstream of G-CSF.
In conclusion, serpinb1 deficiency unleashes unbridled proteolytic activity during inflammation and thereby disables two critical components of the host response to bacterial infection, the neutrophil and the collectin SP-D. The phenotype of the infected serpinb1-deficient mouse, characterized by a normal early antibacterial response that degenerates over time, highlights the delicate balance of protease–antiprotease systems that protect the host against its own defenses as well as invading microbes during infection-induced inflammation.
MATERIALS AND METHODS
Generation of Serpinb1-deficient mice.
The targeting plasmid was designed to disrupt the serpinb1 gene by deletion of exon 7, which encodes 35% of the protein, including the reactive center loop (11). The deletion strategy, schematized in Fig. 1 A, was based on a three loxP site construct and a two-step transfection and selection method (35), using a negative selection cassette (DT-A) (36) and a positive/negative selection cassette (Hyg-TK) (37), provided by J. Shen (Brigham and Women's Hospital, Boston, MA) and C. Beard (Whitehead Institute, Cambridge, MA), respectively. Mouse genomic DNA used in the targeting vector was from a P1-derived artificial chromosome (PAC) clone (A11) from a 129S6/SvEvTac (129S6) background provided by P. Bird (Monash University, Melbourne, Australia). The targeting plasmid was electroporated in 129S6/W4 ES cells (Taconic Farms). ES clones resistant for hygromycin but not expressing DT-A were selected for homologous recombination by Southern blotting using Bgl I restriction and an external probe in intron 3. Selected clones were further tested by PCR P1 for the presence of the loxP site in intron 6. ES clones with the deleted locus were generated from targeted ES clones with three loxP sites by transient Cre expression, including selection with gancyclovir and screening by PCR P2. Two independent clones with a deleted locus were injected into blastocysts, and each generated multiple germline chimeras, which were crossed to WT 129S6 to obtain two independent strains of serpinb1−/− in a pure 129S6 background. The two KO lines were phenotypically identical. All animal studies were approved by the Animal Care and Use Committee of the CBR Institute for Biomedical Research.
Inoculum preparation.
P. aeruginosa strain PAO1, an LPS-smooth laboratory strain originally isolated from a burn wound, was provided by G. Pier (Brigham and Women's Hospital, Boston, MA). P. aeruginosa strain PA M57-15 is a mucoid strain isolated from a cystic fibrosis patient and was provided by A. van Heeckeren (Case Western Reserve University, Cleveland, OH). For each experiment, the bacterial inoculum was freshly prepared from a frozen glycerol stock and grown overnight on tryptic soy agar (TSA). The inoculum was washed in PBS, and the concentration was adjusted by spectro photometry. The actual CFU in the inoculum was determined each time by plating serial dilutions on TSA. Mice were inoculated with 3–8 × 106 CFU/mouse for PAO1 and 2 × 108 CFU/mouse for PA M57-15. Purified human rSERPINB1 in PBS was prepared as previously described (38) and was premixed with the inoculum before intranasal instillation. Adherence assay of PAO1 in the presence of rSERPINB1 were performed as previously described (39).
Lung infection studies.
Groups of mice were sedated with 100 mg/kg ketamine and 10 mg/kg xylazine and intranasally inoculated by applying 10 μl of inoculum onto each nare. In survival studies, mice were monitored at least every 6 h for 7 d. In other studies, mice were killed at the times indicated in the figures for tissue and body fluid harvest. The dissected spleen and right lung were homogenized, serially diluted in 1% proteose peptone, and plated in duplicate on TSA. Aliquots of lung homogenates were stored at −80°C until further analysis. Left lungs were fixed in Bouin's fixative and processed for immunohistochemistry.
LPS model.
Alveolar recruitment of neutrophils was measured in response to 10 μg P. aeruginosa LPS (Sigma-Aldrich) intranasally instilled in sedated mice as described in the previous section.
BAL.
Tracheas were canulated with an 18-gauge angiocath. Lungs were lavaged five times with 0.8 ml of cold sterile PBS. An analysis of resident and recruited cells in response to infection or LPS was performed on cells pooled from the five washes. For studies of cytokines and free MPO in BAL fluid, the first wash was collected separately and centrifuged, and the supernatant was stored at −80°C until analysis.
ELISA.
Concentrations of cytokines and chemokines (TNF-α, IL-1β, KC, and G-CSF) in BAL or lung homogenate were measured in duplicate by ELISA (BioPlex; Bio-Rad Laboratories and R&D Systems).
MPO assay.
MPO activity was measured in triplicate using a spectrophotometric assay in 50 mM potassium phosphate, pH 6, containing 0.167 mg/ml o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide.
Flow cytometry.
Whole-blood, spleen, bone marrow, BAL, and peritoneal cells were stained with fluorochrome-labeled monoclonal antibodies and analyzed by a flow cytometer FACSCalibur (BD Biosciences). Phosphatidyl serine exposure and viability were assessed by AnV and PI staining.
Western blotting and SP-D proteolysis.
Lung homogenates and BAL were resolved by SDS-PAGE under reducing conditions and immunoblotted using rabbit antiserum to NE (Calbiochem), CG (provided by T. Ley, Washington University, St. Louis, MO) (40), SERPINB1 (17), or SP-D (Chemicon). Blots were stripped and restained with rabbit anti–β-actin antibody (Cell Signaling Technology). rrSP-D dodecamers were provided by E. Crouch (Washington University, St. Louis, MO). In vitro activation of neutrophils and proteolysis of SP-D was performed as previously described (16).
Immunohistochemistry.
Tissue sections of fixed and paraffin-embedded lungs were stained with a rabbit monoclonal antibody specific for active caspase-3 (clone 5A1; Cell Signaling Technology), using the manufacturer's recommendations, and detected with the ABC reagents (Vector Laboratories).
Neutrophil culture.
LPS-recruited neutrophils were washed in IMDM containing 1% FCS, 2 mM l-glutamine, 20mM Hepes, and antibiotics. Neutrophils were 85–95% pure, and the remaining cells mainly consisted of alveolar macrophages and ∼1% lymphocytes and eosinophils. Cells were seeded at 1–2 × 106 cells/ml in teflon beakers at 37°C. After 24 h, cells were counted, and cytospin preparations were stained with Wright-Giemsa.
Statistical analysis.
All analyses were performed using Prism software (GraphPad). Kaplan-Meier survival curves were analyzed by the log-rank test. Differences in median bacterial CFUs per milligram of tissue were analyzed by the Mann-Whitney U test. Other data were expressed as means ± SEM and were analyzed by the unpaired Student's t test. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 demonstrates the effect of delivering rSERPINB1 to serpinb1−/− mice inoculated with 8 × 106 CFU of P. aeruginosa. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070494/DC1.
Supplemental Material
Acknowledgments
We thank Jessica Cooley and Minghe Ma for their excellent technical support. We are indebted to Tanweer Zaidi for her help with adherence assays. We are grateful to Alvin Davis for critical discussions. We are grateful to Michael Carroll for advice and help with ES cell work. We are grateful to Steven Shapiro, Heinz Remold, Manjunath Swamy, and Lisa Westerberg for critical review of the manuscript. We thank the following people for reagents: Caroline Beard and Jie Shen (plasmids), Phillip Bird (PAC clones), Erika Crouch (rrSP-D), Timothy Ley (CG antibody), and Gerald Pier and Anna van Heeckeren (P. aeruginosa strains). We thank Joan Stein-Streilein for access to equipment (BioPlex reader).
This study was supported by the National Institutes of Health (grant no. HL66548 to E. Remold-O'Donnell and grant nos. K08 AI50036 and R21 HL79423 to G.P. Priebe) and by a grant from the Cystic Fibrosis Foundation (E. Remold-O'Donnell). C. Benarafa is a Parker B. Francis Fellow in Pulmonary Research.
The authors have no conflicting financial interests.
Abbreviations used: AnV, annexin V; BAL, bronchoalveolar; CG, cathepsin G; ES, embryonic stem; FSC, forward scatter; hpf, high power field; KC, keratino cyte-derived chemokine; MPO, myeloperoxidase; NE, neutrophil elastase; NSP, neutrophil serine protease; PI, propidium iodide; rrSP-D, recombinant rat SP-D; SP-D, surfactant protein–D; TSA, tryptic soy agar.
References
- 1.Reeves, E.P., H. Lu, H.L. Jacobs, C.G. Messina, S. Bolsover, G. Gabella, E.O. Potma, A. Warley, J. Roes, and A.W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297. [DOI] [PubMed] [Google Scholar]
- 2.Belaaouaj, A., K.S. Kim, and S.D. Shapiro. 2000. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 289:1185–1188. [DOI] [PubMed] [Google Scholar]
- 3.Belaaouaj, A., R. McCarthy, M. Baumann, Z. Gao, T.J. Ley, S.N. Abraham, and S.D. Shapiro. 1998. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4:615–618. [DOI] [PubMed] [Google Scholar]
- 4.Tkalcevic, J., M. Novelli, M. Phylactides, J.P. Iredale, A.W. Segal, and J. Roes. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 12:201–210. [DOI] [PubMed] [Google Scholar]
- 5.Chmiel, J.F., and P.B. Davis. 2003. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir. Res. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taggart, C.C., C.M. Greene, T.P. Carroll, S.J. O'Neill, and N.G. McElvaney. 2005. Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. Am. J. Respir. Crit. Care Med. 171:1070–1076. [DOI] [PubMed] [Google Scholar]
- 7.Moraes, T.J., J.H. Zurawska, and G.P. Downey. 2006. Neutrophil granule contents in the pathogenesis of lung injury. Curr. Opin. Hematol. 13:21–27. [DOI] [PubMed] [Google Scholar]
- 8.Remold-O'Donnell, E., J. Chin, and M. Alberts. 1992. Sequence and molecular characterization of human monocyte/neutrophil elastase inhibitor. Proc. Natl. Acad. Sci. USA. 89:5635–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benarafa, C., and E. Remold-O'Donnell. 2005. The ovalbumin serpins revisited: perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc. Natl. Acad. Sci. USA. 102:11367–11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooley, J., T.K. Takayama, S.D. Shapiro, N.M. Schechter, and E. Remold-O'Donnell. 2001. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry. 40:15762–15770. [DOI] [PubMed] [Google Scholar]
- 11.Benarafa, C., J. Cooley, W. Zeng, P.I. Bird, and E. Remold-O'Donnell. 2002. Characterization of four murine homologs of the human ov-serpin monocyte neutrophil elastase inhibitor MNEI (SERPINB1). J. Biol. Chem. 277:42028–42033. [DOI] [PubMed] [Google Scholar]
- 12.Bird, C.H., E.J. Blink, C.E. Hirst, M.S. Buzza, P.M. Steele, J. Sun, D.A. Jans, and P.I. Bird. 2001. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol. Cell. Biol. 21:5396–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooley, J., F. Rubio, M. Sontag, I. Osberg, F. Accurso, and E. Remold-O'Donnell. 2001. MNEI (monocyte/neutrophil elastase inhibitor) is found at increased levels in cystic fibrosis lavage fluid. Pediatr. Pulmonol. Suppl.22:271a. [Google Scholar]
- 14.Yasumatsu, R., O. Altiok, C. Benarafa, C. Yasumatsu, G. Bingol-Karakoc, E. Remold-O'Donnell, and S. Cataltepe. 2006. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:619–627. [DOI] [PubMed] [Google Scholar]
- 15.Woods, D.E., A. Cantin, J. Cooley, D.M. Kenney, and E. Remold-O'Donnell. 2005. Aerosol treatment with MNEI suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Pediatr. Pulmonol. 39:141–149. [DOI] [PubMed] [Google Scholar]
- 16.Hirche, T.O., E.C. Crouch, M. Espinola, T.J. Brokelman, R.P. Mecham, N. DeSilva, J. Cooley, E. Remold-O'Donnell, and A. Belaaouaj. 2004. Neutrophil serine proteinases inactivate surfactant protein D by cleaving within a conserved subregion of the carbohydrate recognition domain. J. Biol. Chem. 279:27688–27698. [DOI] [PubMed] [Google Scholar]
- 17.Rees, D.D., R.A. Rogers, J. Cooley, R.J. Mandle, D.M. Kenney, and E. Remold-O'Donnell. 1999. Recombinant human monocyte/neutrophil elastase inhibitor protects rat lungs against injury from cystic fibrosis airway secretions. Am. J. Respir. Cell Mol. Biol. 20:69–78. [DOI] [PubMed] [Google Scholar]
- 18.Crouch, E., and J.R. Wright. 2001. Surfactant proteins A and D and pulmonary host defense. Annu. Rev. Physiol. 63:521–554. [DOI] [PubMed] [Google Scholar]
- 19.Gardai, S.J., Y.Q. Xiao, M. Dickinson, J.A. Nick, D.R. Voelker, K.E. Greene, and P.M. Henson. 2003. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 115:13–23. [DOI] [PubMed] [Google Scholar]
- 20.von Bredow, C., A. Wiesener, and M. Griese. 2003. Proteolysis of surfactant protein D by cystic fibrosis relevant proteases. Lung. 181:79–88. [DOI] [PubMed] [Google Scholar]
- 21.Hajjar, A.M., H. Harowicz, H.D. Liggitt, P.J. Fink, C.B. Wilson, and S.J. Skerrett. 2005. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am. J. Respir. Cell Mol. Biol. 33:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kooguchi, K., S. Hashimoto, A. Kobayashi, Y. Kitamura, I. Kudoh, J. Wiener-Kronish, and T. Sawa. 1998. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect. Immun. 66:3164–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura, H., K. Yoshimura, N.G. McElvaney, and R.G. Crystal. 1992. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J. Clin. Invest. 89:1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok, V.A., D.L. Bratton, L. Guthrie, and P.M. Henson. 2001. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J. Immunol. 166:6847–6854. [DOI] [PubMed] [Google Scholar]
- 25.Rehm, S.R., G.N. Gross, and A.K. Pierce. 1980. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J. Clin. Invest. 66:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Heeckeren, A.M., M.D. Schluchter, W. Xue, and P.B. Davis. 2006. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am. J. Respir. Crit. Care Med. 173:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelmeier, C., R.C. Hubbard, G.A. Fells, H.P. Schnebli, R.C. Thompson, H. Fritz, and R.G. Crystal. 1991. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J. Clin. Invest. 87:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoller, J.K., and L.S. Aboussouan. 2005. Alpha1-antitrypsin deficiency. Lancet. 365:2225–2236. [DOI] [PubMed] [Google Scholar]
- 29.Fouret, P., R.M. du Bois, J.F. Bernaudin, H. Takahashi, V.J. Ferrans, and R.G. Crystal. 1989. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J. Exp. Med. 169:833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraes, T.J., J. Plumb, R. Martin, E. Vachon, V. Cherepanov, A. Koh, C. Loeve, J. Jongstra-Bilen, J.H. Zurawska, J.V. Kus, et al. 2006. Abnormalities in the pulmonary innate immune system in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 34:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandivier, R.W., V.A. Fadok, P.R. Hoffmann, D.L. Bratton, C. Penvari, K.K. Brown, J.D. Brain, F.J. Accurso, and P.M. Henson. 2002. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 109:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandivier, R.W., C.A. Ogden, V.A. Fadok, P.R. Hoffmann, K.K. Brown, M. Botto, M.J. Walport, J.H. Fisher, P.M. Henson, and K.E. Greene. 2002. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169:3978–3986. [DOI] [PubMed] [Google Scholar]
- 33.Restrepo, C.I., Q. Dong, J. Savov, W.I. Mariencheck, and J.R. Wright. 1999. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 21:576–585. [DOI] [PubMed] [Google Scholar]
- 34.Giannoni, E., T. Sawa, L. Allen, J. Wiener-Kronish, and S. Hawgood. 2006. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 34:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, H., J. Kessler, and J. Shen. 2000. Heterogeneous populations of ES cells in the generation of a floxed Presenilin-1 allele. Genesis. 26:5–8. [PubMed] [Google Scholar]
- 36.Jackson-Grusby, L., C. Beard, R. Possemato, M. Tudor, D. Fambrough, G. Csankovszki, J. Dausman, P. Lee, C. Wilson, E. Lander, and R. Jaenisch. 2001. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat. Genet. 27:31–39. [DOI] [PubMed] [Google Scholar]
- 37.Soriano, P. 1997. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 124:2691–2700. [DOI] [PubMed] [Google Scholar]
- 38.Cooley, J., B. Mathieu, E. Remold-O'Donnell, and R.J. Mandle. 1998. Production of recombinant human monocyte/neutrophil elastase inhibitor (rM/NEI). Protein Expr. Purif. 14:38–44. [DOI] [PubMed] [Google Scholar]
- 39.Pier, G.B., M. Grout, and T.S. Zaidi. 1997. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA. 94:12088–12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacIvor, D.M., S.D. Shapiro, C.T. Pham, A. Belaaouaj, S.N. Abraham, and T.J. Ley. 1999. Normal neutrophil function in cathepsin G- deficient mice. Blood. 94:4282–4293. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.