Abstract
The majority of highly activated CD4 T cell effectors die after antigen clearance, but a small number revert to a resting state, becoming memory cells with unique functional attributes. It is currently unclear when after antigen clearance effectors return to rest and acquire important memory properties. We follow well-defined cohorts of CD4 T cells through the effector-to-memory transition by analyzing phenotype, important functional properties, and gene expression profiles. We find that the transition from effector to memory is rapid in that effectors rested for only 3 d closely resemble canonical memory cells rested for 60 d or longer in the absence of antigen. This is true for both Th1 and Th2 lineages, and occurs whether CD4 T cell effectors rest in vivo or in vitro, suggesting a default pathway. We find that the effector–memory transition at the level of gene expression occurs in two stages: a rapid loss of expression of a myriad of effector-associated genes, and a more gradual gain of expression of a cohort of genes uniquely associated with memory cells rested for extended periods.
Memory is a defining feature of the adaptive immune system. Observations of long-term protection against specific pathogens have been noted for hundreds of years, but the cellular basis of immunological memory has only recently begun to be dissected (1–4). The study of CD4 T cell memory in particular has been hampered by two factors. First, by the relatively low frequencies of memory CD4 T cells of any given specificity compared with antigen-specific memory CD8 T cells (5–7), and second, by the finding that, in several situations, the number of memory CD4 T cells continues to decline with time after antigen encounter (6–9). These characteristics often lead to a paucity of antigen-specific memory CD4 T cells persisting long-term in T cell–replete mice. Alternatively, memory CD4 T cells have been generated by transferring activated CD4 T cells into host mice in which competition for survival signals and niches is reduced, resulting in substantially larger memory cell populations surviving long-term. Such models have been used to define several distinguishing attributes of memory CD4 T cells (3).
After activation, naive CD4 T cells undergo rapid cell divisions after a lag period, and differentiate in multiple steps to become effectors that carry out many effector functions, both through cognate interactions with other cells and by the secretion of cytokines and chemokines. CD4 T cell effectors can be separated into several subsets based on the spectrum of cytokines they produce, including Th1, which is characterized by IFNγ production, and Th2, which is characterized by production of IL-4 and -5 (10, 11). After antigen clearance, the majority of effectors die via apoptosis, and a small population reverts to a resting state to become memory cells (9, 12, 13). Much debate surrounds the details of this process, and several models of memory T cell generation have been proposed (14). Many recent observations also suggest that antigen-specific memory CD4 T cell populations can display a surprising amount of heterogeneity, as assessed by phenotype, function, and anatomical location (15–18). This diversity of subpopulations can complicate analysis of memory cell properties, especially as some of the heterogeneity seen may reflect cells in different states of activation and subsets with different longevity (16, 19).
Memory CD4 T cell responses differ from naive responses not only quantitatively, because of increased precursor frequencies of antigen-specific cells (20, 21), but on a per-cell basis by several qualitative criteria. For example, memory CD4 T cells rapidly produce multiple Th1- and/or Th2-associated cytokines without a lag period, whereas naive cells produce primarily IL-2 with slower kinetics after TCR stimulation. In addition, memory CD4 T cells do not require interactions with MHC II molecules for survival signals, and their life spans far exceed those of their naive precursors (22, 23). Criteria discriminating between effector and memory cells are not as well defined, although a critical difference is an increased resistance to activation-induced cell death (AICD) in resting memory populations (24, 25). It is also unclear if key functional properties, such as susceptibility to AICD, change quickly as effectors come to rest, or more gradually. Because effector and memory CD4 T cells may have very different capacities and mechanisms by which they can contribute to responses against pathogens, it is critical to understand when functional transitions occur. Analyses of gene expression have identified genes that are highly expressed in memory CD8 T cells in human and mouse (26–29). Studies with human CD4 T cells also indicate that memory cells express higher levels of some activation-induced genes than do naive cells (30). Changes in gene expression provide insight into the molecular differences between T cell subsets; however, the kinetics of such changes during memory differentiation are not known.
We evaluate whether the transition from effector to memory CD4 T cell is rapid, or if the acquisition of memory phenotype and function is more gradual. We investigate the progression from effector to memory using well-defined populations of naive cells, highly activated effector cells, short-term 3 d rested effector cells, and effectors rested for at least 60 d in MHC II–deficient (MHC II KO) hosts, resulting in a homogeneous population of quiescent memory CD4 T cells that is maintained long-term in the absence of antigen. We show that the phenotype, as well as several key functional attributes of such memory cells, are already expressed by highly activated effectors after only 3 d of rest, indicating a rapid transition. This rapid transition from effector to memory is observed with both Th1 and Th2 lineage CD4 T cell effectors and occurs when effectors rest 3 d in vitro or in vivo, suggesting a default, cell-intrinsic program. The transition from effector to memory is also rapid in terms of the loss of expression of a large cohort of genes related to effector function and cell cycling. Finally, we show that the expression of a small cohort of genes increases only gradually after rest and is unique to memory CD4 T cells.
RESULTS
Rapid changes in phenotype during transition to memory
The major question we address is to what extent short-term 3 d rested effectors resemble the effectors from which they originate versus the memory cells to which they give rise. Effector populations were generated in vitro from TCR transgenic CD4 T cells to ensure homogeneous populations of highly activated, antigen-experienced cells. Effector cells were washed thoroughly and rested in the absence of antigen, either in vitro or in vivo, in Thy-disparate hosts for 3 d to generate short-term rested effectors. Memory CD4 T cells were generated by transferring effector cells into MHC II KO hosts for an extended period of rest (60 d). We used MHC II KO mice as hosts to generate memory cells because transferred effectors cells receive no antigen-specific stimulation and undergo very little if any homeostatic proliferation, and a sizeable portion of donor cells survive long-term (12, 22).
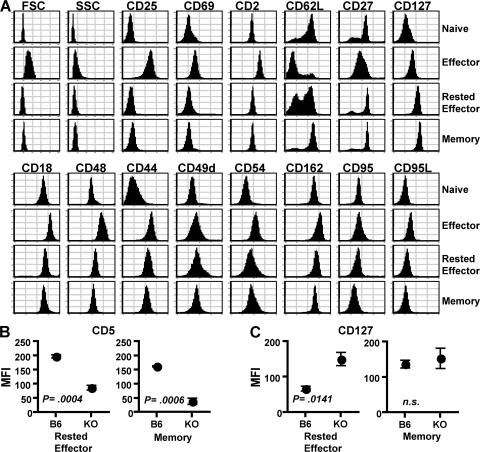
We first characterized the phenotype of naive cells, Th2 effector, and Th2 effectors rested for either 3 (rested effector) or 60 d or more (memory) in MHC II KO hosts. A broad panel of activation markers, adhesion, costimulatory, and cell death/survival molecules were differentially expressed by naive and effector cells (Fig. 1 A). In contrast, memory cells closely resembled naive cells, but were clearly distinguished based on increased expression of CD54 and CD44. Memory cells also up-regulated CD127 (IL-7rα), the expression of which is critical for access to IL-7 and CD4 T cell survival (31, 32). 3 d rested effectors closely resembled memory cells, having down-regulated expression of most effector-associated markers, retained CD44 and CD54 expression, and with up-regulated CD127.
Figure 1.
Cell-surface phenotype of CD4 T cell subsets. (A) Naive AND.Thy1.1 CD4 T cells, 4 d in vitro–generated Th2 effectors, and effectors rested for 3 (Rested Effector) or 60 d (Memory) after transfer into MHC II KO hosts were stained for the indicated markers and analyzed by flow cytometry. All histograms were generated from CD4+, Thy1.1+ donor cells. Results are representative of three separate experiments following subsets originating from the same naive CD4 T cell preparation. Mean fluorescence intensity (MFI) ± the SD of CD5 (B) and CD127 (C) signal of donor cells after 3- (Rested Effector) or 60-d (Memory) transfer into C57BL/6 (B6) or MHC II KO (KO) hosts. P values obtained by an unpaired, two-tailed Student's t test.
To rule out effects of the CD4 lymphopenic environment of MHC II KO hosts on the effector–memory transition, we transferred Th2-polarized effectors into intact B6 hosts. Absolute numbers of transferred cells recovered from B6 hosts were similar to recoveries from MHC II KO hosts 3 d after transfer, but fell dramatically after 60 d, whereas numbers in MHC II KO hosts were maintained (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1). Recent studies support the maintenance of only very low frequencies of memory CD4 T cells of a given clone long-term in T cell–replete mice (33). This difference in the maintenance of memory cells in lymphopenic versus intact hosts is likely caused by competition for IL-7 and other survival factors and niches, or regulation of homeostasis by endogenous memory T cells (32, 34–36). Though rested effectors in intact B6 hosts did not exhibit the prolonged persistence observed in MHC II KO hosts, these cells did persist at significantly higher levels for extended periods than naive cells of the same specificity transferred into B6 hosts (Fig. S2).
Importantly, a rapid transition from effector to memory was also observed in intact, T cell–replete B6 hosts. The phenotype of transferred Th2 effector cells was nearly identical in both B6 and MHC II KO hosts after a 3-d rest, with the notable exceptions of CD5 and CD127; those in normal B6 hosts expressed significantly higher CD5 and lower CD127 than cells rested in MHC II KO hosts (Fig. 1, B and C). The very low numbers of transferred cells routinely detectable in B6 hosts after 60 d of rest precluded extensive analysis of this population, but we did confirm that important markers, such as CD44, CD54, and CD62L, were similarly expressed by memory cells rested for 60 d in either B6 or MHC II KO hosts (unpublished data). Furthermore, when compared with rested effectors generated in B6 hosts, memory cells generated in B6 hosts expressed similar CD5, but higher levels of CD127, which is equivalent to memory cells rested in MHC II KO hosts (Fig. 1, B and C).
We observed a similar rapid shift in phenotype when Th1 effectors were rested for 3 d in vivo (unpublished data). Though effectors of both lineages were similar as measured by the majority of markers analyzed, Th1 and Th2 cells were distinguishable by the differences listed in Table I. However, after 3 d rest, Th1 and Th2 cells appeared virtually identical, and after 60 d, no significant differences were observed using this panel of markers (Table I). These results demonstrate that both Th1 and Th2 effectors undergo rapid, comprehensive phenotypic changes upon antigen clearance, and that resting cells of both lineages are far more similar phenotypically than the effectors that are their precursors, suggesting that certain functional differences between them may be expressed only at the activated effector stage.
Table I.
MFI values of selected markers on Th1 and Th2 subpopulations
| Effector
|
Rested effector
|
Memory
|
||||
|---|---|---|---|---|---|---|
| Th1 | Th2 | Th1 | Th2 | Th1 | Th2 | |
| CD4 | 1,570 ± 141a | 1,004 ± 95b | 1,097 ± 192 | 823 ± 73 | ||
| CD30 | 17 ± 1 | 50 ± 3b | 10 ± 1 | 14 ± 2 | ||
| CD43 | 2,120 ± 377 | 404 ± 104b | 398 ± 45 | 201 ± 25b | 210 ± 72 | 125 ± 22 |
| CD48 | 511 ± 30 | 790 ± 36b | 202 ± 37 | 233 ± 6 | ||
| CD49f | 126 ± 16 | 32 ± 2b | 22 ± 3 | 14 ± 4 | ||
| CD54 | 704 ± 90 | 233 ± 11b | 139 ± 21 | 99 ± 18 | ||
| CD69 | 55 ± 8 | 21 ± 7b | 33 ± 11 | 25 ± 3 | ||
| CD95 | 273 ± 16 | 90 ± 16b | 113 ± 9 | 45 ± 3b | 63 ± 16 | 35 ± 15 |
Average MFI +/− SD of at least three separate experiments
Significant difference between Th1 and Th2 (P < 0.01) by unpaired Student's t test
Cytokine profiles of CD4 T cell subsets
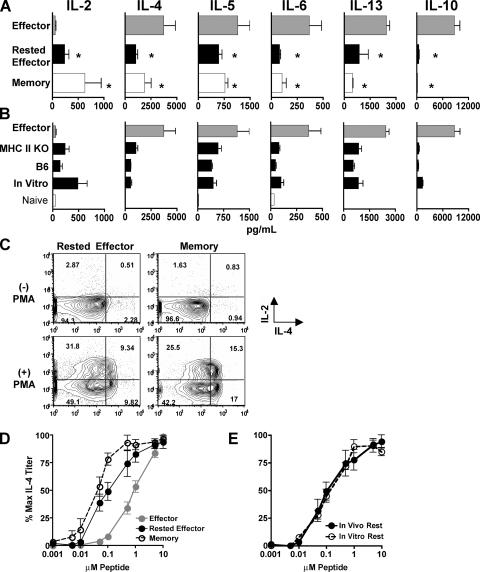
We next compared cytokine production by Th2 effectors, and effectors rested for either 3 (rested effector) or 60 d (memory) in MHC II KO hosts (Fig. 2 A), and by rested effectors generated in either intact B6 hosts, or in vitro (Fig. 2 B). Purified CD4 T cells were stimulated with APCs and peptide for 18 h, during which time naive cells produced only low levels of IL-2 and -6 (Fig. 2 B). As previously reported (9, 37), memory cells produced significantly less IL-4 and -5, but more IL-2 than effectors (Fig. 2 A). Memory cells also produced less IL-6, -13, and strikingly less IL-10 than effectors. Cytokine production from rested effectors and memory cells was very similar and significantly different from effectors, with reacquisition of IL-2, reduction of IL-4, -5, -6, and -13, and loss of IL-10 production (Fig. 2 A). Cytokine production from rested effector and memory populations was also similar, as determined by intracellular cytokine staining (ICCS), with similar numbers of IL-4+, -2+, and double IL-4+/-2+ cells (Fig. 2 C). These results show that, in addition to phenotype (Fig. 1), cytokine production from rested effector and memory populations is similar on a per-cell basis. A similar transition was observed in Th1-polarized cells both on a population and on a per-cell basis with increased IL-2, decreased IFN-γ, and an almost complete loss of IL-10 production by both rested effectors and memory cells compared with Th1 effectors (unpublished data). Effectors rested for 3 d in intact B6 hosts, or rested in vitro for 3 d produced similar amounts and patterns of cytokines as cells rested in MHC II KO hosts (Fig. 2 B).
Figure 2.
Cytokine production from CD4 T cell subsets. (A) AND.Thy1.1 Th2 effectors (Effector) and effectors rested for either 3 (Rested Effector) or 60 d (Memory) in MHC II KO hosts were stimulated with APC and PCCF peptide (5 μM) for 18–20 h. Supernatants were analyzed for cytokines by Luminex; the summary of three experiments is shown ± the SD. * denotes the significant difference from effector cytokine production (P < 0.05) as obtained by an unpaired, two-tailed Student's t test. (B) Effectors rested for 3 d in either MHC II KO or B6 hosts or rested in vitro were cultured with APCs and peptide for 18–20 h. Cytokine production from effectors and naive cells is also shown. (C) Th2-rested effector and memory populations were either stimulated or not with PMA and ionomycin and analyzed for ICCS. Brefeldin A was added after 2 h, and after 4 h cells were stained for IL-2 and -4. (D) Th2 effector, rested effector, or memory cells were cultured with APC, and graded doses of peptide for 18–20 h. IL-4 was measured in supernatants from three separate experiments, and the summary of the percentage of maximal IL-4 titer obtained with each peptide concentration is shown. (E) Comparison of the percent maximal IL-4 response to graded peptide doses by Th2 effectors rested either in vivo or in vitro for 3 d.
Another key feature of primed CD4 T cells is their ability to be optimally restimulated by several-fold lower doses of antigen than is needed for naive cells (38). We measured IL-4 from supernatants of effectors, rested effectors, and memory cells stimulated with graded doses of specific peptide. Results are presented as the percentage of maximal IL-4 production because the populations produced different maximal quantities of IL-4 (Fig. 2 A). Both rested effector and memory cells responded better than effectors to less peptide, producing near maximal IL-4 levels to 10-fold lower antigen doses (Fig. 2 C). Effectors rested 3 d in vitro or in vivo responded identically (Fig. 2 D).
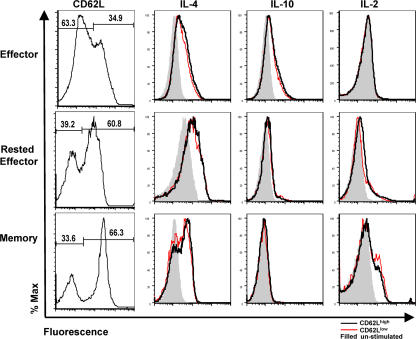
Several reports support the existence of separate subsets of memory T cells based on expression of CD62L and CCR7. It has been suggested that CD62Lhigh, CCR7high cells be termed “central–memory,” and that this subset produces IL-2 in response to restimulation, whereas “effector–memory” cells (CD62Llow and CCR7low) produce little IL-2 and increased amounts of effector cytokines (15). To test for functionally heterogeneous subsets of rested effector and memory cells based on CD62L expression, we restimulated Th2-polarized cells in the presence of TAPI-2, which blocks the activation-induced cleavage of surface CD62L (39) that ordinarily confuses discrete populations based on CD62L expression. This allowed analysis of cytokine production and CD62L expression on a single-cell level using ICCS. CD62Lhigh and CD62Llow populations of effector, rested effector, and memory cells contained nearly identical frequencies of IL-4–, IL-10–, and IL-2–positive cells (Fig. 3), suggesting no obvious functional heterogeneity in cytokine production potential correlating with CD62L expression. This pattern was also observed with Th1-polarized cells when assayed for IFN-γ, IL-2, and IL-10 (unpublished data). We were unable to similarly separate subpopulations based on CCR7 expression, as no significant differences in staining were observed within antigen-experienced CD4 T cell subsets (unpublished data).
Figure 3.
Cytokine production and CD62L expression within CD4 subsets. AND.Thy1.1 Th2 Effectors (Effector), and effectors rested for 3 (Rested Effector) or 60 d (Memory) in MHC II KO hosts were stimulated with PMA and ionomycin in the presence of TAPI-2. Intracellular staining was performed for IL-2, -4, and -10. Histograms representing the cytokine production of CD62Lhigh (black line) and CD62Llow (red line) subsets of each population were generated from CD4+, Thy1.1+ events. Shaded histograms represent unstimulated controls. The percentage of CD62Lhigh/low cells within each population is shown in the far left column.
AICD of CD4 T cell subsets
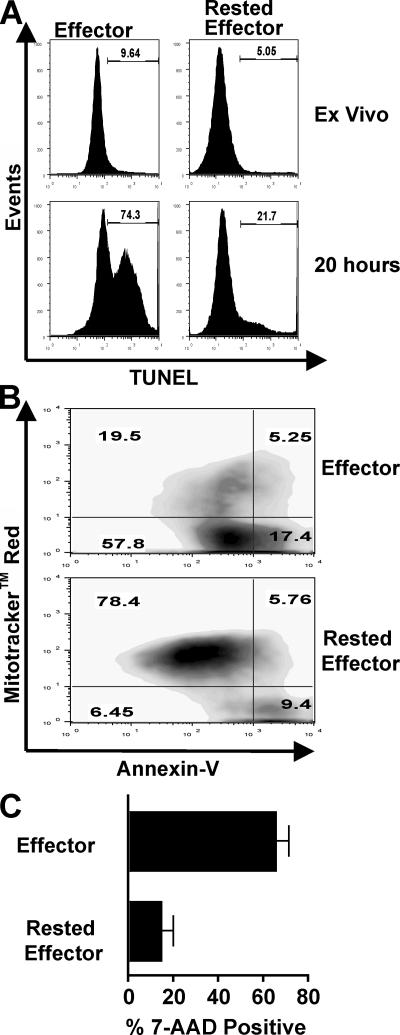
Activated effectors, in contrast to resting naive and memory cells, are highly susceptible to AICD (3, 23, 25, 40). To test whether 3 d–rested effectors resemble sensitive effector or resistant memory cells more, CD4 T cells rested in vivo were purified and restimulated in vitro. As previously reported (40), 20-h restimulation induced significantly more AICD in Th1 relative to Th2 effectors (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1). We concentrated on Th1-polarized cells because of the dramatic level of observed effector AICD relative to Th2 cells. Several pathways can lead to apoptosis of activated T cells (40–42), and we therefore assayed for several cellular criteria associated with apoptosis, including DNA fragmentation (TUNEL staining), nuclear permeability (7-aminoactinomycin-D [AAD] staining), phosphatidylserine exposure on the cell surface (annexin-V staining), and loss of mitochondrial membrane potential (Mitotracker red staining). By all criteria, rested effectors displayed a dramatically reduced level of apoptotic and dead cells compared with effectors, including a greater than threefold reduction in TUNEL+ cells, and a fourfold increase in both Mitotracker+Annexin V− cells and 7-AAD− cells (Fig. 4). Effectors rested in vitro were equally resistant to AICD (unpublished data), and this level of AICD is similar to that previously seen in memory populations (23).
Figure 4.
AICD in CD4 T cell subsets. Highly enriched populations of 4 d Th1 AND.Thy1.1 effectors and 3 d–rested effectors were restimulated for 20 h in vitro. (A) After 20 h, cells were harvested and analyzed for dead and apoptotic cells via TUNEL assay; populations were analyzed before (ex vivo) and after stimulation with platebound Vβ3. (B) Effectors and rested effectors were stimulated with APC and PCCF peptide, analyzed for mitochondrial permeability by Mitotracker red staining, and stained with Annexin V. (C) After 20-h stimulation, populations of effectors and rested effectors were stained with 7-AAD.
Rapid loss of expression of “effector-associated” genes
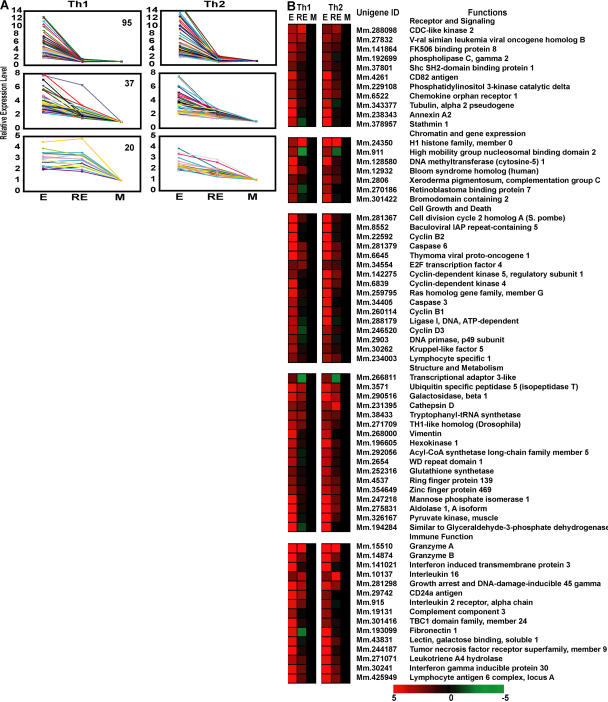
Our functional and phenotypic analyses focused on known attributes and activities of activated and resting CD4 T cells. It is possible that an unbiased, broader investigation might reveal additional differences between rested effector and memory populations. Thus, we compared gene expression profiles of highly purified CD4 T cell subsets from both Th1 and Th2 lineages. In this analysis, we looked at >4,000 lymphocyte-specific genes expressed without restimulation. 250 genes, common in both Th1 and Th2 lineages, were highly expressed in effectors relative to naive cells and were significantly down-regulated in memory cells generated by resting effectors for 60 d in MHC II KO hosts. Strikingly, the majority of these effector-associated genes (84%; 210 of 250) were rapidly down-regulated as a consequence of resting highly activated effectors for only 3 d in vitro (Fig. 5 A and Table S1, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1), with only a fraction (16%; 40 of 250) exhibiting significant differences between such rested effectors and memory cells. Using a different gene filter, 3 d in vitro–rested effectors closely resembled 3 d in vivo–rested effectors (18; Table S2).
Figure 5.
Identification of effector-associated genes lost during the transition from effector to memory. (A) Patterns of down-regulation of effector-associated genes common in Th1 and Th2 lineages. Using rested effectors generated in vitro as intermediates, three patterns of down-regulated expression can be seen (from top to bottom): rapidly down-regulated in rested effector and memory, gradually down-regulated, and down-regulated only in memory. Numbers indicate genes in each group. (B) Functional grouping of effector cell–associated genes. Genes whose expression levels were significantly down-regulated during transition from effector to memory are presented as the normalized intensity ratio between effector and effector (E), effector and rested effector (RE), and effector and memory cells (M). The data were derived from 12 independent measurements of 3 independent hybridizations. Genes with increased expression are shown in red and decreased expression is shown in green. Complete data can be found in Table S1, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1.
Analysis of the function of known effector-associated genes revealed changes in several gene classes. First, growth and apoptosis trends seen in effectors were reversed during 3 d of rest through rapid down-regulation of active growth and cell cycle regulators, including Akt1, cyclins, and cyclin-dependent kinases, as well as caspases and granzymes (Fig. 5 B). Second, a rapid reduction of expression of genes coding for several effector molecules, including surface receptors, adhesion, and other related molecules was observed (Fig. 5 B). Third, we observed expression changes likely to be associated with the transition from a cycling to a resting state, including decreases in cytoskeleton proteins, the decline of glycolysis-related enzymes, and of enzymes in other metabolic pathways (Fig. 5 B). Most of these genes were up-regulated during transition of naive cells to effectors, which is consistent with the designation as effector-associated genes (Table S1).
Gradual acquisition of expression a cohort of “memory-associated” genes
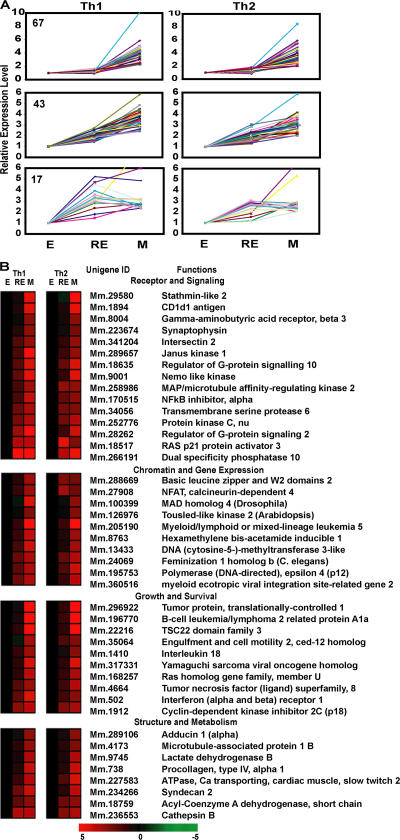
We also identified 144 genes, common in both Th1 and Th2 lineages, which were significantly increased in memory cells. Compared with the rapid loss of expression of effector-associated genes, the gain of expression of memory-associated genes was often more gradual. Only 13% of memory-associated genes (18 of 143; Fig. 6 A and Table S1) were expressed at similar levels by in vitro–rested effectors and memory cells. A similar pattern was observed when 3 d in vivo–rested effectors were compared with memory cells (18; Table S2). The expression level of the majority of memory-associated genes continued to increase as cells continued to rest (113 of 143; 79%; Fig. 6 A and Table S3, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1).
Figure 6.
Identification of memory-associated genes. (A) Patterns of up-regulation of memory-associated genes common in Th1 and Th2 lineages. Using rested effectors generated in vitro as intermediates, three patterns of down-regulated expression can be seen (from top to bottom): up-regulated only in memory, gradually up-regulated, and up-regulated in both rested effector and memory. Numbers indicate genes in each group. (B) Functional grouping of memory cell–associated genes. Genes whose expression levels were significantly up-regulated during transition from effector to memory are presented as the normalized intensity ratio between effector and effector (E), rested effector and effector (RE), and memory and effector cells (M). The data were derived from 12 independent measurements of 3 independent hybridizations. Complete data can be found in Table S2, available at http://www.jem.org/cgi/content/full/jem.20070041/DC1.
Within the cohort of memory-associated genes, we found up-regulation of genes associated with exit from cell cycle, such as cyclin-dependent kinase inhibitor (cdkn2c), as well as genes associated with enhancement of survival (Fig. 6 B). We also found up-regulation of expression of a variety of surface receptors and up-regulation of several intracellular signaling molecules such as Jak1, Ras p21 protein activator (Rasa3), Sac kinase member Lyn, protein kinase C (Prkcn), and dual specificity phosphatase 10 (Dusp10). Finally, we found increases in DNA (cytosine5-)-methyltransferase 3-like (Dnmt3L), which is a regulator of DNA methylation in maternal imprinting during development (43). Thus, in addition to the loss of expression of a great many effector-associated genes, memory cells that survive for ≥60 d up-regulated a cohort of genes to a level greater than that seen at 3 d after the initiation of a resting state.
DISCUSSION
We show that major aspects of the transition from effector to memory CD4 T cell occur rapidly, with effectors adopting the phenotype and functional attributes of resting memory cells within 3 d of antigen and cytokine removal. This transition is mirrored by the rapid down-regulation of a large cohort of effector-associated genes. However, we do find a smaller cohort of memory-associated genes that increases in expression only after an extended period of rest. Our results suggest that this rapid transition represents a largely default, cell-intrinsic program, as similar results were seen in both Th1 and Th2 populations that were rested in vivo, in both intact and MHC II KO hosts, as well as in vitro. These observations support a model in which, other than the removal of antigen, few if any positive signals are required for the transition from the effector to memory mode, indicating that the transition was programmed during activation or effector generation, as has been suggested for other aspects of T cell behavior, including proliferation and contraction (44, 45). Transfer studies of cells stimulated in vitro suggest that programming for memory, like that for effector function, occurs within 1–2 d after initial stimulation (46). Various signals during priming, all of which are present in our effector cultures, may separately and/or together program CD4 T cells for memory.
Recent observations have suggested that some memory CD4 T cells may be maintained long-term by antigen (47), and that two distinct populations of cells, one dependent on and one independent of antigen for long-term survival, might explain heterogeneity seen in some studies of CD4 T cell memory (19). Thus, variables such as TCR avidity might drive competition within a responding polyclonal CD4 T cell population for various survival niches, complicating the analysis of the properties of memory cells. Our analysis of memory cells maintained long-term in MHC II KO hosts is restricted to cells that have the potential for prolonged persistence in the absence of antigen. Using this well-defined, homogeneous population of long-term resting, antigen-experienced cells allows for unambiguous conclusions concerning the transition from activated effector to resting memory cell.
Several factors must regulate and limit the long-term persistence of individual memory CD4 T cells in vivo as indicated by the dramatically lower number of transferred effector cells surviving in intact B6 hosts compared with MHC II KO hosts 60 d after transfer. The mechanisms of homeostasis of memory CD4 T cells are currently not as well defined as for memory CD8 T cells. One extrinsic factor that we have previously identified as being critical for the survival of memory CD4 T cells in the absence of antigen is IL-7 (32, 34). It is interesting that effectors rested for 3 d in intact B6 hosts expressed significantly lower levels of CD127 than cells rested in MHC II KO hosts. It is possible that the low number of transferred cells surviving long-term in B6 hosts is, at least in part, caused by suboptimal access of transferred cells to IL-7 caused by competition with endogenous T memory cells. This interpretation is supported by the finding that cells surviving in either host at 60 d expressed similarly high levels of CD127, implying an equivalent access to IL-7 signaling in surviving long-term memory cells.
The low level of many adhesion and homing molecules expressed on rested effectors suggests that they should traffic differently than activated effectors and should be similar in migration to memory cells. We have previously addressed the homing pattern of effectors and rested effectors after adoptive transfer into naive animals. Although effector CD4 T cells displayed a broad homing pattern to both lymphoid and nonlymphoid sites, rested effectors trafficked preferentially to spleen and lymph node and were only rarely found in tertiary sites (48). The distribution of rested effectors resembled that of transferred memory cells (24, 49).
Several observations indicate that memory CD4 T cells, as compared with effectors, regain the ability to secrete significant amounts of IL-2 upon stimulation (9, 18, 50, 51). We show that the ability to produce IL-2 is regained rapidly after removal of antigen. IL-2 acts as a growth and survival factor in primary CD4 T cell responses (52), and thus may be an essential autocrine or paracrine factor driving secondary expansion. IL-2 is also key for supporting memory CD8 T cell survival (53). The transition from effectors, that do not secrete IL-2, to rested effectors and memory cells that do, thus has the potential to shape very different responses. We also find that both Th1 and Th2 effectors produce significantly more IL-10 than rested effectors and memory cells. It is interesting to speculate that production of IL-10, which is a cytokine associated with immunosuppressive effects (54), may constitute a poorly understood, transient mechanism of immune modulation acting during the peak of highly activated CD4 T cell responses. A further attribute of highly activated effector cells is their high susceptibility to AICD. We report that only a 3-d rest is required for the acquisition of the AICD-resistant phenotype by highly activated effectors, which is an attribute classically associated with memory CD4 T cells.
Observations in a different model system suggest that memory CD4 T cells that were primed and maintained long-term in MHC II KO environments can display impaired effector functions. This finding correlated with increased expression of CD5, CD3, and TCR on cells resting in MHC II KO as opposed to MHC II intact hosts (55). In contrast to Kassiotis et al., we found that CD4 T cell effectors primed in vitro and rested in MHC II KO hosts displayed a decreased level of CD5 compared with cells resting in B6 hosts (Fig. 1 B) and equivalent levels of CD3 and TCR (unpublished data), and we found no evidence of functional impairment.
By taking transcriptional portraits of highly purified populations, we have identified several hundred genes that are differentially expressed during the transition from effector to memory CD4 T cells of both Th1 and Th2 lineages. First, we find a rapid transition from actively dividing effectors to relatively quiescent memory cells that occurs within 3 d of withdrawal of antigen and cytokines, as indicated by down-regulation of cell cycle–activating and up-regulation of cell cycle-arresting genes. Second, rapid changes in expression of adhesion molecules, growth factors and receptors, and signaling molecules are consistent with the rapid shifts in constricted migration patterns, resistance to AICD, and lack of division of memory cells in response to cytokine (12). Finally, a more gradual transition from effector to memory seems to involve the up-regulation of expression of genes involved in chromatin remodeling, resulting in epigenetic changes that, along with differential expression of transcription factors and signaling molecules, ensures the sustained alterations that allow a rapid memory response to proceed in a subsequent encounter with antigen. Although most epigenetic changes occur during cell division, it is possible that certain modifications are initiated in resting memory CD4 T cells (56). In addition, some of the changes in gene expression between rested effector and memory cells may also reflect selection of those resting cells that are most able to survive in the limited environmental niches that support memory T cell survival. Up-regulation of IL-7 receptor (CD127) and increase in Bcl2a1a expression in memory versus rested effector CD4 T cells may be indicative of such selection. Overall, expression patterns of Th1 and Th2 CD4 T cell subsets were strikingly similar, suggesting that underlying events associated with memory cell development are highly conserved. This is further supported by our observations of a near identical surface phenotype on resting Th1 and Th2 cells.
Genes highly expressed in memory cells are of great interest. For example, in view of its role in control of the formation of functional plasma cells (57), cdkn2c might also play a role in functional CD4 T memory cell formation. The role of Dnmt3L may be to suppress the transcription of those genes highly expressed in effector cells by methylation of DNA and/or deacetylation of histones by recruiting Hdac1 into chromatin to keep chromatin structures in an inactive status (58). These genes, and nearly all other memory-associated genes, were weakly expressed in naive CD4 T cells. Thus, Fig. 6 provides a short list of candidates that may explain some aspects of the profound differences between naive and memory CD4 T cells. Further studies are warranted to elucidate the functions of each of these genes in memory T cell generation, function, and homeostasis. Although the cells we obtained after transfer into MHC II KO hosts meet all criteria of “true” memory cells, it will be important to compare their gene expression profile to memory CD4 T cells obtained from animals in which the competition for memory niches can be manipulated, resulting in progressively fewer transferred cells surviving long-term. This comparison might further highlight certain memory-associated genes identified here, or reveal additional genes involved in the homeostasis of memory CD4 T cells.
Overall, gene expression profiles were remarkably similar between naive and memory CD4 T cells; we found only ∼20 genes that were uniquely expressed by memory cells resting in MHC II KO mice for 60 d, compared with naive cells. Thus, although memory cells might express certain genes conferring the ability to survive long-term, they still have much in common with shorter-lived naive CD4 T cells. It is interesting to speculate that long-lived memory CD8 T cells might not resemble naive CD8 T cells to the degree that we have observed with CD4 T cells, as this might explain in part the often observed paucity of long-term memory CD4 T cells as compared with memory CD8 T cells.
Based on our findings, we would suggest that in vitro–generated CD4 T cell effectors, rested by removal of stimulation, are actually early memory cells. We postulate that such cells are generated in vivo as soon as antigen is cleared, causing an abrupt shift from an effector state to a memory state in the host responding to immunization or pathogen exposure. We have assessed the transition from effector to memory in vivo using an influenza infection model in which the response of naive virus-specific TCR transgenic CD4 T cells can be monitored (59). We have previously shown that only the most differentiated cohort of transgenic cells migrates to the lung at the peak of the primary response against the virus, and that these CD4 T cells resemble in vitro generated–Th1 effectors (59). After viral clearance (10–12 d after infection), transgenic cells remaining in the lung at day 14 resemble the rested effectors described in this study, showing reduced cell size, down-regulation of almost all adhesion and costimulatory markers, regained ability to produce IL-2 and loss of IL-10 production upon restimulation, and increased resistance to AICD (unpublished data) (55). These studies show that the transition from highly activated effector to a resting memory-like cell can occur rapidly in vivo.
Our results offer an insight into the special features of the memory CD4 T cell response. First, IL-2 and other effector-associated cytokines are rapidly produced by memory cells, whether they are newly formed (rested effector) or have persisted for some time. These cytokines can provide potent help for B cells, resulting in more rapid antibody production. Effector cytokines may also activate other APC populations with which they interact in a more dramatic way than naive CD4 T cells or highly differentiated effectors, which do not secrete IL-2. Second, the rapid loss of effector properties, including migration to nonlymphoid sites and loss of direct proliferation to cytokines (12), coupled with the shift from sensitivity to insensitivity to AICD, seem to ensure an abrupt switch from the activated effector mode, which acts in peripheral sites but may be pathological, to a more benign resting memory mode, where responses must be reinitiated in the periphery from a small, persistent memory population. Third, the high sensitivity of memory cells to low antigen doses should facilitate more rapid responses to live pathogens, which infect in small numbers and then replicate extensively.
MATERIALS AND METHODS
Mice.
Naive CD4 T cells were obtained from either AND.Thy1.1 transgenic (Tg) mice on a C57BL/6 (B6) background or AND Tg mice crossed to B6-GFP mice (AND.GFP) (60).
AND Tg mice express a TCR (Vα11, Vβ3) recognizing aa 88–104 of pigeon cytochrome c fragment (PCCF). Naive CD4 T cell donors were between 4 and 8 wk of age. Recipients of cell transfers were either intact B6 or MHC II–deficient mice (Aβ−/−) on a B6 background. Mice were obtained from the animal breeding facilities at the Trudeau Institute. All experimental animal procedures were conducted in accordance with the Trudeau Institute Animal Care and Use Committee guidelines.
Naive CD4 T cell isolation.
Naive CD4 T cells were obtained from pooled spleen and lymph node cells of unimmunized TCR Tg mice, as previously described (38). In brief, cells were passed through nylon wool and treated with antibodies against CD8, MHC II, CD11c, and CD24, followed by complement-mediated lysis (Harlan Bioproducts; Pel-Freez Biologicals). Remaining cells were further purified via discontinuous Percoll gradient separation (Sigma-Aldrich). Alternatively, naive cells were purified using positively selecting CD4 MACS beads (Miltenyi Biotec) after red blood cell lysis and Percoll separation. Resulting cells were routinely >95% CD4+, Vα11+, and Vβ3+, displaying a naive phenotype (CD44low, CD62Lhigh). For gene expression analysis, naive AND.GFP CD4 T cells were sorted to >99% purity.
Generation of effector and rested effector populations.
CD4 T cell effectors were generated in vitro, as previously described (12). In brief, 2 × 105/ml naive cells were cultured with 105/ml DCEK-ICAM APC (61) in the presence of 5 μM PCCF and 80 U/ml IL-2 for 4 d in RPMI 1640 media supplemented with 2 mM l-Glutamine, 100 IU penicillin, 100 μg/ml streptomycin (Invitrogen), 10 mM Hepes (Research Organics), 50 μM 2-mercaptoethanol (Sigma-Aldrich) and 7.5% fetal bovine serum (HyClone). Th2 effectors were generated by further adding 200 U/ml IL-4 and 10 μg/ml anti–IFN-γ (XMG1.2) at the initiation of culture; Th1 effectors were generated by further adding 2 ng/ml IL-12 and 10 μg/ml anti–IL-4 (11B11).
In vitro–rested effectors were generated by washing 4-d effectors thoroughly, and reculturing in fresh media for 3 d in the absence of antigen and cytokine. Live cells were isolated by Lympholyte (Cedarlane Laboratories) separation. In vivo–rested effectors were generated by injecting 2 × 107 effectors i.v. into naive hosts. Rested effector and memory cells were reisolated from pooled spleen and lymph nodes. Cells used for functional and phenotypic analysis were stained with αThy1.1 biotin-conjugated antibodies and separated with streptavidin MACS beads, and they were further purified via positive-selecting CD4 MACS beads. The resulting populations were routinely >95% CD4+, Thy1.1+, Vα11+, and Vβ3+. AND.GFP cells, which were used for microarray analysis, were obtained on the basis of GFP expression by FACS sorting using a FACS Vantage DIVA (Becton Dickinson) and were sorted to >99% purity.
Detection of cell-surface molecules by flow cytometry.
Surface staining was done in the dark at 4°C for 25–30 min in PBS supplemented with 1% BSA and 0.1% NaN3 (Sigma-Aldrich). 2.4G2 (anti–mouse CD16/CD32) was added to samples 5 min before staining to block nonspecific binding. The following fluorochrome-labled antibodies were purchased from BD Biosciences, unless otherwise noted: CD2-FITC (clone RM2-5), CD4-APC (RM4-5), CD5-FITC (53–7.3), CD18-PE (C71/16), CD25-PE (PC61), CD27-PE (LG.3A10), CD30-PE (mCD30.1), CD44-FITC (1M7), CD43-PE (1B11), CD48-FITC (HM48-1), CD49d-PE (9C10), CD49f-PE (GoH3), CD54-PE (3E2), CD62L-PE (Mel14), CD69-FITC (H12F3), CD90.1-PerCp (OX-7), CD95(FAS)-PE (Jo2), CD95L(FASL)-PE (MFL3; eBioscience), CD127-PE (A7R34; eBioscience), CD162-PE (2PH1), Vα11-PE (RR3-15), Vβ3-PE (KJ25). Samples were run on a Becton Dickinson FACS Scan, and raw data was analyzed using FlowJo software (Treestar, Inc.).
Detection of secreted and intracellular cytokines.
3 × 105 AND.Thy1.1–purified CD4 T cells were cultured with 1.5 × 105 DECK-ICAM cells and graded concentrations of PCCF (10–.001 μM) for 18 h. Supernatants were assayed for IL-4 by ELISA, as previously described (38). IL-2, -5, -6, -10, and -13 were assayed using Beadlyte Beadmates (Millipore) and the Luminex 100 system (Luminex Corp.). Intracellular cytokine staining was performed as previously described (62). In brief, CD4 T cells were stimulated for 4 h with 10 ng/ml PMA (Sigma-Aldrich) and 50 ng/ml ionomycin (Sigma-Aldrich). 2 h after stimulation, 10 μg/ml Brefeldin A (Sigma-Aldrich) was added for the duration of the culture. Cells were then surface stained and fixed for 20 min with 4% paraformaldehyde (Sigma-Aldrich). After washing in PBS, cells were incubated with 200 μl of 0.1% saponin buffer for 10 min, followed by the addition anti–mouse IL-2, -4, -10, and IFN-γ antibodies (BD Biosciences) for 20 min.
Detection of AICD.
Purified CD4 T cells were assayed for susceptibility to AICD by restimulating highly enriched populations of AND.Thy1.1 CD4 T cells for 20 h in vitro with either APC and peptide or with platebound α-Vβ3 antibody. After 20 h, cells were harvested and assayed for fragmented DNA by TUNEL assay (Invitrogen), for surface phosphatidylserine exposure by Annexin V staining (Invitrogen), for mitochondrial membrane potential by Mitotracker red staining (Invitrogen), or for 7-AAD (eBioscience), as per the manufacturer's instructions.
Construction of cDNA microarray filter and experimental procedure.
To maximize the content of immune-related genes, we constructed a lymphocyte-specific cDNA microarray gene filter consisting of 4,608 cDNA clones. Of the genes on the filter, ∼64% are known genes, 26% are unnamed genes, and 10% are expressed sequence tags. Genes were amplified by PCR and printed onto nylon membranes (SuperCharge Nylon; S&S) using a MicroGrid II printer (BioRobotics; Apogent Discoveries, Inc.). Each cDNA clone was printed twice with one open spot for local background. RNA isolation, cDNA probe synthesis, and labeling were performed as previously described (30). In brief, total RNA was isolated from highly purified CD4 T cells using STAT60 (TEL-TEST, Inc.), and cDNA probes were generated from 5–15 μg of total RNA of cells using Superscript II RNase H-Reverse Transcriptase (Invitrogen) in the presence of 100 μCi α-[33P]dCTP (PerkinElmer Life Sciences) and 500-ng oligo (dT)12-18 primers (Invitrogen). Two gene filters were prehybridized with 10 ml MicroHyb solution (Invitrogen) at 43°C for 2 h; cDNA probes were added into the prehybridized filters, along with 1 ng/ml poly-dA, 1 μg/ml denatured mouse Cot-1, and 200 μg/ml denatured salmon sperm DNA (Invitrogen), and hybridization continued overnight. Filters were washed at 65°C with SSC (2 times), with 1% SDS for 30 min, and with SSC (0.5 times) with 1% SDS for 30 min, and then exposed to a phosphorimaging screen for 2 d. Images were collected from a Typhoon 9410 Imager (GE Healthcare) at 50 μm resolution.
Complete MAIME compliant microarray data is available from GEO (accession no. GSE8352 and NCBI tracking no. 15303196).
Microarray data analysis and RT-PCR.
Images were processed using Array-Pro Analyzer 4.0 software (Media Cybernetics). The resulting intensity values were normalized filter-wide with the median intensity value. Normalized spot intensity was used for the analysis between cell subsets with a modified false discovery rate (FDR) (63, 64). The FDR was set to 0.05, which corresponds to the mean proportion of false positives = 5%. Additional selection criteria, such as intensity fold changes and minimum intensity, were applied to further reduce the FDR. Genes were grouped based on the biological role of each gene using Onto-Express (65). The identity of selected clones was confirmed by sequencing. The differentially expressed genes were further confirmed by real-time quantitative RT-PCR by the standard procedure, as previously described (66).
Online supplemental material.
Fig. S1 summarizes the frequency and absolute number of effector CD4 T cells transferred into either MHC II KO or B6 hosts after 3 or 60 d. Fig. S2 compares the long-term persistence of naive and rested effector CD4 T cells after transfer into intact B6 hosts. Fig. S3 highlights the difference in maximal level of AICD between Th1- and Th2-polarized effector populations. 3 tables are included to provide more extensive information on gene expression studies. Table S1 provides a complete list of genes found to be highly expressed by effector cells of both Th1 and Th2 lineage as compared with rested effector and memory cells (as summarized in Fig. 5).Table S2 summarizes the comparison of gene expression between in vitro– and in vivo–rested effectors. Table S3 provides a complete listing of genes found to be up-regulated in memory compared with effector CD4 T cells (as depicted in Fig. 6). The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20070041/DC1.
Supplemental Material
Acknowledgments
We thank A. Cooper, R. Dutton, D. Jelley-Gibbs, and T. Strutt for critical reading of the manuscript and helpful discussion.
This work was supported by National Institutes of Health (NIH) grant AI-46530 (awarded to S.L. Swain) and by the Trudeau Institute. This work was supported in part by the Intramural Research Program of the NIH, National Institute of Aging.
The authors have no conflicting financial interests.
Abbreviations used: AAD, aminoactinomycin-D; AICD, activation-induced cell death; ICCS, intracellular cytokine staining; MFI, mean fluoresence intensity; PCCF, pigeon cytochrome c fragment; Tg, transgenic.
References
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel, R.M., M.F. Bachmann, T.M. Kundig, S. Oehen, H. Pirchet, and H. Hengartner. 1996. On immunological memory. Annu. Rev. Immunol. 14:333–367. [DOI] [PubMed] [Google Scholar]
- 3.Dutton, R.W., L.M. Bradley, and S.L. Swain. 1998. T cell memory. Annu. Rev. Immunol. 16:201–223. [DOI] [PubMed] [Google Scholar]
- 4.Sprent, J., and C.D. Surh. 2001. Generation and maintenance of memory T cells. Curr. Opin. Immunol. 13:248–254. [DOI] [PubMed] [Google Scholar]
- 5.Cerottini, J.C., and H.R. MacDonald. 1989. The cellular basis of T-cell memory. Annu. Rev. Immunol. 7:77–89. [DOI] [PubMed] [Google Scholar]
- 6.Seder, R.A., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835–842. [DOI] [PubMed] [Google Scholar]
- 7.Homann, D., L. Teyton, and M.B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919. [DOI] [PubMed] [Google Scholar]
- 8.MacLeod, M., M.J. Kwakkenbos, A. Crawford, S. Brown, B. Stockinger, K. Schepers, T. Schumacher, and D. Gray. 2006. CD4 memory T cells survive and proliferate but fail to differentiate in the absence of CD40. J. Exp. Med. 203:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbertson, J., E. Biederman, K.E. Bennett, R.M. Kondrack, and L.M. Bradley. 2002. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J. Immunol. 168:1095–1102. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann, T.R., J.H. Schumacher, N.F. Street, R. Budd, A. O'Garra, T.A. Fong, M.W. Bond, K.W. Moore, A. Sher, and D.F. Fiorentino. 1991. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol. Rev. 123:209–229. [DOI] [PubMed] [Google Scholar]
- 11.Swain, S.L., L.M. Bradley, M. Croft, S. Tonkonogy, G. Atkins, A.D. Weinberg, D.D. Duncan, S.M. Hedrick, R.W. Dutton, and G. Huston. 1991. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol. Rev. 123:115–144. [DOI] [PubMed] [Google Scholar]
- 12.Hu, H., G. Huston, D. Duso, N. Lepak, E. Roman, and S.L. Swain. 2001. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat. Immunol. 2:705–710. [DOI] [PubMed] [Google Scholar]
- 13.Marrack, P., and J. Kappler. 2004. Control of T cell viability. Annu. Rev. Immunol. 22:765–787. [DOI] [PubMed] [Google Scholar]
- 14.Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 16.Kassiotis, G., and B. Stockinger. 2004. Anatomical heterogeneity of memory CD4+ T cells due to reversible adaptation to the microenvironment. J. Immunol. 173:7292–7298. [DOI] [PubMed] [Google Scholar]
- 17.Bingaman, A.W., D.S. Patke, V.R. Mane, M. Ahmadzadeh, M. Ndejembi, S.T. Bartlett, and D.L. Farber. 2005. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur. J. Immunol. 35:3173–3186. [DOI] [PubMed] [Google Scholar]
- 18.Swain, S.L., J.N. Agrewala, D.M. Brown, D.M. Jelley-Gibbs, S. Golech, G. Huston, S.C. Jones, C. Kamperschroer, W.H. Lee, K.K. McKinstry, et al. 2006. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol. Rev. 211:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson, J.M., M. MacLeod, V.S. Marsden, J.W. Kappler, and P. Marrack. 2006. Not all CD4+ memory T cells are long lived. Immunol. Rev. 211:49–57. [DOI] [PubMed] [Google Scholar]
- 20.Whitmire, J.K., M.S. Asano, K. Murali-Krishna, M. Suresh, and R. Ahmed. 1998. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J. Virol. 72:8281–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga, S.M., and R.M. Welsh. 1998. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J. Immunol. 161:367–374. [PubMed] [Google Scholar]
- 22.Swain, S.L., H. Hu, and G. Huston. 1999. Class II-independent generation of CD4 memory T cells from effectors. Science. 286:1381–1383. [DOI] [PubMed] [Google Scholar]
- 23.Swain, S.L. 2000. CD4 T-cell memory can persist in the absence of class II. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain, S.L., M. Croft, C. Dubey, L. Haynes, P. Rogers, X. Zhang, and L.M. Bradley. 1996. From naive to memory T cells. Immunol. Rev. 150:143–167. [DOI] [PubMed] [Google Scholar]
- 25.Inaba, M., K. Kurasawa, M. Mamura, K. Kumano, Y. Saito, and I. Iwamoto. 1999. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J. Immunol. 163:1315–1320. [PubMed] [Google Scholar]
- 26.Kaech, S.M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 111:837–851. [DOI] [PubMed] [Google Scholar]
- 27.Holmes, S., M. He, T. Xu, and P.P. Lee. 2005. Memory T cells have gene expression patterns intermediate between naive and effector. Proc. Natl. Acad. Sci. USA. 102:5519–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willinger, T., T. Freeman, H. Hasegawa, A.J. McMichael, and M.F. Callan. 2005. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J. Immunol. 175:5895–5903. [DOI] [PubMed] [Google Scholar]
- 29.Malek, R.L., H. Sajadi, J. Abraham, M.A. Grundy, and G.S. Gerhard. 2004. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 138:363–373. [DOI] [PubMed] [Google Scholar]
- 30.Liu, K., Y. Li, V. Prabhu, L. Young, K.G. Becker, P.J. Munson, and N. Weng. 2001. Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. J. Immunol. 166:7335–7344. [DOI] [PubMed] [Google Scholar]
- 31.Bradley, L.M., L. Haynes, and S.L. Swain. 2005. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 26:172–176. [DOI] [PubMed] [Google Scholar]
- 32.Kondrack, R.M., J. Harbertson, J.T. Tan, M.E. McBreen, C.D. Surh, and L.M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hataye, J., J.J. Moon, A. Khoruts, C. Reilly, and M.K. Jenkins. 2006. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 312:114–116. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., G. Huston, and S.L. Swain. 2003. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 198:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surh, C.D., O. Boyman, J.F. Purton, and J. Sprent. 2006. Homeostasis of memory T cells. Immunol. Rev. 211:154–163. [DOI] [PubMed] [Google Scholar]
- 36.Dooms, H., and A.K. Abbas. 2006. Control of CD4+ T-cell memory by cytokines and costimulators. Immunol. Rev. 211:23–38. [DOI] [PubMed] [Google Scholar]
- 37.Swain, S.L. 1994. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1:543–552. [DOI] [PubMed] [Google Scholar]
- 38.Rogers, P.R., C. Dubey, and S.L. Swain. 2000. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164:2338–2346. [DOI] [PubMed] [Google Scholar]
- 39.Zaph, C., J. Uzonna, S.M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 10:1104–1110. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, X., T. Brunner, L. Carter, R.W. Dutton, P. Rogers, L. Bradley, T. Sato, J.C. Reed, D. Green, and S.L. Swain. 1997. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185:1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hildeman, D.A., T. Mitchell, T.K. Teague, P. Henson, B.J. Day, J. Kappler, and P.C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744. [DOI] [PubMed] [Google Scholar]
- 42.Green, D.R. 2003. Overview: apoptotic signaling pathways in the immune system. Immunol. Rev. 193:5–9. [DOI] [PubMed] [Google Scholar]
- 43.Hata, K., M. Okano, H. Lei, and E. Li. 2002. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 129:1983–1993. [DOI] [PubMed] [Google Scholar]
- 44.Mercado, R., S. Vijh, S.E. Allen, K. Kerksiek, I.M. Pilip, and E.G.P. Am. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833–6839. [DOI] [PubMed] [Google Scholar]
- 45.Kaech, S.M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dooms, H., E. Kahn, B. Knoechel, and A.K. Abbas. 2004. IL-2 induces a competitive survival advantage in T lymphocytes. J. Immunol. 172:5973–5979. [DOI] [PubMed] [Google Scholar]
- 47.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 48.Swain, S.L., J.N. Agrewala, D.M. Brown, and E. Roman. 2002. Regulation of memory CD4 T cells: generation, localization and persistence. Adv. Exp. Med. Biol. 512:113–120. [PubMed] [Google Scholar]
- 49.Agrewala, J.N., D.M. Brown, N.M. Lepak, D. Duso, G. Huston, and S.L. Swain. 2007. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J. Biol. Chem. 282:6106–6115. [DOI] [PubMed] [Google Scholar]
- 50.Bradley, L.M., D.D. Duncan, K. Yoshimoto, and S.L. Swain. 1993. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J. Immunol. 150:3119–3130. [PubMed] [Google Scholar]
- 51.Bradley, L.M., G.G. Atkins, and S.L. Swain. 1992. Long-term CD4+ memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J. Immunol. 148:324–331. [PubMed] [Google Scholar]
- 52.Zhang, X., L. Giangreco, H.E. Broome, C.M. Dargan, and S.L. Swain. 1995. Control of CD4 effector fate: transforming growth factor β1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J. Exp. Med. 182:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams, M.A., A.J. Tyznik, and M.J. Bevan. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 441:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 55.Kassiotis, G., S. Garcia, E. Simpson, and B. Stockinger. 2002. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 3:244–250. [DOI] [PubMed] [Google Scholar]
- 56.Fann, M., J.M. Godlove, M. Catalfamo, W.H. Wood III, F.J. Chrest, N. Chun, L. Granger, R. Wersto, K. Madara, K. Becker, et al. 2006. Histone acetylation is associated with differential gene expression in the rapid and robust memory CD8(+) T-cell response. Blood. 108:3363–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tourigny, M.R., J. Ursini-Siegel, H. Lee, K.M. Toellner, A.F. Cunningham, D.S. Franklin, S. Ely, M. Chen, X.F. Qin, Y. Xiong, et al. 2002. CDK inhibitor p18(INK4c) is required for the generation of functional plasma cells. Immunity. 17:179–189. [DOI] [PubMed] [Google Scholar]
- 58.Bourc'his, D., G.L. Xu, C.S. Lin, B. Bollman, and T.H. Bestor. 2001. Dnmt3L and the establishment of maternal genomic imprints. Science. 294:2536–2539. [DOI] [PubMed] [Google Scholar]
- 59.Roman, E., E. Miller, A. Harmsen, J. Wiley, U.H. Von Andrian, G. Huston, and S.L. Swain. 2002. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196:957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kusser, K.L., and T.D. Randall. 2003. Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. J. Histochem. Cytochem. 51:5–14. [DOI] [PubMed] [Google Scholar]
- 61.Dubey, C., M. Croft, and S.L. Swain. 1995. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J. Immunol. 155:45–57. [PubMed] [Google Scholar]
- 62.Jelley-Gibbs, D.M., N.M. Lepak, M. Yen, and S.L. Swain. 2000. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J. Immunol. 165:5017–5026. [DOI] [PubMed] [Google Scholar]
- 63.Baldi, P., and A.D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinformatics. 17:509–519. [DOI] [PubMed] [Google Scholar]
- 64.Benjamini, Y., D. Drai, G. Elmer, N. Kafkafi, and I. Golani. 2001. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125:279–284. [DOI] [PubMed] [Google Scholar]
- 65.Khatri, P., S. Draghici, G.C. Ostermeier, and S.A. Krawetz. 2002. Profiling gene expression using onto-express. Genomics. 79:266–270. [DOI] [PubMed] [Google Scholar]
- 66.Hess, K., Y. Yang, S. Golech, A. Sharov, K.G. Becker, and N.P. Weng. 2004. Kinetic assessment of general gene expression changes during human naive CD4+ T cell activation. Int. Immunol. 16:1711–1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.