Abstract
Plasmacytoid dendritic cells (PDCs) play a pivotal role as cytokine-secreting accessory cells in the antimicrobial immune defense. In contrast, the capacity of PDCs to act as antigen-presenting cells in naive T cell priming remains unclear. By studying T cell responses in mice that lack conventional DCs (cDCs), and by the use of a PDC-specific antigen-targeting strategy, we show that PDCs can initiate productive naive CD4+ T cell responses in lymph nodes, but not in the spleen. PDC-triggered CD4+ T cell responses differed from cDC-driven responses in that they were not associated with concomitant CD8+ T cell priming. Our results establish PDCs as a bona fide DC subset that initiates unique CD4+ Th cell–dominated primary immune responses.
T lymphocytes respond to antigenic peptides presented on major MHC molecules. Cytotoxic CD8+ T cells recognize peptides in the context of MHC class I, whereas CD4+ Th cells recognize peptides in complex with MHC class II. Naive T cell activation is restricted to secondary lymphoid organs, such as the spleen and LNs, and is thought to rely on specialized “professional” APCs comprising B cells, macrophages, and DCs. The APC composition of lymphoid organs likely encodes a potential to initiate distinct T cell responses. However, the differential contribution of APC to in vivo immune responses remains poorly understood.
Natural IFN-producing cells (IPCs) are a unique subset of hematopoetic cells that play a critical role in the innate and adaptive immune defense against infections (1). Viruses (or unmethylated CpG DNA) engage Toll-like receptor (TLR) 7 and 9 on IPCs (2) and trigger the secretion of massive amounts of type I interferons (IFN-α, -β, -ω, and -λ) (3). This specialization of IPCs in cytokine production is reflected in their distinct “plasmacytoid” morphology, resembling Ig-secreting plasma cells (4). IPC-derived cytokines are critical for the initiation of early antiviral NK cell responses (5, 6). In addition, IPC-derived type I IFNs boost the ability of conventional DCs (cDCs) to mature and stimulate T cells, thus, promoting antiviral CTL responses (7, 8). Furthermore, IPCs provide a critical costimulatory signal for the CpG-induced activation of cDCs (9, 10). Independent of their cytokine- and CD40L-mediated accessory function to control NK and cDC activities, IPCs have also been proposed to play a direct role as APCs in the onset of T cell stimulation. The constitutive presence of IPCs in lymphoid organs, their expression of MHC molecules, and their acquisition of DC morphology upon culture, has led to the idea that IPCs represent a distinct DC subset, the so-called plasmacytoid DCs (PDCs). However, the original discovery of DCs defined them by the capacity to prime naive T cells (11), and this activity has remained their hallmark. Moreover, recent in vivo DC depletion experiments indicate that CD11chigh cDCs are required to initiate cytotoxic CD8+ T cell responses against intracellular pathogens (12, 13). The potential of IPC/PDCs to prime naive T cells, however, remains controversial. Freshly isolated PDCs are generally poor T cell stimulators. Thus, human blood–derived PDCs do not stimulate naive CD4+ T cells in a MLR, unless cultured in the presence of IL-3 or virus (HSV) (14). Moreover, murine splenic PDCs fail to induce naive T cell proliferation to endogenous antigens, even after virus exposure (15). In contrast, murine peptide-pulsed PDCs derived from Flt-3–driven BM culture or spleens can promote the in vitro expansion of CD4+ T cells and Th cell polarization (16). Adoptive transfer experiments with splenic and BM culture–derived, CpG-matured PDCs showed that PDCs can elicit responses of naive CD8+ T cells to endogenous, but not exogenous, antigens (17). These conflicting results are likely caused by the different source of the PDCs used in the reports. Moreover, none of the studies addressed the in vivo potential of unmanipulated PDCs to prime naive T cells.
Recently, IPC/PDCs have been shown to play a critical role in the control of airway inflammation (18, 19) and allotransplant rejection (20, 21). Although these findings mark PDCs as potential candidates for future adoptive cell therapies, such approaches will require better understanding of the in vivo characteristics of this unique cell type.
We analyze CD4+ and CD8+ T cell responses to antigen challenge in mice that were conditionally depleted of CD11chigh cDCs, but retain IPC/PDCs. We show that depletion of cDCs impairs splenic T cell responses, indicating that PDCs are incapable of T cell priming in this organ. In contrast, the combined use of cDC ablation and a novel PDC-specific antigen-targeting strategy revealed that, in LNs, PDCs can efficiently trigger productive naive CD4+ T cell responses. Interestingly, and in contrast to cDC-driven responses, the PDC-triggered CD4+ T cell stimulations lack a concomitant CD8+ T cell expansion. Our in vivo results, thus, characterize PDCs as bona fide DCs that can initiate unique CD4+ Th cell–dominated primary immune responses.
RESULTS
PDCs are spared from ablation in DTx-treated CD11c-DTR transgenic mice
To investigate differential in vivo functions of APCs in lymphoid organs, we recently developed a diphtheria toxin receptor (DTR)–based system that allows the conditional ablation of CD11chigh cDCs (12). DTR expression in CD11c-DTR transgenic mice is driven by a DNA fragment flanking the Itgax gene (22), which encodes the αX subunit of the CD11c integrin. Murine CD11c expression is found in all cDCs, but has also been reported for certain macrophages (23–25), activated T cells (26), NK cells (27), and plasmablasts (28).
Murine PDCs are defined as being CD11clow B220+ Ly6C+, and, furthermore, can be identified by the PDC-specific antibodies 120G8 (29), 440c (30), and mPDCA-1 (31). To test the DTx sensitivity of CD11c-DTR transgenic CD11clow PDCs, we injected CD11c-DTR transgenic C57BL/6 mice with DTx. Flow cytometric analysis of the spleens 1 d after DTx treatment revealed that PDCs were spared from DTx-induced ablation, whereas cDCs were depleted (Fig. 1 A and Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20062373/DC1). The DTx resistance of PDCs is explained by the absence of expression of the DTR-GFP fusion protein by the bulk population of these cells (Fig. 1 B). Moreover, it was also confirmed for LN PDCs (Fig. 1, C and D; Fig. 3 A; Fig. S1 A) and PDCs located in the bone marrow (Fig. S1 A). This indicates that the 5.5-kb Itgax promoter/enhancer fragment (22) does not carry all transcription control elements required for physiological αX subunit expression in CD11c+ cells (32), and further supports the notion that PDCs and cDCs are genetically distinct entities (33). To investigate whether the toxin treatment results in a functional impairment of PDCs, we tested type I interferon responses. As seen in Fig. 1 E, DTx treatment neither affected the IFN-α response to in vivo CpG treatment nor compromised the ability of PDCs to produce IFN-α in response to an in vitro influenza virus challenge. Notably, we consistently observed in spleens, but not LNs, of CD11c-DTR transgenic C57BL/6 mice a small fraction of mPDCA-1+ 440c+ CD11clow cells that were DTR/GFP+ and DTx sensitive; however, upon in vitro exposure to virus, these cells did not secrete IFN-α (Fig. 1, B and D, and not depicted). Collectively, these results indicate that PDCs of CD11c-DTR transgenic mice are resistant to DTx treatment. CD11c-DTR transgenic mice, thus, provide a unique model to study the role of PDCs in the priming of immune responses in the absence of conventional CD11chigh DCs.
Figure 1.
PDCs are spared from ablation in DTx-treated CD11c-DTR transgenic mice. (A) Flow cytometric analysis of the spleens of untreated controls and CD11c-DTR transgenic mice 1 d after i.v. injection of DTx (4 ng/body weight). cDCs are gated as CD11chigh mPDCA-1neg cells; PDCs are defined as being CD11cint mPDCA-1+. (left) Dot blots show cells gated according to scatter. (right) The bar graph represents analysis of splenic cDCs and PDCs of untreated mice and mice 24 h after treatment (i.p. DTx; 10 μg OVA i.v.). n = 5 for each group. (B) DTR/GFP expression profiles of splenic cDCs and PDCs isolated from CD11c-DTRtg mice that were untreated or treated with DTx. Cells are defined and gated as in A. Graphs are representative of five repeats. Percentage refers to DTx-sensitive GFP+ mPDCA-1+ CD11clow cells (filled area). (C) Flow cytometric analysis of popliteal LNs of control mice CD11c-DTR transgenic mice 1 d after s.c. injection of 20 ng DTx into the hind footpads. (left) Dot blots show cells gated according to scatter. (right) Histogram represents analysis of splenic cDCs and PDCs of untreated mice and mice 24 h after the indicated treatment (i.p. DTx; 10 μg OVA i.v.). n = 5 for each group. (D) DTR-GFP expression profiles of LN PDCs isolated from untreated or treated CD11c-DTRtg mice. Note absence of GFP+ mPDCA-1+ CD11clow subpopulation observed in spleen (B). (E) IFN-α production by splenic PDC and non-PDC cell fractions isolated from noninjected or DTx-injected CD11c-DTRtg mice and incubated in vitro for 20 h in the presence or absence of 400 HAU/ml influenza virus and blood sera analysis of DTx-treated and untreated CD11c-DTRtg mice, 6 h after PBS or 100 μg CpG i.v. injection. Error bars depict the SD.
Figure 3.
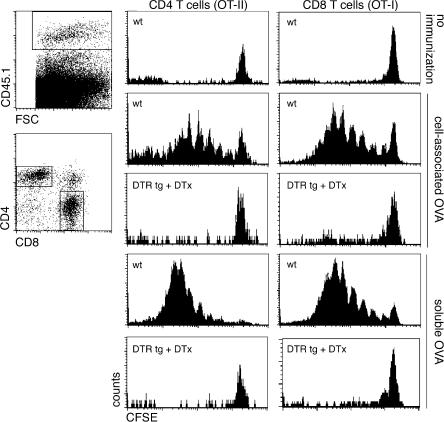
Ablation of cDCs impairs CD8+ T cell priming, but not CD4+ T cell priming, in the LNs. (A) Flow cytometric analysis of the popliteal LNs of CD11c-DTR transgenic mice during the course of a priming experiment. Mice were injected twice with DTx in the hind footpads, on day 1 and 3. The line graph represents the analysis of LN cDCs and PDCs as indicated in the FACS blots (top right). n = 3 for each group. The bottom diagram refers to T cell priming experiments performed in B. (B) Flow cytometric analysis of cotransferred CFSE-labeled CD45.1+ OT-I and -II cells (bottom row, OT-II transfer only) into DTx-treated nontransgenic and CD11c-DTR transgenic mice (CD45.2+) 4 d after s.c. immunization with soluble OVA. Histograms represent cells gated according to scatter, CD45.1, and CD8 or CD4 expression, as indicated in the dot plots. Data show results of 2 representative experiments out of 10 repeats. Graph summarizes percentages of dividing CFSE-labeled CD45.1+ OT-II cells in mice (n = 14) that were treated with DTx and immunized, compared with proliferation in immunized WT control mice (n = 8; set as 100%). Each dot represents an independent mouse.
PDCs fail to prime naive T cells in the spleen
We first investigated the potential of splenic PDCs to stimulate naive T cells in the absence of CD11chigh cDCs. We isolated TCR transgenic OVA-specific CD8+ and CD4+ T cells from OT-I and -II mice, respectively (34, 35). Donor mice carried an allotypic marker (CD45.1) that allowed for detection of the transferred cells in the recipient mice. Before transfer, the T cells were labeled with the intracellular dye CFSE, which allows the monitoring of in vivo proliferation (36). T cells were cotransferred into WT and CD11c-DTR transgenic C57BL/6 mice. The grafted mice were treated with DTx and, 8 h later, immunized by i.v. injection of soluble OVA (10 μg) or OVA-loaded splenocytes (37). 4 d after immunization, the mice were killed and spleens were isolated and analyzed for the proliferation status of the T cell grafts (Fig. 2). All challenges resulted in vigorous expansion of both CD4+ and CD8+ T cell grafts in the WT recipient mice. As previously reported (12), depletion of CD11chigh cDCs impaired the response of CD8+ T cells to cell-associated antigen. Presentation of antigen in the context of MHC class I to CD8+ T cells requires a unique phagosome-to-cytosol pathway resulting in cross-presentation, which is an activity proposed to be uniquely associated with cDCs or cDC subsets (12, 37, 38). In support of this notion, we found that cDCs are also needed for CD8+ T cell priming after challenge with exogenous soluble antigen (Fig. 2). Moreover, depletion of splenic cDCs also abrogated OVA-specific CD4+ T cell responses to cell-associated and soluble antigen, thus extending the study by Tian et al. (39). Importantly, as opposed to a recent study (40), we evaluated the T cell–priming potential of PDCs in the absence of cDCs. Splenic PDCs are unable to prime naive CD4+ and CD8+ T cells in the absence of CD11chigh cDCs.
Figure 2.
cDC ablation impairs splenic CD4+ and CD8+ T cell priming. Flow cytometric analysis of cotransferred CFSE-labeled CD45.1+ OT-I and -II T cell grafts (106 cells each) in DTx-treated nontransgenic and CD11c-DTR transgenic mice (CD45.2) 4 d after i.v. immunization with OVA-loaded splenocytes or soluble OVA. Histograms represent cells gated according to scatter, CD45.1, and CD8 or CD4 surface expression, as indicated in the dot plots. Data show results of one representative experiment out of three.
PDCs can prime naive CD4+ T cells in LNs, but fail to support CD8+ T cell priming
T cell responses are initiated in specialized secondary lymphoid organs, such as the spleen and LNs, which differ considerably in their microarchitecture, reflecting their specialization for the handling of blood- and tissue-borne antigens, respectively. We next investigated whether CD11chigh DCs would also be required in the LNs. Local, s.c. DTx injection results in the rapid depletion of LN-resident cells expressing the DTR-GFP fusion protein, including both CD8α+ and CD8α− CD11chigh cDCs and sinusoidal macrophages (23), but not PDCs (Fig. 1 C; Fig. 3 A; Fig. S1, A–C). Repetitive s.c. DTx injection results in persistent cDC ablation (Fig. 3 A and Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20062373/DC1). To investigate the impact of the LN cDC depletion on T cell responses in this organ, we engrafted WT and CD11c-DTR transgenic C57BL/6 recipient mice with a mixture of OVA-specific CD4+ (OT-II) and CD8+ T cells (OT-I). The mice were subjected to the DTx treatment (which was continued throughout the experiment to maintain cDC depletion; Fig. 3 A) and, 8 h later, immunized by s.c. injection of 10 μg OVA. 3 d after immunization, we isolated the antigen-draining popliteal LNs and analyzed them for the proliferation status of grafted T cells. The adoptively cotransferred CD45.1+ CD4+ and CD8+ T cells readily proliferated in the LNs of DTx-treated WT mice in response to immunization with soluble OVA (Fig. 3 B). Importantly, the DTx-induced cDC depletion abrogated the expansion of CD8+ T cells, indicating that, as in the spleen, priming of naive CD8+ T cells required the presence of cDCs. Surprisingly, however, we observed a proliferative response of the grafted CD4+ T cells in the cDC-depleted LNs (Fig. 3 B). Importantly, this was unlikely to be caused by residual cDCs because the DTx treatment impaired in the same LNs the CD8+ T cell response, which is generally more sensitive to antigen challenge (41). The persistence of the CD4+ T cell priming in the absence of cDCs was also observed in CD11c-DTRtg BALB/c mice, in combination with an OVA-specific TCR transgenic naive CD4+ T cell graft isolated from DO11.10 mice (Fig. S3) (42). Moreover, to confirm our observation in a non-TCR transgenic system and with an antigen other than OVA, we challenged untreated and cDC-depleted DTRtg mice with KLH/CpG and analyzed the resulting response of endogenous CD4+ T cells. 7 d after s.c. antigen challenge, we purified CD4+ T cells from popliteal LNs and subjected them to an in vitro restimulation protocol with KLH-pulsed APC. Again, CD4+ T cells were efficiently primed irrespective of the presence of cDCs (Fig. S2).
Our data show that LNs contain APCs other than CD11chigh DCs or macrophages that are capable of stimulating naive CD4+ T cells. Although PDCs are potential candidates, other MHC class II+ cells could be responsible for the CD4+ T cell priming. By far, the largest MHC class II+ population in the LNs are B lymphocytes, which, even when naive, have been shown to efficiently ingest and process noncognate protein antigens (43). However, the ability of B cells to activate naive CD4+ T cells remains under debate (44–46). Moreover, pMHC encounter on B cells is believed to result in nonproductive T cell priming or tolerization (47). To investigate the role of B cells in our system, we crossed the CD11c-DTR transgene onto a B cell–deficient genetic background (C57BL/10-Igh-6tm1Cgn [μMT]) (48). CD11c-DTRtg:μMT and μMT C57BL/6 mice were engrafted with OVA-specific CD4+ T cells (OT-II) and subjected to the DTx treatment and OVA immunization. B cell deficiency resulted in a reduced recovery of grafted CD4+ T cells in DTx-treated CD11c-DTRtg:μMT and μMT C57BL/6 mice. However, the frequency of OT-II cells that lost the CFSE-label, and thus proliferated in response to the OVA challenge, was unaffected by the absence of B cells (Fig. S4, A and B, available at http://www.jem.org/cgi/content/full/jem.20062373/DC1). CD4+ T cell priming in the LNs, thus, persisted in the combined cells in the absence of B cells and CD11chigh cDCs. Other potential APC candidates are epidermal Langerhans cells (LCs), which are CD11cneg, but up-regulate CD11c expression upon migration to the skin-draining LNs (49). LCs would, hence, be expected to become DTx sensitive by the time they reach the LNs. To nevertheless exclude the possibility that the CD4+ T cell response we observed was caused by residual LCs, we crossed the CD11c-DTR transgenic mice to mice that harbor the DTR gene under the LC-specific Langerin promoter (50). DTx-treatment of LanDTR:CD11cDTR mice results in ablation of cDCs and LCs (unpublished data). However, the response of the adoptively transferred OT-II CD4+ T cells to the OVA challenge persisted (Fig. S4 C). Collectively, these data suggest that cells other than cDCs, macrophages, B cells, or LCs are responsible for the priming of CD4+ T cells, and point at a role for PDCs in antigen presentation.
LN PDCs efficiently acquire and process soluble antigen and prime productive T cell responses
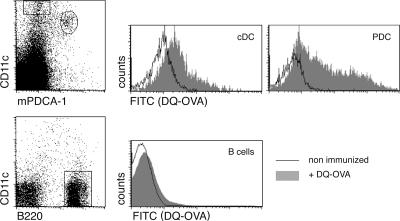
Antigen presentation and CD4+ T cell priming by LN-resident PDCs would require their antigen uptake. Importantly, soluble antigens of low molecular weight (<70 kD) are readily transported into the T cell zone of the LN, i.e., PDC proximity, via a highly developed conduit network (51). To investigate whether LN PDCs have access to antigen, we immunized C57BL/6 WT mice with DQ-OVA and analyzed LN APC populations for antigen uptake. This fluorogenic reagent is undetectable in its unprocessed form because of autoquenching, but becomes fluorescent upon entry into acidic, endosomal cellular compartment. As seen in Fig. 4, DQ-OVA was readily ingested by the LN-resident CD11chigh DCs and subjected to processing as indicated by the induction of the green fluorescent label. PDCs were labeled at least as efficiently as CD11chigh cDCs, whereas B cells remained unaffected by the DQ-OVA challenge. Importantly, as it occurred in naive mice, antigen uptake was independent of anti-OVA antibodies, and hence Fcγ receptors, which have been implied in PDC antigen capture (52). Thus, PDCs have access to antigen in our experimental system, and they could be responsible for the priming of CD4+ T cells in LNs depleted of the CD11chigh DCs.
Figure 4.
In vivo antigen acquisition by PDCs. Flow cytometric analysis of popliteal LNs harvested 24 h after s.c. injection of DQ-OVA into hind footpads. Histograms represent analysis of DQ-OVA uptake by cDCs (CD11chigh mPDCA-1−), PDCs (CD11cint mPDCA-1+), and B cells (CD11c− B220+). Results are representative of two independent experiments.
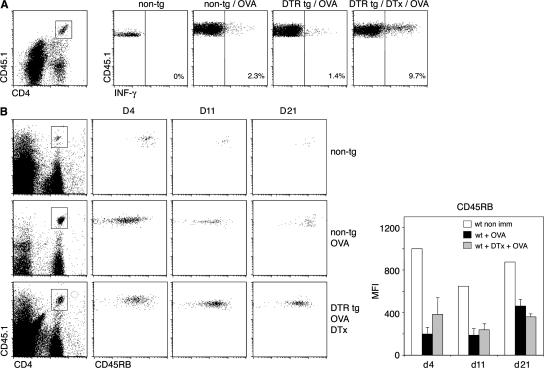
Proliferative T cell expansion can be associated with the generation of cytokine-producing effector T cells, but also abortive T cell responses (53–55). We therefore investigated the quality of the primed LN CD4+ T cells by studying Th cell polarization and long-term T cell survival. To analyze Th cell polarization of the grafted OVA-specific CD4+ T cells, we performed an in vivo restimulation assay by rechallenging the OVA-immunized mice with an i.v. injection of OVA peptide (56). Both in the presence and absence of CD11chigh cDC, CD4+ T cells differentiated efficiently into IFN-γ–producing effector T cells (Fig. 5 A). Abortive T cell stimulation is characterized by a collapse of the antigen-specific T cell population after its initial expansion (53, 54). To test the persistence of CD4+ T cells that were primed in the absence of cDCs, we used mixed CD11c-DTR BM chimeras, which allow for repetitive DTx treatment, and hence extended cDC depletion (57). In both untreated and cDC-depleted mice, the OT-II CD4+ T cell graft clonally expanded and persisted well beyond the initial antigen challenge (Fig. 5 B). Furthermore, in both the presence and absence of cDCs, the grafted naive CD4+ T cells differentiated into T cells of CD45RBlow memory phenotype (58). Collectively, PDCs seem capable of stimulating naive CD4+ T cells, resulting in a persistent response that includes IFN-γ–producing effector T cells of memory phenotype. Importantly, and in contrast to the immune reaction triggered by cDCs, this response is not accompanied by a concomitant CD8+ T cell response.
Figure 5.
CD4+ T cells that are primed in the absence of cDCs differentiate into Th cells with memory phenotype and persist for extended periods of time. (A) CFSE-labeled CD45.1+ OT-II cells were transferred into nontransgenic and CD11c-DTR transgenic mice (CD45.2+). 1 d later, mice were treated s.c. with DTx; 8 h later, they were immunized in their hind footpads with OVA and CpG. 3 d after immunization, IFN-γ production in the popliteal LN was assessed after in vivo restimulation with OVA peptide, 2 h before removal of popliteal LNs. Data represent IFN-γ profiles after gating on CD4+ CD45.1+ lymphocytes. Results are representative of two independent experiments. (B) CFSE-labeled CD45.1+ OT-II cells were transferred into mixed [WT > WT] or [DTRtg > WT] BM chimeras (CD45.2+). The mice were injected s.c. with DTx 1 d after the transfer, and they were immunized 8 h later with OVA injected into their hind footpads. Sequential s.c. DTx injections were performed every second day until day 21. Blood was collected on day 4, 11, and 21 and evaluated for the presence of OT-II cells (CD4+ CD45.1+) and expression of the memory T cell marker CD45RB. Bar graph depicts mean fluorescence intensity values of blood staining for CD45RB on day 4, 11, and 21. Results are representative of two independent experiments.
PDC-specific antigen targeting and PDC-mediated in vivo and in vitro CD4+ T cell priming
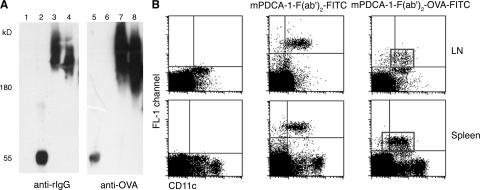
The aforementioned results suggest that LN PDCs efficiently ingest exogenous antigen and are able to initiate productive CD4+ T cell responses. To obtain independent evidence that PDCs can act as APCs, we decided to adopt a strategy involving specific, antibody-mediated, antigen-targeting APCs (53) and conjugate the PDC-specific mPDCA-1 antibody to OVA. To avoid complement-dependent lysis (31), we used a nondepleting F(ab')2 fragment. Functional integrity of the construct was tested by Western blotting. As depicted in Fig. 6 A, the conjugates were readily detected both via the kappa light chain and the OVA-fraction, whereas free OVA or unconjugated mPDCA-1–F(ab')2 fragments were only detected with the anti-rat IgG or the -OVA antibody, respectively. The OVA-conjugated antibody fragment specifically targeted PDC in splenic or LN single-cell suspensions in vitro (unpublished data). To demonstrate in vivo specificity, we took advantage of a FITC conjugate of the mPDCA-1–F(ab')2-OVA construct. Intravenous or s.c. injection of FITC–mPDCA-1–F(ab')2–OVA resulted in specific labeling of CD11clow PDCs in spleens and LNs and spared CD11chigh cDCs (Fig. 6 B).
Figure 6.
Characterization of selective APC targeting via the anti–mPDCA-1–F(ab')2–OVA construct. (A) Western blot analysis of the OVA-conjugated antibody construct. Free OVA and unconjugated or OVA-conjugated anti–mPDCA-1 antibody constructs were resolved by SDS-PAGE (4-12% gradient Tris-glycine gel) and, after immunoblotting, detected with anti–rat and anti-OVA antibody, respectively. Lanes 1 and 5 contain free OVA, lanes 2 and 6 contain the unconjugated anti–mPDCA-1–F(ab')2 antibody fragment, and lanes 3 and 4 and 7 and 8 contain two fractions of the anti–mPDCA-1–F(ab')2–OVA conjugate. (B) Specific in vivo targeting of PDCs with FITC-labeled anti–mPDCA-1–F(ab')2–OVA. Conjugates were injected i.v. or s.c. and, after 3 h, spleens and popliteal and inguinal LNs were isolated. Counterstaining with CD11c was performed on single-cell preparations from untreated (left dot plots) or in vivo–targeted cells (middle and right dot plots). Note that the staining in the FL-1 channel is based on the in vivo–injected, FITC-coupled mPDCA-1–F(ab')2–OVA construct.
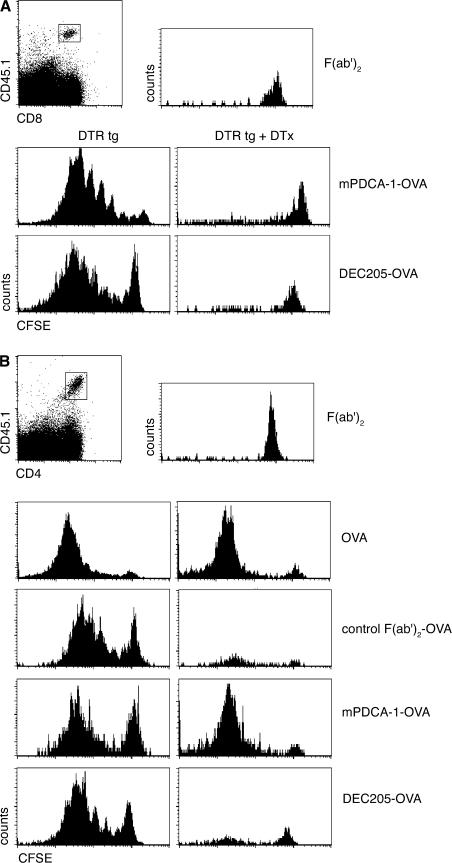
To direct the OVA antigen to both PDCs and cDCs, we used the mPDCA-1–F(ab')2–OVA conjugate (mPDCA-1–OVA) and an OVA conjugate that targets a subset of CD11chigh cDCs via the CD205 receptor (DEC205–OVA) (53, 59). We first investigated the response of OVA-specific CD8+ T cells (OT-I) to the conjugate challenge. As seen in Fig. 7 A, s.c. immunization of untreated CD11c-DTRtg mice with the mPDCA-1–OVA and DEC205–OVA conjugates resulted in comparable responses of the naive CD8+ T cells in the draining popliteal LNs. Importantly, depletion of cDCs abrogated both the DEC205–OVA and the mPDCA-1–OVA–induced CD8+ T cell expansion. Thus, the cDC-driven CD8+ T cell response to the mPDCA-1–OVA conjugate was likely caused by antibody-independent antigen uptake by cDCs or secondary cross-presentation of cellular PDC debris by cDCs. Conventional DC ablation revealed that, in contrast to a recent study (40), even when directly targeted with exogenous antigen, PDCs seem unable to trigger CD8+ T cell responses. Instead, our results support the notion of the general inability of PDCs to channel exogenous antigen into the MHC class I presentation pathway and cross-present (17).
Figure 7.
In vivo antigen targeting of PDCs leads to priming of LN CD4+ T cells, but not CD8+ T cells, in the absence of cDCs. (A) Flow cytometric analysis of transferred CFSE-labeled CD45.1+ OT-I cells into s.c. DTx-treated CD11c-DTR transgenic mice (CD45.2) 4 d after footpad immunization with 8 μg mPDCA-1–OVA (OVA content, ∼7.5%) or 7 ng DEC205–OVA (OVA content, ∼10%). Histograms represent CFSE profiles of cells gated according to CD45.1 and CD8 expression, as indicated in the dot plot. Data show a representative result for three experiments. (B) Flow cytometric analysis of transferred CFSE-labeled CD45.1+ OT-II cells into DTx-treated CD11c-DTR transgenic mice, 4 d after footpad immunization with F(ab)2, OVA, control F(ab)2–OVA, mPDCA-1–F(ab)2–OVA, or DEC20-OVA. Histograms represent CFSE profiles of cells gated according to CD45.1 and CD4 expression, as indicated in the dot plot. Data show one representative experiment out of three.
Next, we analyzed the response of OVA-specific LN CD4+ T cells (OT-II) to conjugate challenge. Like the immunization with OVA, challenge of untreated CD11c-DTRtg mice with the antibody-conjugates resulted in vigorous expansion of the CD4+ T cell grafts. In contrast to the CD8+ T cell responses, and confirming our previous results (Fig. 3), the CD4+ T cell responses to the OVA and mPDCA-1–F(ab')2–OVA challenge resisted the DTx treatment in CD11c-DTR transgenic mice (Fig. 7 B). Importantly, however, the CD4+ T cell response to the DEC205–OVA challenge was impaired by depletion of CD11chigh cDCs. These results provide further evidence that the CD4+ T cell response we observe in the absence of CD11chigh cDCs is driven by PDCs. Notably, the antibodies to DEC205 and mPDCA-1 target distinct surface receptors (60, 61), which likely differ in their potential to promote endocytosis. This precludes any quantitative conclusion on the priming potential of cDCs versus PDCs.
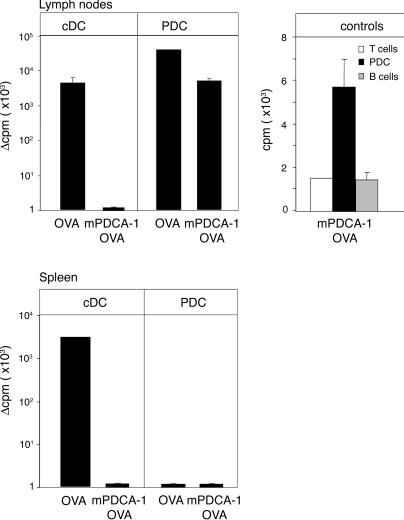
To obtain final proof for the CD4+ T cell priming potential of PDCs, we decided to use an antibody-mediated depletion protocol to test T cell responses in the combined absence of both cDCs and PDCs. However, in our hands, even repetitive injection of mPDCA-1 and anti-Gr1 antibodies (18, 31) resulted in incomplete LN PDC depletion (unpublished data). Therefore, we resorted to an in vivo loading/in vitro readout system. WT BALB/c mice were immunized with OVA or mPDCA-1–OVA. 1 d later, PDCs, cDCs, and B cells were isolated from LNs and spleens, sorted to purity, and tested in vitro for their ability to prime naive OVA-specific CD4+ T cells (DO11.10). As seen in Fig. 8, cDCs isolated from LNs or spleens of OVA-immunized mice readily stimulated T cell proliferation, whereas cDCs and B cells isolated from mPDCA-1–OVA– immunized mice failed to do so. This confirms the specificity of the in vivo antigen-targeting strategy (Fig. 6 B). Importantly, PDCs isolated from LNs of mice immunized with both OVA and mPDCA-1–OVA were able to stimulate the naive CD4+ T cells. Substantiating our in vivo data on the inability of splenic PDCs to prime CD4+ T cells, in the in vitro assay PDCs isolated from spleens of immunized mice also failed to stimulate the T cells. Collectively, our results show that LN PDCs can initiate unique CD4+ Th cell–dominated primary immune responses.
Figure 8.
In vivo antigen targeting of PDCs leads to LN PDC-mediated in vitro CD4+ T cell proliferation. CD11chigh cDCs, PDCs, and B cells were sorted from spleens and LNs of BALB/c mice that were i.v. or s.c. immunized with OVA or mPDCA-1–F(ab')2–OVA, and cultured with OVA-specific CD4+ T cells (DO11.10) for 72 h, after which thymidine incorporation was measured. Results are plotted as the arithmetic mean of cycles per minute (cpm) from triplicate cultures with cells isolated from immunized mice minus the mean cpm of cultures of the respective cell populations isolated from unimmunized mice. The absolute mean cpm for the LN cells were as follows: 4,297 (cDC/OVA); 30,537 (LNPDC/OVA); 5,065 (LNPDC mPDCA-1–OVA); and 2,995 for spleen cells (cDC/OVA). The positive fraction of PDCs contained <0.01% contaminating CD11chigh cDCs. n = 4 for each group. Error bars represent the SD. Controls include responder T cells only and T cells with B cells isolated from PDCA-OVA immunized mice.
DISCUSSION
We analyzed the responses of naive CD4+ and CD8+ T cells to antigen challenge in mice that were depleted of conventional CD11chigh cDCs, but retained PDCs. In support of previous reports (12, 39), we found that the capacity to prime naive CD4+ and CD8+ T cells in the spleen is uniquely restricted to cDCs. Ablation of cDCs, however, revealed the surprising potential of PDCs to support CD4+ T cell responses in the LNs. Importantly, PDCs differed from cDCs in that they could not induce CD8+ T cell priming.
Naive T cells are stimulated in lymphoid organs by specialized, “professional” APCs that induce clonal T cell expansion and differentiation. Efficient eradication of invading microbes requires distinct immune responses, involving both cellular and humoral defense mechanisms. Pathogen identification is believed to result from engagement of pathogen sensors (such as TLRs), which are differentially expressed on APCs and may allow distinct APC subsets to trigger specific responses. Division of labor between APCs, however, remains poorly understood. This is particularly true for IPC/PDCs, which are critical for NK cells and cDC function, but whose in vivo capacity for antigen presentation and T cell priming remains unclear. We reasoned that any potential APC functions of PDCs might be masked by the presence of cDCs, and thus decided to test naive T cell responses in mice that were conditionally depleted of cDCs.
Surprisingly, PDCs could prime CD4+ T cells in the LNs, but not in cDC-depleted spleens. It remains unclear whether this discrepancy results from the distinct organ architecture or reflects an inherent difference between splenic and LN PDCs. In support of the former, in naive mice PDCs are located in the red pulp and T cell area, but rarely in the marginal zone, which is the site of blood-borne antigen entry to the spleen (62). Moreover, when challenged, splenic PDCs form clusters and, compared with cDCs, they show a delayed translocation to the T cell zone (62). This migration might be critical for the spleen, whereas it may not be required in the LNs. Recruitment of PDCs to the LNs occurs via high endothelial venules (HEVs), and thus the cells enter directly into the T cell area (63). Even before antigen-carrying DCs arrive from the periphery, resident LN APCs are known to take up soluble antigens (47), which enter the deeper LNs through an intricate network of conduits (51). Accessibility of antigen to LN PDCs is also highlighted by the observation that these cells readily ingested injected DQ-OVA (Fig. 4), although, interestingly, we failed to label splenic PDCs by intravenous DQ-OVA injection (not depicted). Alternatively, the unique ability of LN PDCs to prime CD4+ T cells might indicate that these cells differ from their splenic counterpart. Indeed, even when we targeted antigen directly to both LN and spleen PDCs and overrode the need for translocation (by an in vitro readout; Fig. 8), splenic PDCs failed to prime CD4+ T cells (Fig. 8). Interestingly, LN PDCs are characterized by a distinct expression pattern of activation markers, such as Sca-1, and are poor producers of IFN-α compared with Sca-1+ BM PDCs (unpublished data). However, a more detailed comparative study will be required to investigate qualitative differences between splenic and LN PDCs. Importantly, the antigen challenge results in a rapid PDC influx to the LN, and in our experimental setup, we do not discriminate between LN-resident and recruited PDCs.
Although we would like to stress that we do not want to compare the efficiency of cDCs and PDCs in T cell priming potential, we establish that PDCs can prime naive CD4+ T cells. Absence of cDCs is arguably an artificial situation unlikely to occur under physiological circumstances. However, we predict that pathogens that specifically target or activate PDCs will elicit exclusive CD4+ T cell responses supporting humoral, but not cytotoxic, immunity. PDC-specific targeting could be mediated by endocytic receptors, such as Siglec-H (40, 64), which are expressed on murine PDCs, but not cDCs. So far, murine PDC-specific pathogen sensors have not been reported, although PDCs are characterized by higher expression of TLR7 and TLR9 (65), which are also expressed by human PDCs, but absent from human interstitial DCs (2, 66). The unique priming of CD4+, but not CD8+, T cell responses by PDCs could result from exclusive presentation of exogenous antigens on MHC II, but not MHC I, molecules. As suggested earlier (17), PDCs might lack the phagosome-to-cytosol pathway required for cross-presentation. Importantly, this would spare the priming PDCs from a unique negative-feedback mechanism through which CTLs kill APCs, and thereby terminate the priming process (67, 68). The inability to cross-present would render PDCs resistant to the CTLs elicited by cDCs. In the course of an ongoing response to a given antigen, the APC balance might therefore be shifted from cDCs to PDCs and, as a result, priming of CD4+ T cells and potentially humoral responses might be favored. As CD4+ T cells also critically contribute to the development of CD8+ T cell memory (69), the APC function of PDCs might have a general impact on the shape of immune responses.
We show that in LNs, PDCs efficiently trigger productive naive CD4+ T cell responses. Interestingly, and in contrast to cDC-triggered responses, PDC-triggered CD4+ T cell responses are not associated with concomitant CD8+ T cell expansion. Instead, PDCs initiate unique CD4+ Th cell–dominated primary immune responses.
MATERIALS AND METHODS
Mice.
CD11c-DTR transgenic (B6.FVB-Tg Itgax-DTR/GFP 57Lan/J) mice (12); OT-I (C57BL/6) TCR transgenic mice harboring OVA-specific CD8+ T cells (42); DO11.10 (BALB/c); and OT-II (C57BL/6) TCR transgenic mice harboring OVA-specific CD4+ T cells (35); B cell–deficient mice (C57BL/10-IgH-6tm1Cgn (μMt) (48) and LanDTR mice carrying the DTR-GFP gene under the Langerin promoter (50). LanDTR:CD11cDTR and DTRtg:μMT mice were obtained by intercrossing of the respective lines. Mixed (DTR > WT) BM chimeras were generated as previously reported (57). For systemic cDC depletion, mice were injected i.p. with 4 ng/g body weight DTx (D-2918; Sigma-Aldrich). For local cDC depletion in popliteal LNs, animals were injected with 20 ng DTx s.c. into hind footpads. If indicated, DTx was applied. All mice were used at the age of 6–12 wk, maintained under specific pathogen-free conditions, and handled according to protocols approved by the Weizmann Institute Animal Care Committee as per international guidelines.
Isolation of CD11chigh DCs and PDCs.
Peripheral LNs or spleens were digested with 1 mg/ml of collagenase D (Roche) for 45 min at 37°C. Tissues were mechanically disrupted in PBS, and for spleens, RBC lysis was performed by incubation with 1 ml ACK buffer (0.15 M NH4Cl, 0.1 M KHCO3, 1 mM EDTA, and PBS) for 1 min at room temperature. PDCs were enriched by MACS cell sorting according to the manufacturer's protocol (Miltenyi Biotec GmbH).
In vitro proliferation assay.
BALB/c mice were immunized i.v. (20 μg OVA or 10 μg mPDCA-1–OVA) and s.c. into hind footpads (10 μg OVA or mPDCA-1–OVA). 24 h later, spleens and popliteal and inguinal LNs were harvested, and cDCs and PDCs were purified by high-speed cell sorting according to CD11c and mPDCA-1 expression using a FACS Aria (Becton-Dickinson). Positive fraction of PDCs contained <0.01% contaminating CD11chigh cDCs. DCs (104) or PDCs (2–3 × 104) were cultured with 105 DO11.10 CD4+ T cells. Cultures were pulsed after 72 h with 1 μCi of [H3]thymidine, and incorporation was measured 16 h later.
IFN-α secretion assay.
MACS-enriched splenic PDCs were cultured at 2 × 106 cells/ml in complete RPMI 1640 for 20 h, with 400 HAU/ml influenza virus A/Texas/1/77 (provided by R. Arnon, The Weizmann Institute, Rehovot, Israel). Supernatants were assayed using an IFN-α ELISA kit (Performance Biomedical Laboratories).
Conjugation of OVA to anti–mPDCA-1 F(ab')2 fragments and specificity test.
Anti–mPDCA-1 monoclonal antibody (rat IgG2b, κ; Miltenyi Biotec GmbH) was digested with Pepsin A (Sigma-Aldrich). After size fractionation (Superdex 200 16/60 gel filtration column; GE Healthcare), F(ab')2 fragments were conjugated to OVA (Sigma-Aldrich) activated with succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (Pierce Chemical Co.) according to the manufacturer's protocol. F(ab')2-OVA conjugates were size fractionated and sterile filtrated (Millex GV filter unit 0.22 μm; Millipore) for in vivo application. Construct integrity was evaluated by Western blot analysis with horseradish peroxidase–conjugated polyclonal rabbit anti–rat Ig (H+L; Jackson ImmunoResearch Laboratories) and horseradish peroxidase–coupled rabbit anti-OVA antibody (Research Diagnostics, Inc.), respectively. OVA content was determined by ELISA to be ∼75 ng OVA/μg mPDCA-1 F(ab')2-OVA conjugate. To demonstrate in vivo specificity, we injected 10–25 μg FITC-conjugated anti–mPDCA-1–F(ab')2–OVA i.v. or into the hind footpads. 3 h later, spleens or pooled popliteal and inguinal LNs were analyzed for PDC-specific targeting.
Immunohistochemistry.
4-μm paraffin sections of popliteal LNs were deparaffinized and treated with 2 ml HCl, H2O2 in 50% methanol, and 50% PBS for 15 min at room temperature. For antigen exposure, sections were microwaved in 10 mM sodium citrate, pH 6.0. After blocking with 20% heat-inactivated normal goat serum and 0.1% Triton X-100 in PBS, sections were incubated overnight at room temperature with polyclonal biotinylated goat anti-GFP antibody (ab6658; Abcam). Staining was revealed with an avidin–biotin peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories). Finally, the sections were stained with hematoxylin-Mayer (Finkelman).
Analysis of T cell proliferation.
TCR transgenic T cells were isolated from spleens and LNs of respective mice, enriched by MACS cell sorting with anti-CD8 or -CD4 antibodies according to the manufacturer's protocol (Miltenyi Biotec GmbH), and labeled with CFSE (C-1157; Invitrogen) (57). CFSE-labeled T cells (1–2 × 106/mouse) were injected into the tail veins of the recipient mice.
Immunization.
Mice were immunized with cell-associated antigen by i.v. injection of 3 × 107 OVA-loaded β2m−/− splenocytes (37). Soluble OVA (Sigma-Aldrich) was injected i.v. (10 μg/mouse) or s.c. into hind footpads (10 μg/footpad). KLH (Sigma-Aldrich) was injected s.c. into hind footpads (10 μg/footpad). The anti–DEC205-OVA conjugate (provided by K. Mahnke, University Hospital Heidelberg, Heidelberg, Germany) was used at a dose of 7 ng/footpad. F(ab')2 and anti–mPDCA-1–F(ab')2–OVA were injected at a dose of 8 μg/footpad. For measurement of Ag uptake, mice were immunized with DQ-OVA (Invitrogen) by hind footpad injection of 10 μg/footpad.
Intracellular cytokine staining.
Mice were immunized with 25 μg OVA in 5 nmol/footpad CpG nucleotide 1668 (TIB MOLBIOL). 2 d later, mice were in vivo restimulated by intravenous injection of 100 μg OVA peptide (residues 323–329) 2 h before the removal of popliteal LNs. The tissue was minced and incubated in 1 mg/ml of collagenase D (Roche) for 20 min at 37°C in medium supplemented with 10 μg/ml brefeldin A (Calbiochem). Cells were fixed in 2% formaldehyde, permeabilized with 0.3% saponin, and stained for anti-CD45.1, -CD4, and –IFN-γ antibodies.
Flow cytometric analysis.
The staining reagents used in this study included the PE-coupled anti-CD4, CD19, CD45.1, CD11c, CD45RB, CD8, and KJ126 (DO11.10 clonotype); biotinylated anti-CD45.1 and mPDCA-1; APC-coupled CD11c, CD4, IFN-γ, and CD8; the FITC-coupled anti-CD4; PE-Cy5.5–coupled anti-B220 (Caltag Laboratories); and PerCP-coupled anti-CD4 and CD8 antibodies. Unless otherwise indicated, the reagents were obtained from PharMingen or eBioscience. The anti–Siglec-H antibody (440c) was provided by M. Colonna (Washington University School of Medicine, St. Louis, MO). Cells were analyzed on a cytometer (FACSCalibur; BD Biosciences) using CellQuest software (BD Biosciences).
Online supplemental material.
Fig. S1 provides additional flow cytometric and histological data concerning the DTx sensitivity and resistance of cDCs and PDCs, respectively. Figs. S2 and S3 show unimpaired PDC-driven in vivo and in vitro CD4+ T cell responses in FVB/N mice (non-TCR transgenic system; Fig. S2) and in BALB/c mice (DO11.10 system; Fig. S3). Fig. S4 shows DTx-resistant CD4+ T cell responses in mice deficient for B cells and cDCs (CD11c-DTRtg:μMT), as well as LCs and cDCs (LanDTR:CD11cDTR). The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20062373/DC1.
Supplemental Material
Acknowledgments
We thank M. Colonna for the anti–Siglec-H antibody (440c), K. Mahnke for the DEC-205 OVA conjugate, and B. Clausen for the Langerin-DTR mice. We thank Guy Shakhar and the members of the Jung laboratory for critical reading of the manuscript, and we are grateful to Adi Ben-Yashar, Y. Chermesh, and O. Amram for technical help and animal husbandry.
This work was supported by the Israel Science Foundation. S. Jung is the incumbent of the Pauline Recanati Career Development Chair and a Scholar of the Benoziyo Center for Molecular Medicine.
The authors have no conflicting financial interests.
Abbreviations used: cDC, conventional DC; DTR, diphtheria toxin receptor; DTx, diphtheria toxin; HEV, high endothelial venule; IPC, IFN-producing cell; LC, Langerhan cell; PDC, plasmacytoid DC; TLR, Toll-like receptor.
References
- 1.Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai, T., S. Sato, K.J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068. [DOI] [PubMed] [Google Scholar]
- 4.Lennert, K., and W. Remmele. Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes. 1958. Acta Haematol. 19:99–113. [DOI] [PubMed] [Google Scholar]
- 5.Trinchieri, G., and D. Santoli. 1978. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 147:1314–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandyopadhyay, S., B. Perussia, G. Trinchieri, D.S. Miller, and S.E. Starr. 1986. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J. Exp. Med. 164:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoneyama, H., K. Matsuno, E. Toda, T. Nishiwaki, N. Matsuo, A. Nakano, S. Narumi, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D.F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015. [DOI] [PubMed] [Google Scholar]
- 9.Shen, H., and A. Iwasaki. 2006. A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN-based vaginal microbicide. J. Clin. Invest. 116:2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwajima, S., T. Sato, K. Ishida, H. Tada, H. Tezuka, and T. Ohteki. 2006. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat. Immunol. 7:740–746. [DOI] [PubMed] [Google Scholar]
- 11.Steinman, R.M., and M.D. Witmer. 1978. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc. Natl. Acad. Sci. USA. 75:5132–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, et al. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst, H.C., and M. van den Broek. 2005. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J. Immunol. 174:3920–3924. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki, N., and Y.J. Liu. 2002. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum. Immunol. 63:1126–1132. [DOI] [PubMed] [Google Scholar]
- 15.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E.R. Unanue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y.J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salio, M., M.J. Palmowski, A. Atzberger, I.F. Hermans, and V. Cerundolo. 2004. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Heer, H.J., H. Hammad, T. Soullie, D. Hijdra, N. Vos, M.A. Willart, H.C. Hoogsteden, and B.N. Lambrecht. 2004. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smit, J.J., B.D. Rudd, and N.W. Lukacs. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe, M., B.L. Colvin, and A.W. Thomson. 2005. Plasmacytoid dendritic cells: in vivo regulators of alloimmune reactivity? Transplant. Proc. 37:4119–4121. [DOI] [PubMed] [Google Scholar]
- 21.Ochando, J.C., C. Homma, Y. Yang, A. Hidalgo, A. Garin, F. Tacke, V. Angeli, Y. Li, P. Boros, Y. Ding, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662. [DOI] [PubMed] [Google Scholar]
- 22.Brocker, T., M. Riedinger, and K. Karjalainen. 1997. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Probst, H.C., K. Tschannen, B. Odermatt, R. Schwendener, R.M. Zinkernagel, and M. Van Den Broek. 2005. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 141:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rijt, L.S., S. Jung, A. Kleinjan, N. Vos, M. Willart, C. Duez, H.C. Hoogsteden, and B.N. Lambrecht. 2005. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallon-Eberhard, A., L. Landsman, N. Yogev, B. Verrier, and S. Jung. 2006. Transepithelial pathogen uptake into the small intestinal lamina propria. J. Immunol. 176:2465–2469. [DOI] [PubMed] [Google Scholar]
- 26.Huleatt, J.W., and L. Lefrancois. 1995. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J. Immunol. 154:5684–5693. [PubMed] [Google Scholar]
- 27.Laouar, Y., F.S. Sutterwala, L. Gorelik, and R.A. Flavell. 2005. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 6:600–607. [DOI] [PubMed] [Google Scholar]
- 28.Hebel, K., K. Griewank, A. Inamine, H.D. Chang, B. Muller-Hilke, S. Fillatreau, R.A. Manz, A. Radbruch, and S. Jung. 2006. Plasma cell differentiation in T-independent type 2 immune responses is independent of CD11c(high) dendritic cells. Eur. J. Immunol. 36:2912–2919. [DOI] [PubMed] [Google Scholar]
- 29.Asselin-Paturel, C., G. Brizard, J.J. Pin, F. Briere, and G. Trinchieri. 2003. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171:6466–6477. [DOI] [PubMed] [Google Scholar]
- 30.Blasius, A., W. Vermi, A. Krug, F. Facchetti, M. Cella, and M. Colonna. 2004. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood. 103:4201–4206. [DOI] [PubMed] [Google Scholar]
- 31.Krug, A., A.R. French, W. Barchet, J.A. Fischer, A. Dzionek, J.T. Pingel, M.M. Orihuela, S. Akira, W.M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 21:107–119. [DOI] [PubMed] [Google Scholar]
- 32.Lindquist, R.L., G. Shakhar, D. Dudziak, H. Wardemann, T. Eisenreich, M.L. Dustin, and M.C. Nussenzweig. 2004. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5:1243–1250. [DOI] [PubMed] [Google Scholar]
- 33.LeibundGut-Landmann, S., J.M. Waldburger, C. Reis e Sousa, H. Acha-Orbea, and W. Reith. 2004. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat. Immunol. 5:899–908. [DOI] [PubMed] [Google Scholar]
- 34.Clarke, S.R., M. Barnden, C. Kurts, F.R. Carbone, J.F. Miller, and W.R. Heath. 2000. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78:110–117. [DOI] [PubMed] [Google Scholar]
- 35.Robertson, J.M., P.E. Jensen, and B.D. Evavold. 2000. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J. Immunol. 164:4706–4712. [DOI] [PubMed] [Google Scholar]
- 36.Parish, C.R. 1999. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol. 77:499–508. [DOI] [PubMed] [Google Scholar]
- 37.den Haan, J.M., S.M. Lehar, and M.J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurts, C., M. Cannarile, I. Klebba, and T. Brocker. 2001. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 166:1439–1442. [DOI] [PubMed] [Google Scholar]
- 39.Tian, T., J. Woodworth, M. Skold, and S.M. Behar. 2005. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J. Immunol. 175:3268–3272. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., A. Raper, N. Sugita, R. Hingorani, M. Salio, M.J. Palmowski, V. Cerundolo, and P.R. Crocker. 2006. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 107:3600–3608. [DOI] [PubMed] [Google Scholar]
- 41.Foulds, K.E., L.A. Zenewicz, D.J. Shedlock, J. Jiang, A.E. Troy, and H. Shen. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168:1528–1532. [DOI] [PubMed] [Google Scholar]
- 42.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 43.Zhong, G., C. Reis e Sousa, and R.N. Germain. 1997. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J. Exp. Med. 186:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, G.S., A. Oxenius, H. Hengartner, C. Benoist, and D. Mathis. 1998. CD4+ T cell responses in mice lacking MHC class II molecules specifically on B cells. Eur. J. Immunol. 28:3763–3772. [DOI] [PubMed] [Google Scholar]
- 45.Reis e Sousa, C., and R.N. Germain. 1999. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J. Immunol. 162:6552–6561. [PubMed] [Google Scholar]
- 46.Attanavanich, K., and J.F. Kearney. 2004. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 172:803–811. [DOI] [PubMed] [Google Scholar]
- 47.Itano, A.A., S.J. McSorley, R.L. Reinhardt, B.D. Ehst, E. Ingulli, A.Y. Rudensky, and M.K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 19:47–57. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura, D., and K. Rajewsky. 1992. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 356:154–156. [DOI] [PubMed] [Google Scholar]
- 49.Ruedl, C., P. Koebel, M. Bachmann, M. Hess, and K. Karjalainen. 2000. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J. Immunol. 165:4910–4916. [DOI] [PubMed] [Google Scholar]
- 50.Bennett, C.L., E. van Rijn, S. Jung, K. Inaba, R.M. Steinman, M.L. Kapsenberg, and B.E. Clausen. 2005. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sixt, M., N. Kanazawa, M. Selg, T. Samson, G. Roos, D.P. Reinhardt, R. Pabst, M.B. Lutz, and L. Sorokin. 2005. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 22:19–29. [DOI] [PubMed] [Google Scholar]
- 52.Benitez-Ribas, D., G.J. Adema, G. Winkels, I.S. Klasen, C.J. Punt, C.G. Figdor, and I.J. de Vries. 2006. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after FcγRII-mediated uptake. J. Exp. Med. 203:1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itano, A.A., and M.K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. [DOI] [PubMed] [Google Scholar]
- 55.Sporri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163–170. [DOI] [PubMed] [Google Scholar]
- 56.Reinhardt, R.L., A. Khoruts, R. Merica, T. Zell, and M.K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105. [DOI] [PubMed] [Google Scholar]
- 57.Zaft, T., A. Sapoznikov, R. Krauthgamer, D.R. Littman, and S. Jung. 2005. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J. Immunol. 175:6428–6435. [DOI] [PubMed] [Google Scholar]
- 58.Yang, C.P., S.M. Sparshott, D. Duffy, P. Garside, and E.B. Bell. 2006. The phenotype and survival of antigen-stimulated transgenic CD4 T cells in vivo: the influence of persisting antigen. Int. Immunol. 18:515–523. [DOI] [PubMed] [Google Scholar]
- 59.Mahnke, K., Y. Qian, J. Knop, and A.H. Enk. 2003. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 101:4862–4869. [DOI] [PubMed] [Google Scholar]
- 60.Swiggard, W.J., A. Mirza, M.C. Nussenzweig, and R.M. Steinman. 1995. DEC-205, a 205-kDa protein abundant on mouse dendritic cells and thymic epithelium that is detected by the monoclonal antibody NLDC-145: purification, characterization, and N-terminal amino acid sequence. Cell. Immunol. 165:302–311. [DOI] [PubMed] [Google Scholar]
- 61.Blasius, A.L., E. Giurisato, M. Cella, R.D. Schreiber, A.S. Shaw, and M. Colonna. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260–3265. [DOI] [PubMed] [Google Scholar]
- 62.Asselin-Paturel, C., G. Brizard, K. Chemin, A. Boonstra, A. O'Garra, A. Vicari, and G. Trinchieri. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoneyama, H., K. Matsuno, Y. Zhang, T. Nishiwaki, M. Kitabatake, S. Ueha, S. Narumi, S. Morikawa, T. Ezaki, B. Lu, et al. 2004. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16:915–928. [DOI] [PubMed] [Google Scholar]
- 64.Blasius, A.L., and M. Colonna. 2006. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 27:255–260. [DOI] [PubMed] [Google Scholar]
- 65.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. [DOI] [PubMed] [Google Scholar]
- 66.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 67.Hermans, I.F., D.S. Ritchie, J. Yang, J.M. Roberts, and F. Ronchese. 2000. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J. Immunol. 164:3095–3101. [DOI] [PubMed] [Google Scholar]
- 68.Wong, P., and E.G.P. Am. 2003. Feedback regulation of pathogen-specific T cell priming. Immunity. 18:499–511. [DOI] [PubMed] [Google Scholar]
- 69.Janssen, E.M., E.E. Lemmens, T. Wolfe, U. Christen, M.G. von Herrath, and S.P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. [DOI] [PubMed] [Google Scholar]
- 70.Webster, B., E.H. Ekland, L.M. Agle, S. Chyou, R. Ruggieri, and T.T. Lu. 2006. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 203:1903–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.