Abstract
Nitrite (NO2 −) is an intrinsic signaling molecule that is reduced to NO during ischemia and limits apoptosis and cytotoxicity at reperfusion in the mammalian heart, liver, and brain. Although the mechanism of nitrite-mediated cytoprotection is unknown, NO is a mediator of the ischemic preconditioning cell-survival program. Analogous to the temporally distinct acute and delayed ischemic preconditioning cytoprotective phenotypes, we report that both acute and delayed (24 h before ischemia) exposure to physiological concentrations of nitrite, given both systemically or orally, potently limits cardiac and hepatic reperfusion injury. This cytoprotection is associated with increases in mitochondrial oxidative phosphorylation. Remarkably, isolated mitochondria subjected to 30 min of anoxia followed by reoxygenation were directly protected by nitrite administered both in vitro during anoxia or in vivo 24 h before mitochondrial isolation. Mechanistically, nitrite dose-dependently modifies and inhibits complex I by posttranslational S-nitrosation; this dampens electron transfer and effectively reduces reperfusion reactive oxygen species generation and ameliorates oxidative inactivation of complexes II–IV and aconitase, thus preventing mitochondrial permeability transition pore opening and cytochrome c release. These data suggest that nitrite dynamically modulates mitochondrial resilience to reperfusion injury and may represent an effector of the cell-survival program of ischemic preconditioning and the Mediterranean diet.
Ischemia/reperfusion (I/R) injury is characterized by several cellular events, including the release of tissue enzymes, oxidative modification of essential proteins and lipids, and a dysregulated inflammatory response, which ultimately leads to tissue necrosis and apoptosis (1, 2). On a subcellular level, the mitochondrion is vital to tissue viability, and mitochondrial damage plays a central role in the progression of pathology after I/R. During I/R, mitochondrial ATP synthesis is decreased (leading to depletion of tissue high-energy phosphate stores) (3), enzymes of the respiratory chain are damaged (leading to diminished inner membrane potential) (4, 5), the permeability transition pore is opened (6), and upon reperfusion, reactive oxygen species (ROS) generation is increased. The mechanisms underlying cytoprotection of a large number of I/R therapeutic agents involves the regulation of mitochondrial function either through the modulation of membrane potential, ROS formation, or the activity of the ATP-sensitive potassium channel. Accumulating data suggest that modulation of mitochondrial function is particularly important in ischemic preconditioning, where cytoprotection is evident from hours to days after the nonlethal ischemia-activated cell-survival programs (5, 7).
The circulating molecule nitrite (NO2 −) has been shown to mediate potent cytoprotection after I/R injury in the heart, liver, and brain when administered during ischemia or immediately before reperfusion (8–11). For example, in murine models of myocardial and hepatic infarction, nanomole doses (1.2–48 nmol) of nitrite reduced infarction volume and apoptosis by >50% compared with controls (8, 11); similar effects have been observed in the isolated perfused rat heart (10). More recently, in a rat model of stroke, 48–480 nmol of nitrite reduced cerebral infarct volume by ∼75% and enhanced neurological functional recovery (9). Although the effects of nitrite are undoubtedly potent, the mechanisms orchestrating cytoprotection are unknown.
Emerging data demonstrate that nitrite is an important endocrine reservoir of NO that is reduced to bioactive NO along a physiological pH and oxygen gradient by several mechanisms, including enzymatic reduction by hemoglobin, myoglobin (12–14), components of the mitochondrial respiratory chain (15), and xanthine oxidoreductase (16), as well as nonenzymatically by acidic disproportionation (17, 18). Although NO is known to protect tissues from I/R injury, its therapeutic window is limited in terms of dose, source, and duration of exposure (19–23). In addition, the ability of the enzyme NO synthase to generate NO during I/R is compromised because of the requirement for oxygen as a substrate. Conversely, the conditions during I/R when oxygen is limiting and the tissue becomes acidotic are optimal for the reduction of nitrite to NO. Consistent with the idea of nitrite-dependent NO generation during I/R, the NO scavenger 2-phenyl-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) reversed nitrite-dependent cytoprotection in all previously published studies (8–10).
NO is a well-characterized regulator of mitochondrial function, with nanomolar concentrations reversibly inhibiting cytochrome c oxidase (24, 25), regulating ROS formation (26, 27), initiating biogenesis (28, 29), and limiting apoptotic cytochrome c release (30, 31). Given the central role of mitochondria during I/R, as well as the concept that its modulation may enhance ischemic tolerance during preconditioning, we hypothesized that (a) the cytoprotective effects of nitrite mimic and may mediate the ischemic preconditioning cell-survival program and that (b) the cytoprotective effects of nitrite occur at the level of the mitochondrion, by enhancing reperfusion respiration and energetics and limiting ROS-mediated cellular dysfunction. Finally, considering the fact that nitrate intake in the Mediterranean diet is associated with both nitrite uptake into plasma and a reduction in cardiovascular morbidity and mortality, we evaluated whether dietary nitrite may modulate the organ response to I/R.
RESULTS
Nitrite mimics ischemic preconditioning in vivo
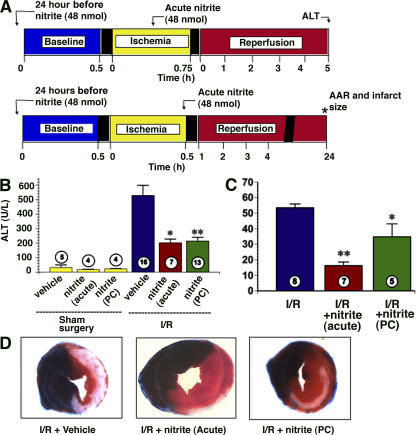
Transient nonlethal ischemia, termed the ischemic preconditioning “trigger,” evokes cellular resilience to I/R (32). Importantly, there exist two temporal windows of I/R tolerance: acute, or classical, ischemic preconditioning, lasting minutes to a few hours after the ischemic trigger, and delayed ischemic preconditioning, evident 1–3 d after the transient ischemic trigger. Although nanomole doses of nitrite mediate cytoprotection when administered during ischemia or immediately before reperfusion (8, 9), it is not known whether nitrite can temporally replicate delayed preconditioning. To test this, mice were administered one bolus intraperitoneal injection of nitrite (48 nmol) or saline and subjected to either myocardial infarction or hepatic ischemia 24 h later (Fig. 1 A). After hepatic I/R, serum alanine aminotransferase (ALT) levels, representing hepatic injury, increased (525 ± 40 U/liter) compared with sham surgical controls (50 ± 10 U/liter). Nitrite administered both acutely and 24 h before ischemia attenuated the increase in ALT (210 and 220 ± 30 U/liter, respectively) to a similar degree (Fig. 1 B). Similarly, nitrite administration acutely or 24 h before ischemia significantly reduced myocardial infarct size (18 ± 4 and 35 ± 14%, respectively) compared with saline-treated mice (53 ± 5%; Fig. 1, C and D). Thus nitrite exerts potent cytoprotective effects after I/R injury temporally analogous to ischemic preconditioning.
Figure 1.
Nitrite mediates both acute and delayed cytoprotection after I/R injury to the heart and liver. (A) Model of hepatic ischemia and myocardial infarction in which nitrite or saline was administered either 24 h before or during ischemia. (B) Plasma ALT levels 5 h into reperfusion in mice after sham surgery, sham surgery with nitrite treatment, ischemia with acute nitrite treatment, or ischemia with nitrite preconditioning (PC). (C) Infarct size as a percentage of area at risk 24 h after myocardial infarction in the absence of nitrite (I/R), with nitrite treatment 5 min before reperfusion (acute), or nitrite treatment 24 h before myocardial infarction (PC). (D) Representative sections of myocardium stained with Evan's blue and triphenyltetrazolium chloride 24 h after infarction in mice receiving no nitrite (I/R + vehicle), acute nitrite treatment, or nitrite preconditioning. *, P < 0.05 in comparison with the I/R + vehicle group; **, P < 0.01 versus the I/R + vehicle group.
Nitrite-dependent protection occurs at the mitochondrial level
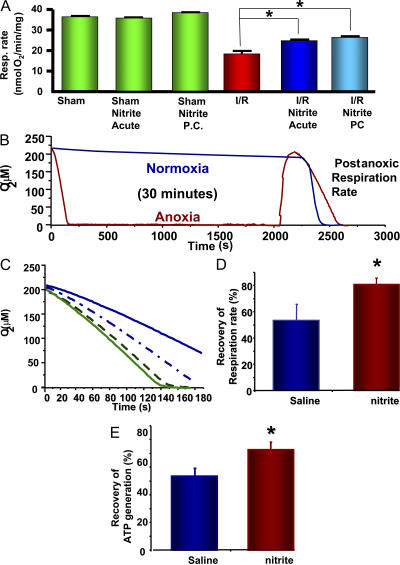
To elucidate the mechanism of cytoprotection in the heart and liver, we examined mitochondria isolated from the liver of mice after hepatic I/R and polarographically measured their respiratory rate. State 3 respiratory rates, using succinate as a substrate, correlated inversely with the measured ALT levels. Mice subjected to sham surgery in the presence and absence of nitrite had the lowest ALT levels and the highest mitochondrial respiratory rates (34–40 nmol O2/min/mg of protein), whereas those subjected to I/R showed an ∼50% decrease in respiratory rate (18 ± 4 nmol O2/min/mg). Nitrite administration (48 nmol) during or 24 h before ischemia increased state 3 succinate-dependent mitochondrial respiration (25 ± 2 and 27 ± 2 nmol O2/min/mg, respectively) after I/R (Fig. 2 A).
Figure 2.
Nitrite preconditioning protects from ischemic damage at the mitochondrial level. (A) Respiratory rates (in the presence of succinate and ADP) of liver mitochondria isolated from mice 5 h after being subjected to hepatic I/R in the presence and absence of acute and preconditioning nitrite. (B) In vitro model of mitochondrial anoxic/reoxygenation. Red trace is mitochondria subjected to anoxia. Blue trace is mitochondria subjected to normoxia for 30 min. (C) Representative respiration traces of 1 mg/ml of mitochondria isolated from rats preconditioned with saline (continuous lines) or 480 nmol nitrite (dashed lines) before (green) and after (blue) anoxia. (D and E) Postanoxic respiration (D) and ATP synthesis (E) rates expressed as a percentage of preanoxic rate for mitochondria isolated from rats preconditioned with saline or nitrite. All experiments are means ± SEM of at least n = 3 independent experiments. *, P < 0.01.
In an attempt to evaluate whether this protection occurs at the mitochondrial level versus an effect on cellular injury resulting in secondary, indirect mitochondrial protection, we examined isolated rat mitochondria in the absence of other cellular organelles subjected to an in vitro model of I/R injury. In these experiments, mitochondria isolated from rat liver were used because of their robust activity and abundant quantity in comparison with mitochondria isolated from mice. The mitochondria (1 mg/ml) were stimulated to respire in state 3 through complex II by the addition of 5 mM succinate and 1 mM ADP until all the oxygen in the chamber was consumed. After 30 min of anoxia, the mitochondria were reoxygenated, washed, and resupplied with substrate. The mitochondrial respiratory rate was decreased by ∼50% in comparison with the preanoxic respiratory rate and compared with control mitochondria suspended in normoxic buffer for 30 min (Fig. 2, B and C). This diminished respiratory rate recapitulates that seen in vivo and implicates mitochondrial damage induced by anoxia/reoxygenation. The isolated mitochondrial experiments were then repeated after one intraperitoneal injection of either nitrite (480 nmol) or saline to rats 24 h before hepatic mitochondrial isolation. Although the preanoxic complex II-dependent respiratory rate was similar with and without prior nitrite administration (85 ± 12 and 80 ± 10 nmol O2/min/mg, respectively), mitochondria from the nitrite-treated rats had significantly higher postanoxic respiration rates (67 ± 12 nmol O2/min/mg) versus saline-treated rats (45 ± 6 nmol O2/min/mg; Fig. 2 C). Examination of the pre- and postanoxic rates demonstrates that the saline group sustained a 48 ± 6% drop in respiration rate, whereas the nitrite group decreased by only 20 ± 4% (Fig. 2 D).
To exclude mitochondrial uncoupling as a major component in differential postanoxia respiratory rates, the rate of ATP formation was determined. Preanoxic rates of ATP production were similar with or without nitrite administration. However, after anoxia, mitochondria from the saline-treated rats recovered 52 ± 4% of ATP synthesis, whereas mitochondria from nitrite-treated rats recovered 78 ± 2% (Fig. 2 E). These data suggest that nitrite-mediated delayed cytoprotection occurs at the level of the mitochondrion.
Nitrite does not regulate mitochondrial biogenesis
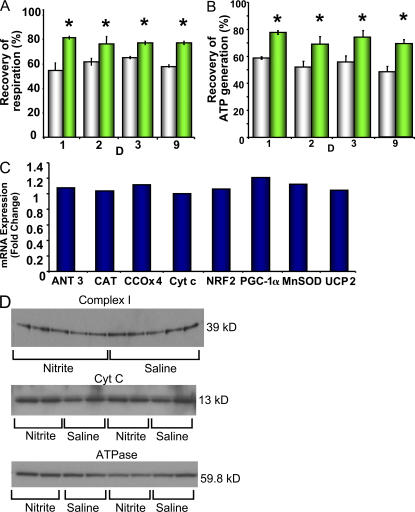
Because the half-life of nitrite in blood is only 11 min (33), we considered that nitrite may mimic delayed preconditioning through the transcriptional regulation of nuclear-encoded mitochondrial functioning genes (34, 35). Moreover, recent studies have shown that NO activates the mitochondrial biogenesis regulatory program (28, 29). To explore this possibility, we evaluated the gene expression profiles in response to daily nitrite administration for 1, 2, 3, and 9 d before mRNA extraction. The gene expression profiles of the nuclear genes encoding mitochondrial biogenesis regulatory proteins, antioxidant enzymes, uncoupling proteins, and respiratory chain enzymes were quantified. Although nitrite-dependent protection was maintained in the mitochondria throughout this exposure time (Fig. 3, A and B), nitrite-treated rats showed no change in mRNA expression of genes encoding transcriptional regulators of the mitochondrial biogenesis program (PGC-1α and NRF2), respiratory proteins (cytochrome c oxidase, cytochrome c, and adenine nucleotide translocase), or in antioxidant defense mediators (catalase, superoxide dismutase, and uncoupling protein 2) in comparison with saline-treated rats (Fig. 3 C). Mitochondrial protein expression (Fig. 3 D) and mitochondrial number and morphology (unpublished data) remained constant despite nine consecutive days of nitrite treatment. These data show that nitrite-dependent protection is not mediated by changes in gene transcription or translation that directly modulate the mitochondrial biogenesis program. Similarly, a single dose of nitrite administered 5 h before the extraction of hepatic tissue demonstrated no significant up-regulation of genes encoding cyclooxygenase-2 and inducible NO synthase (iNOS; 1.66 ± 0.02 fold change [P = 0.08] and 1.335 ± 0.09 fold change [P = 0.23], respectively; n = 10). As cyclooxygenase-2 and iNOS are established mediators of delayed preconditioning (36), a partial role of this signaling cascade in nitrite-dependent delayed cytoprotection cannot be completely excluded without further study.
Figure 3.
Nitrite-dependent cytoprotection does not modulate mitochondrial biogenesis. Rats were given one intraperitoneal injection (480 nmol) of saline or nitrite daily for nine consecutive days. (A and B) Recovery of respiration (A) and ATP (B) generation rates of mitochondria isolated from these rats after 30 min of anoxia in vitro (white, saline; green, nitrite). (C) Relative expression of genes in the livers of nitrite-treated rats on day 9 presented as the fold change in gene expression compared with saline-treated rats. (D) Protein expression of the 39-kD subunit of complex I, cytochrome c, and ATPase subunit B in the livers of rats after 9 d of nitrite or saline treatment. All experiments are means ± SEM of at least n = 3 independent experiments. *, P < 0.01.
Acute in vitro nitrite treatment protects mitochondria from I/R injury
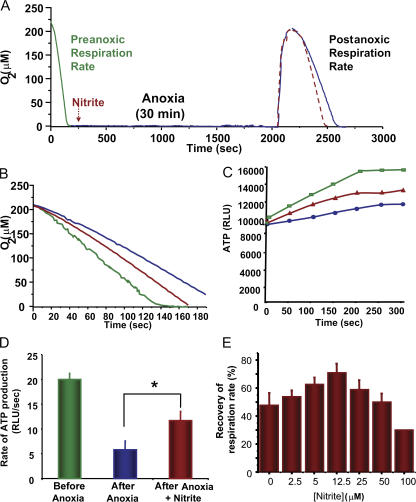
The lack of evidence for nitrite-dependent regulation of mitochondrial protein expression suggested that the protective effect of nitrite on mitochondria was most likely caused by the posttranslational modification of existing mitochondrial proteins. We hypothesized that if posttranslational modification was indeed responsible, mitochondrial protection of respiration and ATP synthesis should be observed even with acute nitrite treatment of mitochondria in vitro. To test this, we subjected isolated mitochondria from untreated rats to 30 min of anoxia followed by reoxygenation in the presence and absence of nitrite (Fig. 4 A). As predicted, nitrite-treated (10 μM) mitochondria had both higher complex II–dependent state 3 respiration (78 ± 12 nmol O2/min/mg; Fig. 4, A and B) and ATP production (16 ± 6 relative light units/s; Fig. 4, C and D) rates than control mitochondria (54 ± 4 nmol O2/min/mg and 9.8 relative light units/s, respectively) after anoxia/reoxygenation. Interestingly, nitrite-dependent protection from the anoxia-induced drop in respiration rate showed a biphasic dose–response curve (0–100 μM), with peak protection occurring at a concentration of 12.5 μM (Fig. 4 E). This biphasic response is identical in shape and concentration to previously observed dose–response curves for nitrite-mediated cytoprotection in vivo, and the concentration of 12.5 μM nitrite conferring peak protection to the mitochondria parallels the tissue concentration of nitrite shown to orchestrate peak protection in vivo (8).
Figure 4.
Acute nitrite treatment protects mitochondria against I/R injury. (A) Model of in vitro mitochondrial damage showing preanoxic (green) and postanoxic rates in the absence (blue) and presence (red) of nitrite. Arrow denotes the addition of 10 μM nitrite. Respiration rates (B) and ATP generation rates (C) before anoxia (green) and after anoxia in the presence of saline (blue) and nitrite (red). (D) Quantification of rate of ATP generation before and after anoxia. (E) Recovery of respiration rate with increasing concentrations (0–100 μM) of nitrite added during anoxia. All experiments are means ± SEM of at least n = 3 independent experiments. *, P < 0.01. RLU, relative light units.
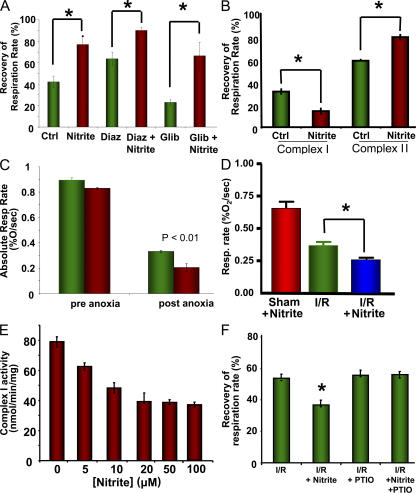
It is well established that modulation of mitochondrial ATP-dependent potassium (KATP) channels during I/R can affect tissue viability. To determine whether nitrite mediates cytoprotection by regulating KATP channels, we tested the effects of nitrite treatment during anoxia/reoxygenation in the presence of diazoxide, a KATP channel opener, and glibenclamide, an inhibitor of this channel. In our model, opening of the KATP channel with 200 μM diazoxide was protective, with a 63 ± 6% recovery of respiration after anoxia in comparison with 42 ± 5% in the control. Likewise, inhibition of the KATP channel with 100 μM glibenclamide resulted in a significant decrease in postanoxic recovery of respiration (22 ± 3%). Although both of these pharmacologic agents had independent effects on postanoxic respiration rates, neither drug inhibited nitrite-dependent protection of mitochondria (Fig. 5 A), suggesting that the KATP channel was not the target for nitrite-mediated cytoprotection.
Figure 5.
Nitrite treatment inhibits complex I. (A) Recovery of complex II–dependent respiratory rate in mitochondria that were untreated (Ctrl), treated with 10 μM nitrite alone, 200 μM diazoxide (Diaz), diazoxide and nitrite, 150 μM glibenclamide (Glib), or glibenclamide and nitrite during anoxia. (B) Recovery of respiration of mitochondria respiring on succinate (complex II) or glutamate/malate (complex I) and ADP in the absence (green) and presence (red) of 10 μM nitrite during anoxia/reoxygenation. (C) Absolute respiratory rates before and after anoxia of mitochondria from rats treated with nitrite (green) or saline (red) 24 h earlier. (D) Respiratory rates of mitochondria isolated from mice 5 h after they were subjected to hepatic I/R in vivo. (E) Complex I activity of isolated mitochondria treated with increasing concentrations of nitrite. (F) Recovery of complex I respiration rate in untreated mitochondria or treatment with 20 μM nitrite, 100 μM PTIO, or nitrite and PTIO. All experiments are means ± SEM of n = 3 experiments. *, P < 0.01.
Nitrite inhibits complex I after I/R
Although we have observed a nitrite-dependent protection of the complex II respiratory rate after anoxia/reoxygenation, it is important to also consider the effects of nitrite on complex I–dependent respiration, because complex I is known to be a primary site of both injury and ROS production after I/R (37). To test the effects of nitrite on complex I, we measured glutamate/malate-supported (5 mM each) state 3 respiration in isolated mitochondria before and after anoxia/reoxygenation. After anoxia/reoxygenation, both complex I– and II–supported respiration showed a decrease in respiration rate, though complex I showed a much greater decrease in rate (62 ± 4 vs. 35 ± 3% recovery), consistent with the increased susceptibility of complex I to I/R damage. However, in contrast to observed effects on complex II respiration, respiration through complex I was inhibited (20 vs. 35% recovery in control mitochondria), not enhanced, by nitrite treatment during anoxia/reoxygenation (Fig. 5 B). Interestingly, the same effect was observed in the postanoxic respiration rate of mitochondria isolated from rats treated with 480 nmol nitrite 24 h before isolation, though there was no significant effect of nitrite treatment on preanoxic respiration rate (Fig. 5 C). This trend was recapitulated in mitochondria isolated from mice that were subjected to hepatic I/R in vivo (Fig. 5 D). Spectrophotometric measurement of complex I–dependent oxidation of NADH confirmed that nitrite treatment (0–100 μM) of isolated mitochondria during anoxia concentration-dependently inhibited the enzymatic activity of complex I (Fig. 5 E), whereas we saw no substantial effect on the isolated activities of complex II or IV (unpublished data).
NO can S-nitrosate critical thiols on complex I, leading to an inhibition of its activity (38–40). This inhibition of complex I is proposed to exert cytoprotection during I/R, potentially through the modulation of ROS production. We found that 200 μM of the NO scavenger PTIO reversed the nitrite-dependent inhibition of complex I respiration after in vitro anoxia/reoxygenation, suggesting that the modification and subsequent inhibition of complex I was NO dependent (Fig. 5 F).
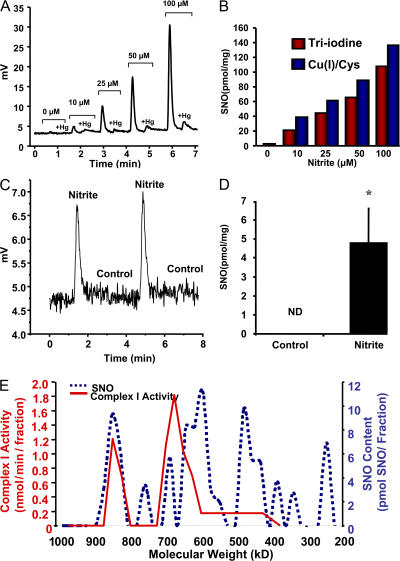
To identify the type of NO-dependent posttranslational modification, we measured protein S-nitrosothiols (SNOs), iron-nitrosyl, and N-nitrosamine complexes from isolated mitochondria treated with 0–100 μM nitrite during anoxia/reoxygenation using gas-phase ozone-based reductive chemiluminescence with both triiodide and copper/cysteine reductants (Fig. 6, A and B). Both reductive methods confirmed that nitrite treatment increased mitochondrial protein S-nitrosation in a concentration-dependent manner from 0.05–160 pmol/mg of protein, suggesting that S-nitrosation of mitochondrial proteins could be responsible for nitrite-dependent cytoprotection. Consistent with this thesis, isolated mitochondria from rats treated with 480 nmol nitrite 24 h before isolation had detectable S-nitrosation (4.8 ± 3 pmol/mg), whereas no significant S-nitrosation could be measured in saline-treated controls (Fig. 6, C and D).
Figure 6.
Nitrite S-nitrosates complex I. (A) Representative chemiluminescence trace of nitrite-treated mitochondria injected into triiodine before and after pretreatment with mercuric chloride (+Hg). (B) Quantitation of S-nitrosation in mitochondria from A (red) and an identical experiment using copper (I)/cysteine as a reductant instead of triiodine (blue). (C) Representative copper (I)/cysteine–based chemiluminescence trace of mitochondria isolated from rats treated with 480 nmol of saline or nitrite 24 h earlier. (D) Quantification of traces similar to those shown in C. (E) Protein was extracted from rat liver mitochondria and loaded onto a Superose 6 size-exclusion column. The SNO and complex I activity of each fraction was then determined and are represented as picomoles of SNO per fraction and complex I activity of the neat fractions, respectively. Mitochondria subjected to ischemia without NO2 − treatment resulted in no SNO detection (not depicted). All experiments are means ± SEM of at least n = 3 independent experiments. *, P < 0.01.
To further characterize the target of S-nitrosation, nitrite-treated (20 μM) mitochondria were fractionated by Superose 6 gel filtration, and SNO levels were measured by reductive chemiluminescence in each fraction. Significant S-nitrosation (∼10 pmol) of the 900-kD mitochondrial fraction was observed in the nitrite-treated mitochondria, which includes complex I proteins and activity (39), whereas no S-nitrosation was detected on this fraction in control mitochondria (Fig. 6 E). Although nitrite appeared to S-nitrosate additional targets on the electron transport chain, these data suggest that nitrite-dependent S-nitrosation and NO-mediated thiol oxidation of complex I are responsible for the inhibition of complex I activity after anoxia-reoxygenation both in vivo and in vitro.
Nitrite-dependent complex I inhibition limits reperfusion ROS generation and aconitase inactivation
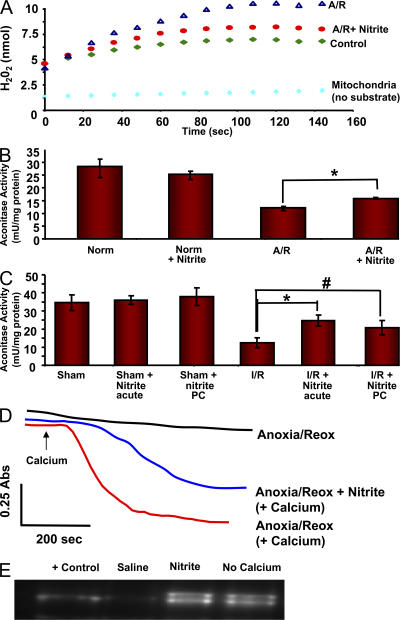
Complex I is a primary site of mitochondrial ROS production, with superoxide generation by forward and reverse electron transport (41, 42). Inhibition of complex I may confer cytoprotection potentially because of the limitation of oxidative protein damage by the inhibition of ROS production by this complex (39, 43, 44). To determine whether nitrite-dependent inhibition of complex I diminishes mitochondrial ROS generation, we subjected mitochondria to 30 min of anoxia in the presence and absence of nitrite 10 μM and measured H2O2 production by the oxidation of amplex red at reoxygenation. After I/R, H2O2 production was increased in isolated mitochondria (9.9 ± 0.5 nmol/min/mg) in comparison with preanoxic levels (3 ± 0.5 nmol/min/mg). However, this increase was attenuated (4.5 ± 1.4 nmol/min/mg) by nitrite treatment (Fig. 7 A).
Figure 7.
Nitrite decreases oxidative damage in mitochondria. (A) Representative traces of ROS production measured by amplex red in mitochondria without substrate (light blue) in the presence of glutamate/malate and ADP before anoxia/reoxygenation (green), and after anoxia/reoxygenation in the presence (red) and absence (blue) of 20 μM nitrite. (B) Aconitase activity of mitochondria during normoxia and after anoxia/reoxygenation in the presence and absence of 20 μM nitrite. (C) Aconitase activity in liver tissue from mice 5 h after they were subjected to hepatic I/R or sham surgery in the presence and absence of 48 nmol of acute or preconditioning nitrite. (D) Representative traces of calcium-induced pore opening of mitochondria subjected to anoxia/reoxygenation in the presence of 20 μM nitrite. Traces are in the absence of calcium (black), in the absence of nitrite treatment (red), or with nitrite treatment (blue). (E) Western blot analysis of cytochrome c remaining in mitochondria from the conditions shown in D. All experiments are means ± SEM of at least n = 3 independent experiments. *, P < 0.01; #, P < 0.05.
The Fe-S containing mitochondrial enzyme aconitase is highly susceptible to oxidative damage. We measured the activity of this enzyme as an index of oxidative protein damage, with a lowered activity indicating greater oxidative damage. Consistent with a nitrite-dependent decrease in ROS production, mitochondria subjected to anoxia/reoxygenation sustained more oxidative damage (13 ± 1.1 mU/mg of protein) than control mitochondria (28 ± 4 mU/mg), whereas those treated with nitrite during anoxia were significantly protected (18 ± 1.8 mU/mg; Fig. 7 B). These effects translated to entire livers taken from mice 5 h after in vivo hepatic I/R, in which the I/R-induced increase in oxidative damage was diminished by nitrite treatment either acutely or 24 h before the ischemic episode (48 nmol; Fig. 7 C).
Nitrite treatment prevents opening of the permeability transition pore and cytochrome c release
Opening of the mitochondrial permeability transition pore has been linked to the release of cytochrome c and is important in initiating the mitochondrial apoptotic pathway. Because oxidants have been shown to sensitize the permeability transition pore (45, 46), we determined whether the cytoprotective effects of nitrite could in part be caused by nitrite-dependent protection against pore opening after I/R. Isolated mitochondria were subjected to 30 min of anoxia in the presence or absence of 20 μM nitrite, 10 μM calcium was added to stimulate pore opening during reperfusion, and mitochondrial swelling was spectrophotometrically monitored as a measure of pore opening. In the presence of calcium, mitochondrial pore opening was facilitated, as indicated by a rapid drop in absorbance. However, in mitochondria treated with nitrite during anoxia, pore opening was delayed after the addition of calcium and occurred at a considerably slower rate and to a lesser extent than in the absence of nitrite (Fig. 7 D).
To assess whether nitrite limits cytochrome c release, the mitochondria were centrifuged 1 min after the addition of calcium, and the amount of cytochrome c remaining in the mitochondria was measured. In control mitochondria, calcium-induced pore opening elicited the loss of cytochrome c from control mitochondria, whereas nitrite-treated mitochondria were protected from cytochrome c release (Fig. 7 E).
Oral nitrite administration confers resistance to hepatic and cardiac I/R injury
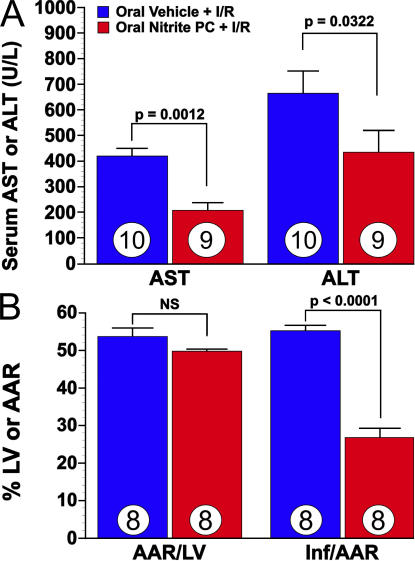
Several chemical agents, including rotenone and amobarbitol, are known to inhibit complex I and confer protection to mitochondria after I/R. However, these agents are not practical therapeutic candidates and certainly are not naturally occurring compounds. Conversely, nitrite forms from the reduction of dietary nitrate by oral bacterial flora and is a potential cardioprotective agent in the nitrate-rich Mediterranean diet (47, 48). To evaluate whether oral nitrite would promote preconditioning cytoprotection after I/R injury, we next tested oral dosing of nitrite. To begin to explore this, nitrite or saline vehicle was administered to mice via oral gavage 24 h before in vivo hepatic or cardiac I/R. Both liver and heart I/R were evaluated 24 h after a single oral gavage of 200 μl of 100 mg/liter sodium nitrite. This would correspond to 9.6 μmol of sodium nitrite per kilogram. Because the total body load of nitrite after ingestion of a nitrate-rich meal (i.e., 100 g of spinach) amounts to >1 μmol/kg (47), our dose is consistent with the daily nitrite intake on the Mediterranean diet. This regimen replicated the cytoprotection evoked by the earlier intraperitoneal administration of nitrite used in the previous experiments performed in this study (Fig. 8, A and B).
Figure 8.
Hepatic and myocardial I/R injury are attenuated in mice after oral nitrite therapy. (A) The effects of oral nitrite preconditioning (PC) on the severity of hepatic I/R injury (measured as aspartate aminotransferase [AST] and ALT) in mice. Mice received nitrite via oral gavage at 24 h before 45 min of hepatic ischemia and 5 h of reperfusion. Numbers for each group are shown inside the bars. (B) Bar graph of oral nitrite preconditioning and myocardial I/R injury in mice. Mice received oral nitrite at 24 h before 30 min of ischemia and 24 h of reperfusion. The myocardial area-at-risk (AAR) per total left ventricle (LV) was not significantly different between study groups (NS). The myocardial infarct size (Inf) per area-at-risk was significantly (P < 0.0001) reduced in the oral nitrite preconditioning group when compared with the oral vehicle group. Eight animals were investigated in each group.
DISCUSSION
These data demonstrate that nanomole doses of nitrite mediate both acute cytoprotection and mimic delayed preconditioning in the heart and liver in vivo. Mechanistically, both acute and delayed nitrite-dependent cytoprotection occur at the mitochondrial level, with the inhibition of mitochondrial complex I by S-nitrosation limiting mitochondrial ROS generation, oxidative protein damage to the electron transport chain, cytochrome c release, and cellular and tissue infarction.
Interestingly, NO production has been shown to evoke acute (classical) preconditioning and is required in the delayed ischemic preconditioning cell-survival program (19, 49). This occurs through increased endothelial NOS activity after acute ischemic preconditioning and via induction of iNOS 24 h later for remote preconditioning (50). Relevant to the current study, nitrite levels increase in a biphasic manner after both acute and delayed ischemic preconditioning, with maximal increases observed during the I/R tolerant periods (50). In this study, we show that administration of nitrite at either of these preconditioning time points confers potent cytoprotection in vivo and modulates mitochondrial function. In addition, previous studies have shown complex I inhibition (51), as well as an increase in mitochondrial S-nitrosation (39), after ischemic preconditioning of the rat heart. Given these data, it is intriguing to consider that nitrite could serve as both an endogenous reservoir for NO production and an effector molecule mediating the cell-survival effects of both acute and delayed ischemic preconditioning. Consistent with such NO-dependent signaling, PTIO, a direct NO scavenger, has been observed to inhibit the cytoprotective effects of nitrite in cardiac (10, 20), liver (20), and brain (9) I/R.
NO itself has been shown to directly protect cultured rat neonatal cardiomyocyte mitochondria when administered in time periods compatible with both classical and delayed preconditioning (52, 53). Previous studies show that NO modulates mitochondrial Ca2+ handling that in turn diminishes reoxygenation-associated Ca2+ overload (52, 53). In this manuscript, we demonstrate that nitrite administration directly modifies the electron transfer chain complex I activity in association with S-nitrosation of this complex. This is associated with blunted reperfusion/reoxygenation ROS-mediated injury. The putative mechanisms that could confer this phenotype include the direct inhibition of complex I–generated ROS or via the modest reduction in the mitochondrial membrane potential that may result from the partial inhibition of complex I activity (5).
An apparent paradox is evident with respect to complex I inhibition and ROS generation. In brief, in an aerobic environment with adequate oxidative phosphorylation, substrate, and reducing equivalents, the direct inhibition of complex I with either rotenone (54) or by preformed SNOs (40) results in complex I–derived ROS production. Conversely, when complex I is inhibited in the context of I/R by rotenone, the production of ROS is decreased during the oxidation of complex I substrate, in parallel with the preservation of mitochondrial content and the rate of oxidation through cytochrome c oxidase (55). This mitochondrial “protective” response to complex I inhibition during ischemia has been replicated by the use of amobarbital (a reversible inhibitor of complex I) (43), by S-nitrosoglutathione–dependent transnitrosation in the Langendorff perfused heart (39), and in this study, by the naturally occurring anion nitrite. Collectively, these data suggest that transient inhibition of complex I in the I/R milieu can have protective effects associated with reduced ROS production during reperfusion. In contrast, chronic inhibition of complex I under an otherwise physiologic milieu can be cytotoxic and associated with elevated ROS production (56).
Interestingly, nitrite appears to be unique among the aforementioned agents in that it does not inhibit complex I activity during normal physiology but only inhibits complex I activity in the setting of I/R injury. These data suggest that the modification of complex I by these near physiological concentrations of nitrite is dynamic and only operational in the setting of ischemia. The mechanisms whereby low-dose nitrite-mediated modification of complex I is “fine-tuned” to have no appreciable inhibitory effect under normal homeostatic conditions but sufficient inhibitory effects in the context of hypoxic or redox stress are currently unknown and require further investigations. However, such dynamic inhibition of complex I allows for sustained mitochondrial oxidative phosphorylation through complex II during I/R while limiting ROS generation, mitochondrial calcium overload, and the release of cytochrome c (37, 52, 57).
The chemistry of nitrite-dependent nitrosation of complex I will also require further study, considering the fact that the NO radical will not directly nitrosate reduced thiol (one-electron oxidation is required). There are several possibilities that might be unique to the mitochondria. First, direct nitrosation of thiols by nitrous acid: the inner membrane space has an estimated pH of ∼7.2, with the matrix having a pH of ∼7.9. Although minimal nitrous acid should form at pH 7.2 (the pKa of nitrite is ∼3.2), any nitrous acid that formed would occur in the inner membrane space and potentially at complexes I, III, IV, and V, which actively pump protons. The hydrophobic transmembrane environment of these complexes would stabilize both nitrous acid, N2O3, and the formed SNO. Second, NO production from nitrite reduction could secondarily react with nitrogen dioxide or superoxide formed from mitochondrial oxidation pathways and could form nitrosating intermediates such as N2O3 or ONOO−. Third, we have recently found that nitrite binds to and reacts with metheme proteins, such as methemoglobin, to produce an NO2 radical–like intermediate that can then react with NO to form N2O3 (58); N2O3 would directly nitrosate complex I. This would provide a metal-based pathway to S-nitrosation by nitrite. Although these pathways remain theoretical, the mechanisms of S-nitrosation by nitrite are currently an active area of research in the NO field.
It is tempting to speculate that nitrite is the endogenous molecule that regulates ischemic ROS formation at complex I after reperfusion. Such an innate mechanism could represent an evolved response to limit tissue injury during birth (for the fetus and mother), traumatic hemorrhage, and extreme exercise. From a therapeutic standpoint, nitrite would represent an ideal candidate to confer mitochondrial and, by extension, cellular resilience to I/R injury through the partial inhibition of complex I. The therapeutic potential of nitrite is underscored as follows: (a) this anion is a chemically stable endogenous reservoir for NO, (b) the required therapeutic concentration is in the nanomolar range, and (c) it is also readily available in diet (i.e., the Mediterranean diet) and, as shown in our study, its protective effect is clearly observed with oral intake.
Consistent with an innate physiological role for nitrite in modulating the stress response, the cytoprotective effects of nitrite in the heart and liver are measurable at nitrite doses <1.2 nmol in murine models of myocardial infarction and hepatic I/R (8). These doses increase plasma nitrite levels by <10% and are consistent with increases observed after the ingestion of a standard leafy green salad (47, 48) or after regular moderate exercise (59–61). Furthermore, mice with diminished basal plasma nitrite concentrations are more susceptible to I/R injury, an effect that is attenuated by administration of exogenous nitrite (11). These data suggest that a diet rich in nitrate and nitrite may have profound cytoprotective effects and, most provocatively, could constitute the “active” ingredient of the cardioprotective Mediterranean diet (47, 48).
In conclusion, we have shown that nitrite potently mediates cytoprotection after I/R of the mammalian heart and liver at the mitochondrial level through the transient inhibition of complex I and subsequent limitation of oxidative damage. The acute and remote temporal windows of nitrite-dependent cytoprotection suggest that nitrite may be a central mediator in ischemic preconditioning. Collectively, these studies reveal a global role for nitrite in the regulation of ischemic responses at the subcellular level. Finally, its efficacy as an oral preparation at near physiologic doses suggests a role for nitrite as a dietary cardioprotective agent.
MATERIALS AND METHODS
Chemicals.
All reagents were obtained from Sigma-Aldrich unless otherwise noted.
Animals.
8–10-wk-old male C57BL6/J mice (The Jackson Laboratory) were used in accordance with the Animal Care and Use Committee of AECOM. Male Sprague-Dawley rats (250-500 g; Harlan) were used in accordance with the Animal Care and Use Committee of the National Heart Lung Blood Institute.
Hepatic I/R.
Mice were anesthetized with 100 mg/kg ketamine and 8 mg/kg xylazine injected intraperitoneally. A midline laparotomy incision was performed, and mice were heparinized (100 μg/kg intraperitoneally) to prevent blood clotting. Microaneurysm clamps were used to completely clamp the hepatic artery and portal vein, causing ischemia of the left lateral and median lobes of the liver, as previously described (8). The liver was kept moist with normal saline for 45 min, after which the microaneurysm clamp was removed to allow reperfusion. Mice were sutured, and serum was collected 5 h later for measurement of liver transaminase levels.
Myocardial infarction.
Mice were anesthetized by injection of 50 mg/kg ketamine and 50 mg/kg pentobarbital and were then intubated with polyethylene tubing connected to a rodent ventilator, after which the respiratory rate was set to 122 breaths per minute. A median sternectomy was performed, and the left main coronary artery was completely ligated witha 7-0 silk suture mounted on a tapered needle, as previously described (8). After 30 min, the suture was removed, and animals were reperfused for 24 h.
Mitochondrial isolation and respiration.
Liver mitochondria were isolated by differential centrifugation in a buffer (250 mM sucrose, 10 mM Tris, 1 mM EGTA, pH 7.4) at 4°C, as previously described (62). To measure respiration of isolated mitochondria, 1 mg/ml of protein was suspended in respiration buffer (120 mM KCl, 25 mM sucrose, 10 mM Hepes, 1 mM EGTA, 1 mM KH2PO4, 5 mM MgCl2) in a stirred, sealed chamber fit with a Clark-type oxygen electrode (Instech Corp.) connected to a data recording device (DATAQ Systems).
In vitro anoxia/reoxygenation.
The in vitro anoxia/reoxygenation protocol was adapted from Ozcan et al. (63). State 3 respiration was initiated, and mitochondria were allowed to consume oxygen until the chamber became anoxic. The mitochondria were left in this anoxic state for 30 min. To reoxygenate, mitochondria were centrifuged and resuspended in oxygenated buffer containing fresh substrate and ADP and allowed to respire once again. Postanoxic respiratory rate was expressed as a percentage of preanoxic rate and called recovery of respiration.
ATP generation.
ATP synthesis in isolated mitochondria suspended in respiration buffer with 15 mM succinate and 0.5 mM ADP was measured by monitoring the luminescence of luciferase/luciferin over time using the ATP detection kit from Invitrogen.
Complex I activity.
Complex I activity was determined in isolated mitochondria by spectrophotometrically (340 nm) monitoring the oxidation of 100 μM NADH in the presence of 10 μM coenzyme Q1 in the presence and absence of 25 μM rotenone.
Detection of S-nitrosation.
To measure S-nitrosation, 10 mg of isolated mitochondria treated with nitrite was lysed with a solution of 1% NP-40 and 100 μM diethylenetriamene pentaacetate (DTPA). Half of the sample was immediately injected into copper/cysteine-based reductive chemiluminescence, which is specific for S-nitrosated protein adducts. The other half of the protein was divided into three parts: one left untreated, one treated with 10% acidified sulfanilamide to eliminate nitrite, and one treated with 5 mM mercuric chloride and 10% acidified sulfanilamide (v/v) to eliminate nitrite and SNOs. The three fractions were injected into a vessel containing triiodine and connected inline to an NO chemiluminescence detector (Seivers), as previously described (64).
ROS generation.
H2O2 generation was spectrophotometrically assayed by monitoring the oxidation of amplex red to product resofurin at 585 nm using the Amplex Red Hydrogen Peroxide/Peroxidase assay kit (Invitrogen).
Mitochondrial fractionation.
3 mg of mitochondrial protein was extracted in 60 μl of buffer (10 mM Hepes, pH 7.4, 1 mM EDTA, 100 μM DTPA, 1.6% lauryl-maltoside). 25 μl of sample (∼1.25 mg of protein) was loaded onto a 1 × 24 cm Superose 6 size-exclusion column, run at a flow rate of 0.5 ml/min, with the running buffer (10 mM Hepes, pH 7.4, 1 mM EDTA, 100 μM DTPA). 500-μl fractions were collected, and 475 μl was immediately subjected to chemiluminescent SNO analysis, as described in Detection of S-nitrosation, with the remaining 25 μl being used for protein determination by a bicinchoninic acid–based protein assay. The column was calibrated by running high-range molecular mass standards (68–669 kD; GE Healthcare).
Aconitase activity.
Mitochondria were lysed by three cycles of freeze/thaw, and aconitase activity was measured by spectrophotometrically monitoring the formation of NADPH at 340 nm using the Bioxytech Aconitase-340 kit (Oxis Research).
Permeability transition pore opening and cytochrome c release.
After being subjected to anoxia/reoxygenation in the presence or absence of nitrite, 1 mg/ml of mitochondria was given fresh substrate and spectrophotometrically monitored at 540 nm at 37°C. After 100 s, 10 μM calcium chloride was added to each cuvette, and monitoring was continued. 1 min after the addition of calcium, pore opening was suspended by the addition of 500 μM EGTA and 20 μM cyclosporine A, and the mitochondria were centrifuged at 10,000 g for 5 min. The mitochondrial pellets were subjected to Western blot analysis using an anticytochrome c antibody (Santa Cruz Biotechnology, Inc.).
Oral nitrite studies.
Sodium nitrite was dissolved in saline at a concentration of 100 mg/liter. 24 h before either hepatic or myocardial ischemia, mice were administered 200 μl of the 100 mg/liter sodium nitrite stock solution or vehicle (saline) orally with the aid of a 20-gauge oral gavage needle. The hepatic and myocardial I/R injuries were performed as described in Hepatic I/R and Myocardial infarction.
Acknowledgments
We would like to thank Dr. Xeuxing Yu at the National Heart Lung Blood Institute Histology Core Facility for his assistance with the processing and analysis of tissue for electron microscopy. We would also like to thank Tish Murphy, Toren Finkel, and Robert Balaban for helpful comments and suggestions about the experimental data.
These studies were supported by grants from the National Institutes of Health (RO1 HL-60849 to D.J. Lefer and F32 DK-077380-01 to J.W. Calvert) and the American Diabetes Association (7-04-RA-59 to D.J. Lefer).
David J. Lefer and Mark T. Gladwin are named on a provisional patent for the use of sodium nitrite in cardiovascular disease. The authors have no further conflicting financial interests.
D.J. Lefer and M.T. Gladwin contributed equally to this work.
Abbreviations used: ALT, alanine aminotransferase; iNOS, inducible NO synthase; I/R, ischemia/reperfusion; PTIO, 2-phenyl-tetramethylimidazoline-1-oxyl-3-oxide; ROS, reactive oxygen species; SNO, S-nitrosothiol.
References
- 1.Braunwald, E., and R.A. Kloner. 1985. Myocardial reperfusion: a double-edged sword? J. Clin. Invest. 76:1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefer, A.M., and D.J. Lefer. 1996. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc. Res. 32:743–751. [PubMed] [Google Scholar]
- 3.Rouslin, W., C.W. Broge, and I.L. Grupp. 1990. ATP depletion and mitochondrial functional loss during ischemia in slow and fast heart-rate hearts. Am. J. Physiol. 259:H1759–H1766. [DOI] [PubMed] [Google Scholar]
- 4.Rouslin, W. 1983. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am. J. Physiol. 244:H743–H748. [DOI] [PubMed] [Google Scholar]
- 5.Sack, M.N. 2006. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc. Res. 72:210–219. [DOI] [PubMed] [Google Scholar]
- 6.Kim, J.S., L. He, T. Qian, and J.J. Lemasters. 2003. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr. Mol. Med. 3:527–535. [DOI] [PubMed] [Google Scholar]
- 7.Honda, H.M., P. Korge, and J.N. Weiss. 2005. Mitochondria and ischemia/reperfusion injury. Ann. NY Acad. Sci. 1047:248–258. [DOI] [PubMed] [Google Scholar]
- 8.Duranski, M.R., J.J. Greer, A. Dejam, S. Jaganmohan, N. Hogg, W. Langston, R.P. Patel, S.F. Yet, X. Wang, C.G. Kevil, et al. 2005. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 115:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung, K.H., K. Chu, S.Y. Ko, S.T. Lee, D.I. Sinn, D.K. Park, J.M. Kim, E.C. Song, M. Kim, and J.K. Roh. 2006. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 37:2744–2750. [DOI] [PubMed] [Google Scholar]
- 10.Webb, A., R. Bond, P. McLean, R. Uppal, N. Benjamin, and A. Ahluwalia. 2004. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA. 101:13683–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiva, S., X. Wang, L.A. Ringwood, X. Xu, S. Yuditskaya, V. Annavajjhala, H. Miyajima, N. Hogg, Z.L. Harris, and M.T. Gladwin. 2006. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2:486–493. [DOI] [PubMed] [Google Scholar]
- 12.Cosby, K., K.S. Partovi, J.H. Crawford, R.P. Patel, C.D. Reiter, S. Martyr, B.K. Yang, M.A. Waclawiw, G. Zalos, X. Xu, et al. 2003. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9:1498–1505. [DOI] [PubMed] [Google Scholar]
- 13.Huang, Z., S. Shiva, D.B. Kim-Shapiro, R.P. Patel, L.A. Ringwood, C.E. Irby, K.T. Huang, C. Ho, N. Hogg, A.N. Schechter, and M.T. Gladwin. 2005. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 115:2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiva, S., Z. Huang, R. Grubina, J. Sun, L.A. Ringwood, P.H. MacArthur, X. Xu, E. Murphy, V.M. Darley-Usmar, and M.T. Gladwin. 2007. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100:654–661. [DOI] [PubMed] [Google Scholar]
- 15.Castello, P.R., P.S. David, T. McClure, Z. Crook, and R.O. Poyton. 2006. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 3:277–287. [DOI] [PubMed] [Google Scholar]
- 16.Li, H., A. Samouilov, X. Liu, and J.L. Zweier. 2001. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J. Biol. Chem. 276:24482–24489. [DOI] [PubMed] [Google Scholar]
- 17.Weitzberg, E., and J.O. Lundberg. 1998. Nonenzymatic nitric oxide production in humans. Nitric Oxide. 2:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Zweier, J.L., P. Wang, A. Samouilov, and P. Kuppusamy. 1995. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1:804–809. [DOI] [PubMed] [Google Scholar]
- 19.Bolli, R. 2001. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J. Mol. Cell. Cardiol. 33:1897–1918. [DOI] [PubMed] [Google Scholar]
- 20.Duranski, M.R., J.W. Elrod, J.W. Calvert, N.S. Bryan, M. Feelisch, and D.J. Lefer. 2006. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 291:H2980–H2986. [DOI] [PubMed] [Google Scholar]
- 21.Jones, S.P., and R. Bolli. 2006. The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell. Cardiol. 40:16–23. [DOI] [PubMed] [Google Scholar]
- 22.Jones, S.P., J.J. Greer, A.K. Kakkar, P.D. Ware, R.H. Turnage, M. Hicks, R. van Haperen, R. de Crom, S. Kawashima, M. Yokoyama, and D.J. Lefer. 2004. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 286:H276–H282. [DOI] [PubMed] [Google Scholar]
- 23.Nakano, A., G.S. Liu, G. Heusch, J.M. Downey, and M.V. Cohen. 2000. Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J. Mol. Cell. Cardiol. 32:1159–1167. [DOI] [PubMed] [Google Scholar]
- 24.Brown, G.C., and C.E. Cooper. 1994. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 356:295–298. [DOI] [PubMed] [Google Scholar]
- 25.Cleeter, M.W., J.M. Cooper, V.M. Darley-Usmar, S. Moncada, and A.H. Schapira. 1994. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345:50–54. [DOI] [PubMed] [Google Scholar]
- 26.Brookes, P., and V.M. Darley-Usmar. 2002. Hypothesis: the mitochondrial NO(*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: an alternative function for cytochrome C oxidase. Free Radic. Biol. Med. 32:370–374. [DOI] [PubMed] [Google Scholar]
- 27.Poderoso, J.J., M.C. Carreras, C. Lisdero, N. Riobo, F. Schopfer, and A. Boveris. 1996. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch. Biochem. Biophys. 328:85–92. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli, E., E. Clementi, C. Paolucci, V. Cozzi, C. Tonello, C. Sciorati, R. Bracale, A. Valerio, M. Francolini, S. Moncada, and M.O. Carruba. 2003. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 299:896–899. [DOI] [PubMed] [Google Scholar]
- 29.Nisoli, E., S. Falcone, C. Tonello, V. Cozzi, L. Palomba, M. Fiorani, A. Pisconti, S. Brunelli, A. Cardile, M. Francolini, et al. 2004. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. USA. 101:16507–16512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookes, P.S., E.P. Salinas, K. Darley-Usmar, J.P. Eiserich, B.A. Freeman, V.M. Darley-Usmar, and P.G. Anderson. 2000. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J. Biol. Chem. 275:20474–20479. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y.M., T.H. Kim, D.W. Seol, R.V. Talanian, and T.R. Billiar. 1998. Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J. Biol. Chem. 273:31437–31441. [DOI] [PubMed] [Google Scholar]
- 32.Yellon, D.M., and J.M. Downey. 2003. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 83:1113–1151. [DOI] [PubMed] [Google Scholar]
- 33.Dejam, A., C.J. Hunter, M.M. Pelletier, L.L. Hsu, R.F. Machado, S. Shiva, G.G. Power, M. Kelm, M.T. Gladwin, and A.N. Schechter. 2005. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 106:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLeod, C.J., A.P. Jeyabalan, J.O. Minners, R. Clevenger, R.F. Hoyt Jr., and M.N. Sack. 2004. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation. 110:534–539. [DOI] [PubMed] [Google Scholar]
- 35.McLeod, C.J., I. Pagel, and M.N. Sack. 2005. The mitochondrial biogenesis regulatory program in cardiac adaptation to ischemia—a putative target for therapeutic intervention. Trends Cardiovasc. Med. 15:118–123. [DOI] [PubMed] [Google Scholar]
- 36.Shinmura, K., Y.T. Xuan, X.L. Tang, E. Kodani, H. Han, Y. Zhu, and R. Bolli. 2002. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ. Res. 90:602–608. [DOI] [PubMed] [Google Scholar]
- 37.Lesnefsky, E.J., S. Moghaddas, B. Tandler, J. Kerner, and C.L. Hoppel. 2001. Mitochondrial dysfunction in cardiac disease: ischemia–reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 33:1065–1089. [DOI] [PubMed] [Google Scholar]
- 38.Brown, G.C., and V. Borutaite. 2004. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta. 1658:44–49. [DOI] [PubMed] [Google Scholar]
- 39.Burwell, L.S., S.M. Nadtochiy, A.J. Tompkins, S. Young, and P.S. Brookes. 2006. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 394:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dahm, C.C., K. Moore, and M.P. Murphy. 2006. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 281:10056–10065. [DOI] [PubMed] [Google Scholar]
- 41.Lambert, A.J., and M.D. Brand. 2004. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miwa, S., J. St-Pierre, L. Partridge, and M.D. Brand. 2003. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic. Biol. Med. 35:938–948. [DOI] [PubMed] [Google Scholar]
- 43.Chen, Q., S. Moghaddas, C.L. Hoppel, and E.J. Lesnefsky. 2006. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J. Pharmacol. Exp. Ther. 319:1405–1412. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, E.R., F. Hurrell, R.J. Shannon, T.K. Lin, J. Hirst, and M.P. Murphy. 2003. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem. 278:19603–19610. [DOI] [PubMed] [Google Scholar]
- 45.Brookes, P.S., and V.M. Darley-Usmar. 2004. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am. J. Physiol. Heart Circ. Physiol. 286:H39–H46. [DOI] [PubMed] [Google Scholar]
- 46.Landar, A., S. Shiva, A.L. Levonen, J.Y. Oh, C. Zaragoza, M.S. Johnson, and V.M. Darley-Usmar. 2006. Induction of the permeability transition and cytochrome c release by 15-deoxy-Delta12,14-prostaglandin J2 in mitochondria. Biochem. J. 394:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gladwin, M.T., A.N. Schechter, D.B. Kim-Shapiro, R.P. Patel, N. Hogg, S. Shiva, R.O. Cannon III, M. Kelm, D.A. Wink, M.G. Espey, et al. 2005. The emerging biology of the nitrite anion. Nat. Chem. Biol. 1:308–314. [DOI] [PubMed] [Google Scholar]
- 48.Lundberg, J.O., M. Feelisch, H. Björne, E. Jansson, and E. Weitzberg. 2006. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 15:359–362. [DOI] [PubMed] [Google Scholar]
- 49.Cohen, M.V., X.M. Yang, and J.M. Downey. 2006. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc. Res. 70:231–239. [DOI] [PubMed] [Google Scholar]
- 50.Xuan, Y.T., X.L. Tang, Y. Qiu, S. Banerjee, H. Takano, H. Han, and R. Bolli. 2000. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am. J. Physiol. Heart Circ. Physiol. 279:H2360–H2371. [DOI] [PubMed] [Google Scholar]
- 51.da Silva, M.M., A. Sartori, E. Belisle, and A.J. Kowaltowski. 2003. Ischemic preconditioning inhibits mitochondrial respiration, increases H2O2 release, and enhances K+ transport. Am. J. Physiol. Heart Circ. Physiol. 285:H154–H162. [DOI] [PubMed] [Google Scholar]
- 52.Rakhit, R.D., M.H. Mojet, M.S. Marber, and M.R. Duchen. 2001. Mitochondria as targets for nitric oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation. 103:2617–2623. [DOI] [PubMed] [Google Scholar]
- 53.Smart, N., M.H. Mojet, D.S. Latchman, M.S. Marber, M.R. Duchen, and R.J. Heads. 2006. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 69:164–177. [DOI] [PubMed] [Google Scholar]
- 54.Kushnareva, Y., A.N. Murphy, and A. Andreyev. 2002. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 368:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesnefsky, E.J., Q. Chen, S. Moghaddas, M.O. Hassan, B. Tandler, and C.L. Hoppel. 2004. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 279:47961–47967. [DOI] [PubMed] [Google Scholar]
- 56.Tretter, L., I. Sipos, and V. Adam-Vizi. 2004. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem. Res. 29:569–577. [DOI] [PubMed] [Google Scholar]
- 57.Chen, Q., and E.J. Lesnefsky. 2006. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic. Biol. Med. 40:976–982. [DOI] [PubMed] [Google Scholar]
- 58.Gladwin, M.T., N.J. Raat, S. Shiva, C. Dezfulian, N. Hogg, D.B. Kim-Shapiro, and R.P. Patel. 2006. Nitrite as a vascular endocrine nitric oxide resevoir that contributes to hypoxic signaling, cytoprotection and vasodilation. Am. J. Physiol. Heart Circ. Physiol. 291:H2026–H2035. [DOI] [PubMed] [Google Scholar]
- 59.Edwards, D.G., R.S. Schofield, S.L. Lennon, G.L. Pierce, W.W. Nichols, and R.W. Braith. 2004. Effect of exercise training on endothelial function in men with coronary artery disease. Am. J. Cardiol. 93:617–620. [DOI] [PubMed] [Google Scholar]
- 60.Maeda, S., T. Miyauchi, T. Kakiyama, J. Sugawara, M. Iemitsu, Y. Irukayama-Tomobe, H. Murakami, Y. Kumagai, S. Kuno, and M. Matsuda. 2001. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 69:1005–1016. [DOI] [PubMed] [Google Scholar]
- 61.Maeda, S., T. Tanabe, T. Otsuki, J. Sugawara, M. Iemitsu, T. Miyauchi, S. Kuno, R. Ajisaka, and M. Matsuda. 2004. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens. Res. 27:947–953. [DOI] [PubMed] [Google Scholar]
- 62.Shiva, S., P.S. Brookes, R.P. Patel, P.G. Anderson, and V.M. Darley-Usmar. 2001. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 98:7212–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozcan, C., E.L. Holmuhamedov, A. Jahangir, and A. Terzic. 2001. Diazoxide protects mitochondria from anoxic injury: implications for myopreservation. J. Thorac. Cardiovasc. Surg. 121:298–306. [DOI] [PubMed] [Google Scholar]
- 64.Yang, B.K., E.X. Vivas, C.D. Reiter, and M.T. Gladwin. 2003. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic. Res. 37:1–10. [DOI] [PubMed] [Google Scholar]