Abstract
The phosphatase of regenerating liver-1, PRL-1, gene was detected in a screen for foveal cone photoreceptor-associated genes. It encodes a small protein tyrosine phosphatase that was previously immunolocalized to the photoreceptors in primate retina. Here we report that in cones and cone-derived cultured cells both PRL-1 activity and PRL-1 gene expression are modulated under oxidative stress. Oxidation reversibly inhibited the phosphatase activity of PRL-1 due to the formation of an intramolecular disulfide bridge between Cys104 within the active site and another conserved Cys, Cys49. This modulation was observed in vitro, in cell culture and in isolated retinas exposed to hydrogen peroxide. The same treatment caused a rapid increase in PRL-1 expression levels in cultured cells which could be blocked by the protein translation inhibitor, cycloheximide. Increased PRL-1 expression was also observed in living rats subjected to constant light exposure inducing photooxidative stress. We further demonstrated that both oxidation and overexpression of PRL-1 upon oxidative stress are greatly enhanced by inhibition of the glutathione system responsible for cellular redox regulation. These findings suggest that PRL-1 is a molecular component of the photoreceptor's response to oxidative stress acting upstream of the glutathione system.
Keywords: Phosphatase, Oxidation/reduction, Cysteine, Retina, Photoreceptors, Light damage
1. Introduction
We originally detected phosphatase of regenerating liver-1, PRL-1, in a screen for genes expressed in foveal cone photoreceptors which are concentrated in the center (macula) of the primate retina and are responsible for high-acuity vision [1]. The PRL-1 gene is located on human chromosome 6q12, within a region known to harbor at least two macular dystrophy loci [2, 3]. The corresponding protein, PRL-1, is localized to the outer segments of red and green, but not blue, cone photoreceptors in retina [1].
PRL-1, is a member of a family of non-classical protein tyrosine phosphatases (PTPs) that also includes PRL-2 and PRL-3 [4, 5]. PRL phosphatases represent a type of small (140 to 180 amino acids), prenylated PTP [6–8]. The catalytic domain of PRLs shares similarity with dual-specificity PTP domains, which in other phosphatases dephosphorylate both tyrosines and serine/threonines and lack any distinct domains. PRLs share a high degree (>75%) of amino acid sequence identity within their family, but there is less than 20% homology within the catalytic domain of PRL-1 and that of other PTPs [8–10]. PRLs can be tethered on the membrane surface via the C-terminal farnesylation [5, 7, 8]. Outside of the PRL family, PRL-1 shares the highest homology (~40%) with the dual-specificity PTPs, Cdc14p and PTEN [8]. PTEN also functions as a lipid phosphatase [11]. In spite of these homologies, the dual-specificity or lipid phosphatase activity of PRL-1 has not been previously addressed.
PRL-1 is expressed in both proliferating and terminally differentiated tissues including normal embryo, regenerating liver, fetal liver, adult gastric epithelium, brain and skeletal muscle cells [1, 12–16]. PRL-1 appears to play a growth-associated role in regulating cell proliferation, tumorgenesis, and/or differentiation. It is apparently regulated in a cell cycle-dependent manner, by interacting with tubulin and modulating spindle dynamics during mitosis, or with the nuclear transcription factor, ATF7, to regulate the mitotic progression [17, 18]. Expression of PRL-1 has been shown to be elevated in proliferating cells and during inflammation [19] and is regulated by P53 and early growth-response-1 [20, 21].
In postmitotic cells, where there is no requirement for cell division-associated roles, PRL-1 is also expressed at significant levels but its function(s) is completely unknown. Clues relating to the function of PRL-1 derive from recent studies showing that several PTPs can be regulated by oxidative modulation [10, 22–27]. The target of this modulation is the conserved cysteine (Cys) residue within the catalytic site which can be oxidized, either reversibly or irreversibly, resulting in the loss of the PTP activity [23, 27, 28]. This mechanism may compliment the known effect of oxidative stress in which protein tyrosine phosphorylation is stimulated by exposure to free radicals (40).
Because there is no mitosis in the mature retina, we focused our functional studies of PRL-1 in photoreceptors on exploring its role in oxidative regulation. Even under normal conditions, the eye is subjected to oxidative stress resulting from normal light exposure and the high rate of oxidative metabolism in the retina. Cumulative effects of oxidative stress have been implicated in a number of retinal pathologies including age-related macular degeneration and some inherited macular dystrophies [29–31]. Under oxidative stress, production of reactive oxygen species may also regulate signal transduction in photoreceptors by inactivating PTPs that could, in turn, up-regulate protein phosphorylation.
In the present study, we demonstrate that in photoreceptors and cultured photoreceptor-derived cells, PRL-1 is indeed modulated by oxidation caused either by treatment with hydrogen peroxide (H2O2) or by exposure of living retinas to oxidative stress associated with continuous illumination. We confirmed that this modulation is based on a specific reversible formation of an intramolecular disulfide bridge involving the catalytic cysteine and accompanied by inactivation of phosphatase activity. Furthermore, we revealed two novel aspects of this regulation. First, the expression level of PRL-1 in the retina and a photoreceptor-derived cell line increases under oxidative stress and can be blocked by inhibition of protein translation. Second, there is a clear linkage between the oxidation status of PRL-1 and glutathione (GSH) system, a major redox (reduction-oxidation) regulation pathway of the cell [32]. The GSH system is engaged in constant regeneration of oxidized PRL-1 to the reduced state, which is otherwise very prominent under stress.
2. Materials and Methods
2.1. Isolation of Retinal RNA
Human retinal RNA for PRL-1 constructs was isolated from 4 mm trephine punches of macula retina. Rat retinal RNA for quantitative RT-PCR (qRT-PCR) was isolated from retinas of control and light-exposed rats. Trizol reagent (Invitrogen, Carlsbad, CA) was used with glycogen added as a carrier, as previously described [33]. Following isolation, total RNA was DNase-treated using DNA-free DNase (Ambion, Austin, TX) and RNA yields were quantitated by fluorescence at 530 nm using RiboGreen (Molecular Probes, Eugene, OR). First-strand cDNAs were synthesized from the total RNA (1 µg/reaction) with an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), as described by the manufacturer.
2.2. Expression and Purification of Recombinant PRL-1
Plasmid constructs for expressing recombinant wild type (WT) PRL-1 and PRL-1 mutants with a C-terminal 6xHis affinity tag were generated. Full-length human PRL-1 cDNA was amplified by RT-PCR of human retinal cDNA with PRL-1 primers based on GenBank Accession Number NM 003463 forward primer, 5’-ATGGCTCGAATGAACCGCCCA-3’ and reverse primer (including sequence for 6xHis in bold) 5’-TTAGTGGTGATGGTGATGATGTTGAATGCAACAGTTGTTTCTATG-3’ and cloned in frame into the prokaryotic expression vector, pETBlue-1, using a pETBlue-1 AccepTor Vector kit (Novagen, San Diego, CA) to generate the pETBlue1-hisPRL-1 construct. Cys104Ser (C104S), Cys49Ser (C49S) and Cys99Ser (C99S) mutants of PRL-1 were generated using the pETBlue1-hisPRL-1 construct as template DNA with a Quik-Change™ Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer’s instruction manual. Every expression construct, including the PRL-1 Cys mutants, was verified by DNA sequencing performed at the Duke University DNA Analysis Facility.
WT and mutant His-tagged PRL-1 expressing plasmids were transformed into E. coli Tuner™ (DE3) pLacI cells (Novagen) for protein expression. His-tagged proteins were purified on ProBond resin (Invitrogen) using non-denaturing conditions. Briefly, sonicated cells lysates were incubated with ProBond resin for 2 h at 4°C. Following incubation, ProBond resins were washed with 20 mM imidazole and eluted with 250 mM imidazole (pH 8.0) buffer into 50 mM Tris plus 300 mM NaCl.
2.3. Preparation and Purification of PRL-1 Antibody
The polyclonal antibody against PRL-1 was generated by immunizing rabbits with purified recombinant His-tagged PRL-1. Rabbit immunizations were performed by Rockland Laboratories (Gilbertsville, PA). The anti-PRL-1 antibody was affinity purified from rabbit antiserum using Protein A sepharose CL-4B (Amersham Bioscience, Piscataway, NJ) and NHS-activated Sepharose 4 Fast Flow (Amersham Bioscience) coupled with purified recombinant His-PRL-1 protein according to the manufacturer’s instructions.
2.4. Phosphatase Assays
Phosphatase activity assays were based on assays using the fluorogenic phosphatase substrate, 6, 8-difluoro-4-methylumbelliferyl phosphate (DiFMUP, Molecular Probes), a preferred aromatic substrate for PRL-1 [34, 35]. Various concentrations of purified protein were incubated with 50 µM DiFMUP in 50 mM Tris-HCl pH8.0, 50 mM NaCl, 25 mM 3-4-morpholino propanesulphonic acid (MOPS), 1 mM DTT and 0.05% Tween-20 in a 100 µl reaction. The reaction was incubated at 37°C for 30 min and fluorometric measurements were taken at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. The phosphatase inhibition assays were performed with or without a selective tyrosine phosphatase inhibitor sodium orthovanadate (Na3VO4), a selective serine/ threonine (Ser/Thr) phosphatase inhibitor sodium fluoride (NaF) or okadaic acid.
Detection of phosphatase specificity was based on assays using the chemically synthesized phosphopeptides: END(pY)INASL, DADE(pY)LIPQQG and RRA(pT)VA (Promega, Madison, WI). Purified protein was incubated with 100 µM phosphopeptides in 50 mM Imidazole-HCl (pH 7.2) and 2 mM DTT (for phosphotyrosine peptides) or 250 mM Imidazole (pH 7.2), 0.1% β-mercaptoethanol, 1 mM EGTA, 25 mM MgCl2 and 0.5 mg/ml bovine serum albumin (BSA) (for the phosphoserine peptide) in a 50 µl reaction at a final concentration of 2 mg/ml. The reaction was incubated at 37°C for 30 min followed by incubation with the addition of 50 µl Molybdate Dye/ Additive mixture (provided in kit) at room temperature for 15 min. The phospholipid phosphatase assays were performed using a Malachite Green phosphatase assay kit (Echelon Biosciences, San Jose, CA) with a water soluble D-myo-phosphatidylinositol 3,4,5-trisphosphate substrate (diC8-PIP3; Echelon Biosciences). Purified human PTEN protein (Upstate, Charlottesville, VA) was used as an active phospholipid phosphatase control. Various amounts of purified protein (100 ng – 1000 ng) and 100 ng PTEN were incubated with 200 µM diC8-PIP3 in a phosphate free assay buffer containing 100 mM Tris-HCl, pH 8.0 and 2 mM DTT in 50 µl reactions in a microplate. The reaction was incubated at 37°C for 30 min followed by incubation with 200 µl malachite green solution for 15 min at room temperature. The release of phosphate was then measured at an absorbance of 620 nm [36]. The amount of free phosphate was calculated from the standard curve performed with standard phosphate solution in each assay.
2.5. In Vitro H2O2 Oxidation and DTT Reduction Assays
For oxidation assays with H2O2, purified PRL-1 proteins were freshly dialyzed into buffer containing 50 mM Bis-Tris, 50 mM Tris, and 100 mM sodium-acetate, pH7.0, prior to inactivation with H2O2 [37]. Dialyzed PRL-1 adjusted to a final concentration of 2 mg/ml was incubated in the absence or presence of 100, 200, 500 µM H2O2 in a total volume of 100 µl for 10 min at room temperature. The reactions were stopped by adding 12.5 U of catalase (Sigma, Milwaukee, WI) to consume H2O2 (should consume 200 nmol H2O2/s). PRL-1 reactions treated with 500 µM H2O2 were further incubated with 4 mM DTT for 30 min at room temperature. Following treatment, 50 µl per reaction was used for determining PTP activity. In order to minimize the impact of natural oxidation to the protein stock, freshly purified and dialyzed recombinant PRL-1 was used in each assay of enzymatic activity.
2.6. MALDI-TOF Mass Spectrometry
WT PRL-1 and C104S PRL-1 mutant were separated on a SDS-PAGE under non-reducing conditions following incubation with or without 1 mM H2O2 at room temperature for 30 min. The oxidized band and reduced band of each recombinant PRL-1 were visualized by silver staining. Protein bands of interest were excised from the gel and subjected to destaining, in-gel tryptic digestion and peptide extraction using a Mass-Prep digestion robot (Micromass, UK). The peptides were analyzed by matrix assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS) using a Voyager DE-Pro (Applied Biosystems, Foster City, CA).
2.7. Cell Culture and Treatments
The mouse cone photoreceptor-derived 661W cells, kindly provided by Dr. Al-Ubaidi (University of Oklahoma, OK) [38], were grown in DMEM supplemented with 10% FBS and an antibiotic-antimycotic (Gibco, Carlsbad, CA). For most assays cultures were seeded in a six-well plate or 35 mm culture dish at a concentration of 1 × 105 cells in 2 ml growth media and grown to 85% to 90% confluence. Cells used for H2O2 oxidation assays, were supplemented with varying concentrations of H2O2 added to the media for 30 min or various durations at 37°C [39]. For GSH depletion in 661W cells prior to the exposure to H2O2, 1 mM buthionine sulfoximine (BSO, Sigma), a specific GSH synthesis inhibitor, was used to treat cells for 17 h before the cells were exposed to H2O2 as indicated. GSH levels were obtained using a GSH detection Kit from Calbiochem. After treatments with H2O2, cells were washed twice with 2 ml PBS containing 10 U catalase to consume H2O2 and then lysed in 100 µl extraction buffer containing 40 mM N-ethylmaleimide (NEM; Pierce, Rockford, IL; to prevent thiol-disulfide exchange), 100 mM Tris-HCL, PH 7.5, 2% SDS and 1mM phenylmethylsulfonyl fluoride (PMSF) on ice for 5 min. Lysates were collected and briefly sonicated. Protein translation was inhibited by cycloheximide treatment of 661 W cells with 100 µg/ml cycloheximide (Sigma) for 1 hour prior to H2O2 treatment. After exposure to 1 mM H2O2 for increasing times, the cells were washed and lysed as described above.
2.8. Retina Tissue Treatment with H2O2
Fresh porcine eyes were obtained from a local abattoir. The anterior segment including cornea, iris, lens, ciliary body were obliquely dissected out with scissors, vitreous humor was gently removed. The remaining eyecup with retina attached was incubated with 10 mM or 100 mM H2O2 in DMEM without phenol red at 37°C for 30 min [40]. Then the media was removed and the retinas were rinsed with phosphate buffered saline (PBS). Following washes, the retinas were removed gently and lysed in buffer containing 40 mM NEM, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 1mM PMSF and protease inhibitor cocktail tablet (Complete, Mini EDTA-free; Roche Diagnostics, Indianapolis, IN). NEM was added to the lysis buffer in order to alkylate free SH groups thus protecting them from any further oxidation during tissue processing. Note that in vivo disulfide bonded cysteines could not be alkylated during the NEM treatment unless they were initially reduced.
2.9. Animals and Light Exposure
Adult albino Sprague-Dawley rats (weight 180~200 g) were purchased from Harlan (Indianapolis, IN). Rats were maintained in accordance with the Institutional Animal Care and Use Committee at Duke University and the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. Rats were housed conventionally under ambient conditions (12 h dark, 12 h light) and fed standard rat chow and water ad libitum. Experimental animals were exposed to constant cool white light (720 lux measured at the level of the cages) for up to 7 days and either sacrificed immediately following light exposure (between 1 pm and 2 pm to minimize circadian fluctuations in the measurements) or returned to normal cyclic light for 9 weeks before sacrificing. Eyes were removed and the anterior segments and the lens were cut away. In the animals analyzed immediately following constant light exposure retinas were isolated from the eyecup and were either lysed in extraction buffer containing 100 mM Tris-HCl and 2% SDS followed by Western blot analysis, or stored in RNAlater (Ambion) at −20°C for RNA isolation and qRT-PCR analysis. For animals returned to cyclic light for 9 weeks the eyecups were postfixed in a mixture of 2% glutaraldehyde and 2% paraformaldehyde overnight, followed by dehydration through a graded series of ethanols. Then the eyecups were embedded in Spurr’s low-viscosity epoxy resin. Semithin (1 µm) sections were obtained with an ultramicrotome (Leica, Heidelberg, Germany). The sections were attached to glass slides, stained with methylene blue and cover slipped for histologic analysis.
2.10. Western Blots
Protein concentrations in the samples were determined using a DC Protein Assay Kit assay (Bio-Rad) with BioRad protein Assay standard II BSA as the standard. Protein samples were denatured in XT sample buffer (Bio-Rad) under reducing (boiling for 5 min in Bio-Rad XT reducing agent) or non-reducing conditions and separated on 12 % Bis-Tris gels. Proteins were transferred to PVDF membranes, blocked in 10% fat-free milk and incubated with 0.4 µg/ml affinity-purified anti-PRL-1. Blots were incubated with a peroxidase-conjugated secondary antibody (Jackson lab, Bar Harbor, ME) and bands were visualized using the ECL Plus chemiluminescent detection kit (Amersham Bioscience). When needed, blots were reprobed with rabbit anti-thioredoxin (Abcam, Cambridge, MA), or mouse monoclonal anti-γ-tubulin (Sigma, Saint Louise, MO).
2.11. Real-Time Quantitative Reverse Transcription-PCR (qRT-PCR)
Real-time quantitation of PRL-1 mRNA was performed, with intron-spanning primers, using an iCycler iQ detection system (Bio-Rad, Hercules, CA) with SYBR Green, as previously described [33, 41]. First-strand cDNAs were synthesized from equal amounts of total RNA (1 μg/reaction) with a cDNA synthesis kit (iScript) as described by the manufacturer (Bio-Rad). Expression levels were normalized to the geometric mean of the endogenous references, γ-tubulin (TUBG1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein L13a (RPL13A) [42] and calculated by the comparative threshold cycle (CT) method (2−ΔΔCT) [43]. PCR primer sequences for each gene analyzed are available upon request.
3. Results
Protein Tyrosine Phosphatase Specificity of PRL-1
The PTP activity of PRL-1 has been documented in previous studies [4]. However, a systematic study of its phosphoamino acid specificity has not yet been reported. Theoretically, PRL-1 could act as a traditional PTP or as a dual-specificity PTP (which dephosphorylates phospho-tyrosyl, -seryl and -threonyl residues [44]), because the sequence of its catalytic domain, VHCVAGLGR, matches either PTP type [45, 46]. In order to systematically address the substrate specificity of PRL-1 in vitro we expressed recombinant, His-tagged PRL-1 in E coli. It showed robust, concentration dependent phosphatase activity with the non-peptide, synthetic substrate, DiFMUP (Figure 1A). Importantly, a serine substitution of the essential conserved cysteine, Cys104, within the catalytic motif of PRL-1 [47] results in complete loss of phosphatase activity (Figure 1A). Biochemical evidence for the PTP specificity of WT PRL-1 was obtained using several selective phosphatase inhibitors and phosphopeptide substrates. The selective tyrosine phosphatase inhibitor, Na3VO4, completely inactivated PRL-1 at concentrations of 0.1 mM, whereas the Ser/Thr phosphatase inhibitors, NaF and okadaic acid, had no reliable effect (Figure 1B). Experiments with phosphopeptides also revealed a strong PRL-1 preference for phosphotyrosine-containing peptides, END(pY)INASL and DADE(pY)LIPQQG, whereas no activity was detected with the phosphothreonine-containing peptide, RRA(pT)VA (Table 1).
Figure 1.
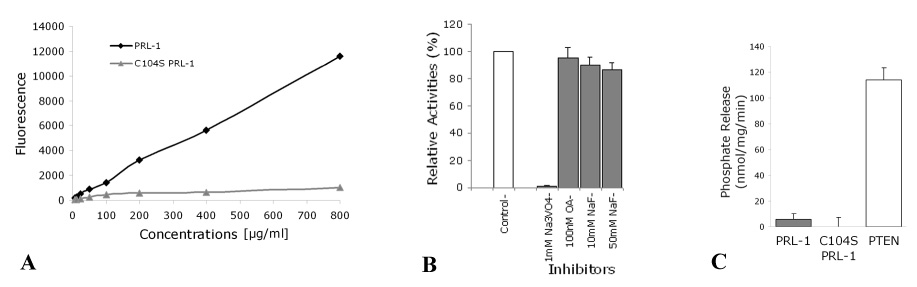
Protein tyrosine phosphatase specificity of recombinant PRL-1. (A) Phosphatase activities were measured using the phosphatase substrate, DiFMUP (50 µM) whose reaction product, DiFMU, has an excitation/emission maxima of ~358 /450 nm. The starting concentration of recombinant PRL-1s was 800 µg/ml. The enzymes were serially diluted in reaction buffer (25 mM MOPS, 50 mM NaCl, 1 mM DTT and 0.05% Tween-20). The activity of C104S mutant PRL-1 was completely abolished. (B) Concentrations of the inhibitors used as indicated on the x-axis and activities relative to that in the absence of any inhibitors (100%) are shown. (C) Recombinant WT PRL-1, C104S PRL-1 and PTEN were incubated with 200 µM water soluble diC8-PIP3 substrate at 37°C for 30 min followed by incubation with 200 µl malachite green solution for 15 min at room temperature. Release of phosphate was measured at an absorbance of 620 nm. The amount of free phosphate was calculated from the standard curve derived using phosphate solution standards. Activities are expressed as nmol of phosphate released per min per mg. Amount of enzyme used in the assay are indicated on the x-axis. Results are means (±SE) from representative experiments. Na3VO4, sodium orthovanadate; NaF, sodium fluoride; OA, okadaic acid.
Table 1.
Substrate specificity of PRL-1
| Substrate | Detectable activity |
|---|---|
| ENDpYINASL | + |
| DADEpYLIPQQG | + |
| RRA(pT/S)VA | − |
| Phospholipids | − |
PRL-1 also shares 32% homology with human PTEN, a dual-specificity PTP known to dephosphorylate phospholipid substrates including phosphatidylinositol-3,4,5-triphosphate (PIP3). However, direct assays showed very little PRL-1 activity toward PIP3 under conditions where the same amount of PTEN hydrolyzed PIP3 at a high rate (Figure 1C). Together, the experiments in Figure 1 argue that PRL-1 is a tyrosine phosphatase with no appreciable phosphoserine/threonine or phospholipid activity.
In Vitro Reversible Oxidative Inactivation of PRL-1 by H2O2
The Cys within the catalytic site of PTPs (Cys104 in PRL-1) has a low pKa and is therefore more susceptible to oxidation [48]. In vitro incubation of purified, recombinant, WT PRL-1 with H2O2 resulted in a concentration-dependent decrease in phosphatase activity (Figure 2A). A significant, nearly 70%, inactivation was observed following a 10 min exposure to 500 µM H2O2 (Figure 2A). This inactivation was reversible since the subsequent incubation of PRL-1 with the reducing agent, DTT (4 mM), resulted in complete restoration of PRL-1 phosphatase activity (Figure 2B). Freshly purified, recombinant PRL-1 migrates as single band as in SDS gel. H2O2 treated PRL-1 migrates as two bands that is reversible by DTT (Figure 2B,top panel).
Figure 2.

In vitro reversible oxidative inhibition of PRL-1. (A) PRL-1 dialyzed in buffer containing 50 mM Tris, 50 mM Bis-Tris, 100 mM Na-Acetate (pH 7.0), adjusted to a final concentration of 2 mg/ml was incubated in the absence or presence of H2O2 in a total volume of 100 µl for 10 min at room temperature. The reaction was stopped by adding 12.5 U catalase, and the PRL-1 phosphatase activity was measured immediately after as described in experimental procedures using DiFMUP. (B) PRL-1 reactions, partially inactivated by 500 µM H2O2, were further incubated with 4 mM DTT for 30 min at room temperature. Remaining PTP activity was assayed with DiFMUP as described for Fig. 1 and expressed as % activity relative to the untreated control PRL-1. Treated PRL-1 was also shown on silver-stained SDS gel (top panel). Results are means from three independent experiments.
Formation of an Intramolecular Disulfide Bond between Cys49 and Cys104 in Oxidized PRL-1
SDS PAGE analysis of WT PRL-1 under reducing conditions (4 mM DTT), yields a single protein band (Figure 3, top panel). However, PRL-1 preincubation with increasing concentrations of H2O2 results in the appearance of a second band with an increased electrophoretic mobility (Figure 3). The reversibility of this phenomenon argues that the second band originates from protein reduction rather than protein cleavage. A similar mobility shift has previously been documented as a modification of PTEN and results from a protein conformation change induced by oxidation of sulfhydryl groups [39].
Figure 3.

Intramolecular disulfide bond formation between Cys104 and Cys49. Recombinant WT and Cys mutant PRL-1s with or without the presence of varying concentrations (as indicated) of H2O2 or DTT, were subjected in SDS-PAGE under non-reducing conditions followed by silver staining. The reduced and oxidized forms of PRL-1 have different electrophoretic mobilities. Redu-, reduced; Oxi-, oxidized.
We proposed, by analogy, that the oxidized form of PRL-1 migrates faster than the reduced form, putatively due to a more compact protein structure, as a result of intramolecular disulfide bonding between cysteines. To test this hypothesis directly we generated an array of PRL-1 mutants in which individual cysteine residues were replaced with serines. The PRL-1 protein contains 6 cysteines. Among these, we focused on three, Cys49, Cys99 and Cys104, which are conserved in all three members of the PRL family. We tested these recombinant mutant PRL-1s for their abilities to be modified by oxidation. Each form of mutant PRL-1 was incubated with various concentrations of H2O2 followed by gel electrophoresis and silver staining (Figure 3). Unlike WT PRL-1, the C104S and C49S PRL-1 mutants migrate as single bands following exposure to H2O2 suggesting that these cysteines could participate in intramolecular disulfide bonding (Figure 3). In contrast, the C99S mutant migrated faster in the presence of H2O2 and therefore could be oxidized. This excludes this residue from potential participation in intramolecular disulfide bonding. Interestingly, the susceptibility of this mutant to oxidation was higher than that of the WT PRL-1 with nearly 100% of the mutant C99S oxidized with 5 mM H2O2 (compared to ~ half of WT PRL-1 oxidized, Figure 3).
Direct evidence that the Cys104 and Cys 49 residues participate in the oxidation-induced formation of an intramolecular disulfide bridge in PRL-1 were obtained by MALDI-TOF MS. Purified recombinant PRL-1 was incubated with or without 1 mM H2O2 and run on a SDS-PAGE under non-reducing conditions. The bands were trypsin-digested and analyzed by MALDI-TOF. The active-site peptide containing Cys104 and another peptide containing Cys49 were reliably detected in the digest (Table 2).
Table 2.
MALDI-MS detection of intramolecular disulfide bond formation in oxidized WT PRL-1
| Enzyme | peptide containing C49 | Active-site peptide (C104) | Cross-linked disulfide peptides |
|---|---|---|---|
| PRL-1 (WT) | VCEATYDTTLVEK | EEPGCCIAVHCVAGLGR | NONE detected |
| Reduced | 1471.69850 ± 0.0782 | 1713.7869 ± 0.0713 | |
| PRL-1 (WT) | VCEATYDTTLVEK | ||
| H2O2 1 mM, | 1471.69850 ± 0.0782 | VCEATYDTTLVEK | |
| 30 min | EEPGCCIAVHCVAGLGR | | | |
| 1713.7869 ± 0.0713 | EEPGCCIAVHCVAGLGR | ||
| 3183.3762 | |||
| VCEATYDTTLVEK | FREEPGCCIAVHCVAGLGR | VCEATYDTTLVEK | |
| 1471.69850 ± 0.0782 | 2016.9563 ± 0.0269 | | | |
| FREEPGCCIAVHCVAGLGR | |||
| 3483.3157 | |||
| PRL-1 | VCEATYDTTLVEK | EEPGCCIAVHSVAGLGR | NONE detected |
| (C104S) | 1471.69850 ± 0.0298 | ||
| Reduced | |||
| PRL-1 | VCEATYDTTLVEK | EEPGCCIAVHSVAGLGR | NONE detected |
| (C104S) | 1471.69850 ± 0.0298 | ||
| H2O2 1 mM, | |||
| 30 min | |||
In the oxidized form of PRL-1 two new peptides were detected at expected molecular masses of 3183.3762 and 3483.3157. These masses correspond to two predicted peptides cross-linked by a disulfide bond between Cys104 and Cys49, respectively (Table 2). These findings confirm interpretation of the oxidized mobility shifts in the WT PRL-1 as the result of the formation of a disulfide bond between Cys104 and Cys49. Our findings corroborate those obtained in 2 recent independent studies [10, 34].
H2O2-mediated Oxidation of Endogenous PRL-1 in 661W Cells and in Retina Tissue
To determine whether the oxidative modulation of PRL-1 also occurs in living cells, we examined whether PRL-1 could be oxidized in a photoreceptor-derived cell line (661W) or in porcine retina. Both of these preparations show robust endogenous expression of PRL-1. The data shown in Figure 4 indicates that incubation of both preparations with H2O2 causes the mobility shift in PRL-1 corresponding to the transition between its reduced and oxidized forms.
Figure 4.

Oxidative status of PRL-1 in 661W cells (A, B) and porcine retina (C) exposed to H2O2. (A) Mouse photoreceptor-derived 661W cells were grown to 90% confluence. Cells were either incubated with varying concentrations of H2O2 (as indicated) for 30 min or (B) incubated with 1 mM H2O2 for varying times (as indicated). (C) Porcine retinas in eyecups were incubated with indicated concentrations of H2O2 (added to DMEM) for 30 min. Cell or retinal lysates were alkylated with 40 mM NEM to prevent disulfide exchange. Proteins were resolved by SDS-PAGE followed by immunoblot analysis with anti-PRL-1 antibody. Equal sample loading was monitored by reprobing the Western blot with anti-γ-tubulin antibodies (data not shown).
The H2O2 concentration dependence assays conducted with 661W cells (Figure 4A) showed the oxidized form of PRL-1 is initially detectable at 0.2 mM H2O2 and becomes predominant at H2O2 concentrations exceeding 0.5 mM. At these high H2O2 concentrations PRL-1 oxidation is extremely rapid and complete within 5 minutes of incubation (Figure 4B). The amount of H2O2 required to oxidize endogenous PRL-1 in the retina was higher (Figure 4C). This may be due to reduced accessibility of H2O2 to the photoreceptors in the eyecup preparations used for these experiments or due to a more robust anti-oxidant system in situ.
Glutathione Levels Alter Oxidative Status of PRL-1 in 661W Cells
GSH is a major cellular redox regulator. To examine the potential contribution of GSH to the regulation of PRL-1 oxidation, we depleted GSH in cells using BSO. BSO is a specific inhibitor of γ-glutamylcysteine synthetase, a key enzyme in GSH synthesis. Pretreatment of 661 W cells with 1 mM BSO for 17 h depleted GSH levels by 75% (data not shown). In these cells, PRL-1 was oxidized at lower concentrations of H2O2 than untreated cells (Figure 5). The data in Figure 5 show that 0.2 mM H2O2 causes only a minor PRL-1 oxidation in control cells (‘-BSO’ lanes), but a rapidly developing, nearly complete oxidation in the GSH-depleted cells (‘+BSO’ lanes).
Figure 5.
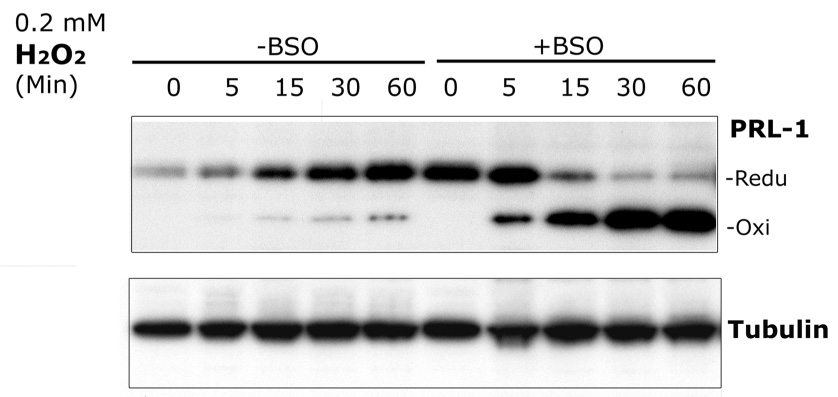
Time-dependent increase in PRL-1 protein levels in 661W cells exposed to low concentrations of H2O2. 661W Cells without pretreatment (-BSO) or pretreated with 1 mM BSO (+BSO) for 17 h, were incubated with 0.2 mM H2O2 for the indicated times. Equal amounts of protein from each sample were separated by SDS-PAGE followed by immunoblot analysis with anti-PRL-1. Equal sample loading was monitored by reprobing the Western blot with anti-γ-tubulin antibodies.
Oxidative Stress Increases PRL-1 Expression in 661W Cells
In addition to oxidative modulation of PRL-1 in 661W cells, nalysis of reduced and oxidized PRL-1 protein levels in Figure 4 (A, B) revealed that PRL-1 protein levels increased with increasing H2O2 concentrations and/or incubation times. We also observed this effect when 661W cells were treated with lower concentrations of H2O2 (0.2 mM; Figure 5). Pretreatment with BSO also increased endogenous PRL-1 levels (Figure 5, time 0, +BSO lane). To examine whether this effect required translation of new protein we treated cells with 100 µg/ml cycloheximide 1 hour prior to treatment with 1mM H2O2 and observed that no increase in PRL-1 protein levels were observed when translation was inhibited (Figure 6).
Figure 6.
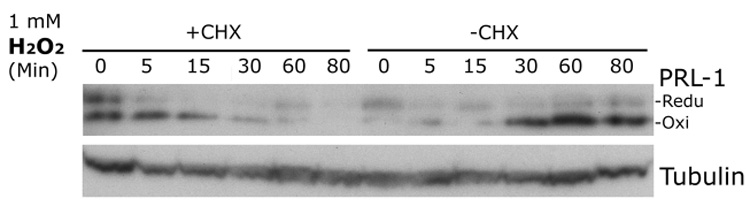
Influence of the translation inhibitor cycloheximide (CHX) on the time-dependent increase in PRL-1 protein levels in 661 W cells exposed to H2O2. 661W cells were exposed to 1 mM H2O2 for increasing times. One set of cells was pretreated with 100 µg/ml CHX for one hour prior to exposure to H2O2 (+CHX) and the other set were treated only with the H2O2 (-CHX). Equal amounts of protein from each well were separated by SDS-PAGE followed by immunoblot analysis with anti-PRL-1 and equal sample loading was monitored by reprobing with anti-tubulin antibodies.
Effect of Constant Light Exposure on PRL-1 in the Rat Retina
To explore oxidative modulation of PRL-1 in photoreceptors in vivo, we used an experimental model of oxidative stress caused by exposing animals to constant light (photooxidative damage paradigm modified from LaVail et al. [49]). Albino rats were exposed to constant white light for a period of 0, 2, 5, or 7 days as described in Experimental Procedures. This did not cause any noticeable morphological changes in their retinas immediately following the exposure (Figure 7A), but resulted in a complete loss of photoreceptors 9 weeks later (Figure 7B).
Figure 7.

Light microscopy of rat retina following 7 days constant light exposure (A) Rat retina exposed to 7 days constant light (B) Rat retina exposed for 7 days to constant light, at nine weeks after return to normal 12 hr cyclic light. RPE, retinal pigment epithelium; OS, outer segments; ONL, outer nuclear layer of photoreceptors; INL, inner nuclear layer, D, days. Scale bars equal 20 microns.
Following light exposure, PRL-1 mRNA and protein levels were measured in the retinas. PRL-1 mRNA levels, analyzed by qRT-PCR, increased at 2, 5 days and 7 days of constant light exposure to nearly 2.5 times the initial level (Figure 8A). PRL-1 protein expression levels were queried on Western blots and were also shown to be increased at each increasing length of light exposure to approximately the same extent after 7 days of constant illumination. Interestingly, all the PRL-1 at each time point was reduced, suggesting that this photooxidative insult causes a compensatory increase in protein expression level but does not saturate its capacity to resist oxidative stress. This behavior of PRL-1 parallels that of a well-established marker of oxidative stress, thioredoxin (Trx) [50], which also showed an increase in expression level after 5 and 7 days of light exposure (Figure 8B).
Figure 8.
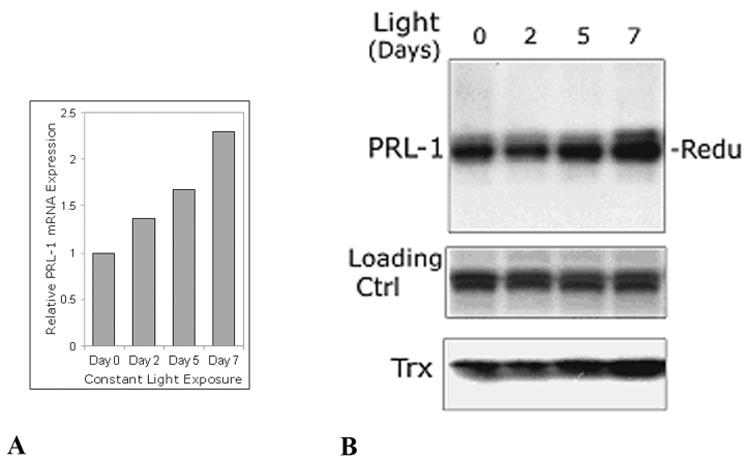
Increasing expression of PRL-1 mRNA (A) and protein (B) in rat retinas due to constant moderate light exposure. Albino rats were exposed to the constant cool white light for 2, 5 and 7 days. Rats kept in normal cyclic light/dark served as control animals. (A) Real-time qRT-PCR summaries of PRL-1 gene expression in constant light-exposed retinas (Day 2,5,7) relative to expression in retina at Day 0 (no constant light) (B) Equal amounts protein (50 µg) of each retinal lysate was subjected to SDS-PAGE followed by immunoblot analysis with anti-PRL-1 antibody. Data are representative of three independent experiments. Trx, thioredoxin; Loading ctrl, non-specific bands on the same blot used as loading control.
4. Discussion
The central observation obtained in this study is that the expression level and redox status of the small phosphatase, PRL-1, undergo dramatic changes when photoreceptors and photoreceptor-derived cells are subjected to oxidative stress. The potential involvement of PRL-1 in managing the stress response in these cells will be discussed.
Reversible Redox Regulation is a General Theme in Modulation of Protein Tyrosine Phosphatase Activity
Direct evidence supporting the role of reversible oxidation of Cys residues has recently been described for the regulation of several phosphatases that leads to a rapid, reversible, stimulus-induced inactivation of PTPs [22–27]. The catalytic sites of PTPs contain a conserved Cys that is directly involved in catalysis and has a low pKa that is susceptible to oxidation [23]. In the classical PTP, PTP1B, formation of a sulphenyl-amide bond through the bonding of sulfur on the Cys to the backbone nitrogen of a neighboring serine, was detected following oxidation [27]. In some non-classical PTPs, including PTEN, Cdc25 and low molecular weight (LMW) PTPs, oxidation by H2O2 induced formation of an intramolecular disulfide bond between the Cys in the catalytic domain and another proximal Cys [22, 37, 51]. In general, oxidation of Cys moieties to their sulfenic acid (Cys-SOH) form is reversible. Therefore, intramolecular bonding in response to oxidation is thought to protect the Cys from further irreversible oxidation to a sulfenic acid (Cys-SO2H) or sulfonic acid (Cys-SO3H) form [23, 52].
Our results are in total agreement with two recent independently conducted studies indicating that PRL-1 oxidation leads to the formation of a disulfide bridge between the catalytic Cys104 and Cys49 [10, 34]. This reaction completely abolishes PRL-1 catalytic activity and could be reversed by reducing agents. We complimented in vitro studies of PRL-1 oxidative modulation with the demonstration that PRL-1 naturally expressed in cone photoreceptor-derived 661W cells could also be oxidized (Figure 4A). Interestingly, this novel result indicates that the Cys99 moiety also modulates the oxidation of the catalytic Cys104. Substitution of Cys99 to Ser resulted in higher sensitivity to the H2O2–induced oxidation in vitro than WT PRL-1, suggesting that Cys99 can facilitate intramolecular disulfide bond formation (Figure 3). Based on the crystal structure of PRL-1 the Cys99 is residue is in close proximity to the C104 residue [10], raising the possibility that the change in the PRL-1 protein conformation induced by the C99S mutation is close enough to influence the disulfide bonding between C104 and C49.
Potential Connection Between PRL-1 Oxidation and the Glutathione Cellular Redox System
The ability of endogenous PRL-1 to undergo redox modulation in 661W cells enabled us to test the connection between this phenomenon and the function of the glutathione (GSH) system, the major determinant of the cellular redox status of protein thiols [32, 53]. GSH is the non-protein thiol present in highest concentrations in the cell where it acts as an antioxidant, as a free radical scavenger, protecting protein sulfhydyls. Previous reports indicate that other non-classical PTPs, such as LMW-PTP, can be reactivated by the GSH system after being subjected to exogenous oxidative stress [54]. We showed that the GSH system specifically controls the redox state of PRL-1 in living cells by demonstrating that its selective reduction inhibitor, BSO, dramatically increases PRL-1 oxidation in 661W cells exposed to H2O2. This indicates that GSH acts downstream of PRL-1 by continuously regenerating it to its reduced form. In the absence of GSH the entire pool of PRL-1 is rapidly transformed into its inactive oxidized form.
Another significant result obtained in the same experiment is that treatment of 661W cells with BSO resulted in a rapid increase in the PRL-1 expression level (Figure 5). This suggests that PRL-1 is an early component of the cellular response to oxidative stress and in the absence of the normal homeostatic regeneration by the GSH system the cell attempts to replenish the pool of its reduced form by rapid biosynthesis. This is further supported by the results in Figure 6 showing that the increase in PRL-1 observed in response to oxidative stress is protein translation dependent.
Potential Involvement of PRL-1 in Defense Against Oxidative Stress in the Retina
Oxidative stress is an established risk factor for various retinal degenerations, particularly in the region of the macula where the cone photoreceptors are most densely concentrated and high acuity vision occurs [55, 56]. This susceptibility to oxidative damage is thought to be due to the high consumption of oxygen, high content of polyunsaturated fatty acids, daily turnover of photoreceptor outer segment disc membranes and continuous light exposure which produces free radicals [55]. Although oxidative stress in the retina cannot be quantitatively measured in vivo, enhanced susceptibility to retinal photodynamic injury via oxidative stress is convincingly established in animal models [57, 58]. One such model is based on exposure of a rodent to constant light of moderate intensity for a period of several days. Although no immediate morphological changes are observed during exposure, severe photoreceptor degeneration takes place within 2 to 3 months after these animals are returned to normal cyclic light ([49] and Figure 7). Photooxidative stress is thought to be a major contributing factor to this degeneration because the major redox systems are activated [50, 57] and antioxidants have been used to reduce the damaging effects of constant light [50, 59].
In this model of light damage, both mRNA and protein levels of PRL-1 increased proportionally to the duration of light exposure to at least 2 times the initial level (Figure 8). Interestingly, all of the PRL-1 in the retinal lysates obtained from control and light exposed rats was reduced. This suggests that with this level of oxidative stress the in vivo cellular defense mechanisms regulating the redox state of PRL-1, presumably the GSH system based on our results obtained in cell culture, are not overwhelmed. Yet, the cells are still responding to stress by boosting PRL-1 biosynthesis, so that the retina levels of both PRL-1 and GSH [50] are increased under photooxidative stress.
One important question raised by our experiments is whether the putative protective role of PRL-1 is directly based on its ability to retain its phosphatase activity, or simply its ability to be oxidized by free radicals formed in the cell under the conditions of stress. Although the amount of GSH in the cell overwhelmingly exceeds the amount of PRL-1, the redox properties of the latter may be such that it is more sensitive to oxidative changes and therefore PRL-1 may be used by photoreceptors as the first echelon of defense against oxidative stress. Understanding the roles of the PRL-1 phosphatase activity and its ability to undergo reversible redox transformation in the cellular response to oxidative stress is a clear goal of future experiments.
In summary, our data support the hypothesis that PRL-1 is a molecular component of the cone photoreceptor’s response to oxidative stress, which causes PRL-1 oxidation and induces its expression. Under oxidative stress, production of reactive oxidation species can act as potential second messengers of signal transduction in cells inactivating protein tyrosine phosphatases that may, in turn, uncouple self-regulatory mechanisms of protein tyrosine kinases down-regulating the protein phosphorylation in photoreceptors. This work provides novel insights into modulation of PRL-1 in oxidative stressed retina and photoreceptors, suggesting potential roles of PRL-1 in photoreceptor cell survival under these conditions.
Acknowledgements
The authors gratefully acknowledge the following funding agencies: The NEI, National Institutes of Health Grant R01EY11286 (to CBR), P30EY0054722 NEI Core Grant, Research to Prevent Blindness (RPB) Career Development Award (CBR), and a RPB Core Grant to Duke Eye Center.
The following abbreviations were used
- PRL-1
phosphatase of regenerating liver
- PTP
protein tyrosine phosphatase
- H2O2
hydrogen peroxide
- Cys
cysteine
- Ser
serine
- Thr
threonine
- DiFMUP
6, 8-difluoro-4-methylumbelliferyl phosphate
- Na3VO4
sodium orthovanadate
- NaF
sodium fluoride
- BSA
bovine serum albumin
- MALDI-TOF-MS
matrix assisted laser desorption ionization-time of flight-mass spectrometry
- AMS
4-acetamido-4'-maleimidylstilbene-2,2'-disulfonic acid
- NEM
N-ethylmaleimide
- BSO
buthionine sulfoximine
- OS
outer segment
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- GSH
glutathione
- Trx
thioredoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yarovinsky TO, Rickman DW, Diamond RH, Taub R, Hageman GS, Rickman C Bowes. Expression of the protein tyrosine phosphatase, phosphatase of regenerating liver 1, in the outer segments of primate cone photoreceptors. Brain Res Mol Brain Res. 2000;77:95–103. doi: 10.1016/s0169-328x(00)00045-0. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y, Genin A, Spinner NB, Diamond RH, Taub R. The gene encoding human nuclear protein tyrosine phosphatase, PRL-1. Cloning, chromosomal localization, and identification of an intron enhancer. J Biol Chem. 1998;273:17286–17295. doi: 10.1074/jbc.273.27.17286. [DOI] [PubMed] [Google Scholar]
- 3.Gehrig A, Felbor U, Kelsell RE, Hunt DM, Maumenee IH, Weber BH. Assessment of the interphotoreceptor matrix proteoglycan-1 (IMPG1) gene localised to 6q13-q15 in autosomal dominant Stargardt-like disease (ADSTGD), progressive bifocal chorioretinal atrophy (PBCRA), and North Carolina macular dystrophy (MCDR1) J Med Genet. 1998;35:641–645. doi: 10.1136/jmg.35.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond RH, Cressman DE, Laz TM, Abrams CS, Taub R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14:3752–3762. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Q, Hong W, Tan YH. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem Biophys Res Commun. 1998;244:421–427. doi: 10.1006/bbrc.1998.8291. [DOI] [PubMed] [Google Scholar]
- 6.Bardelli A, Saha S, Sager JA, Romans KE, Xin B, Markowitz SD, Lengauer C, Velculescu VE, Kinzler KW, Vogelstein B. PRL-3 expression in metastatic cancers. Clin Cancer Res. 2003;9:5607–5615. [PubMed] [Google Scholar]
- 7.Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, Crowell PL, Crowell DN. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 1996;110:49–55. doi: 10.1016/s0304-3835(96)04459-x. [DOI] [PubMed] [Google Scholar]
- 8.Zeng Q, Si X, Horstmann H, Xu Y, Hong W, Pallen CJ. Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma membrane and the early endosome. J Biol Chem. 2000;275:21444–21452. doi: 10.1074/jbc.M000453200. [DOI] [PubMed] [Google Scholar]
- 9.Kozlov G, Cheng J, Ziomek E, Banville D, Gehring K, Ekiel I. Structural insights into molecular function of the metastasis-associated phosphatase PRL-3. J Biol Chem. 2004;279:11882–11889. doi: 10.1074/jbc.M312905200. [DOI] [PubMed] [Google Scholar]
- 10.Jeong DG, Kim SJ, Kim JH, Son JH, Park MR, Lim SM, Yoon TS, Ryu SE. Trimeric structure of PRL-1 phosphatase reveals an active enzyme conformation and regulation mechanisms. J Mol Biol. 2005;345:401–413. doi: 10.1016/j.jmb.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 12.Diamond RH, Cressman DE, Laz TM, Abrams CS, Taub R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14:3752–3762. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano S, Fukuyama H, Fukumoto M, Kimura J, Xue JH, Ohashi H, Fujita J. PRL-1, a protein tyrosine phosphatase, is expressed in neurons and oligodendrocytes in the brain and induced in the cerebral cortex following transient forebrain ischemia. Brain Res Mol Brain Res. 1996;40:105–115. doi: 10.1016/0169-328x(96)00035-6. [DOI] [PubMed] [Google Scholar]
- 14.Diamond RH, Peters C, Jung SP, Greenbaum LE, Haber BA, Silberg DG, Traber PG, Taub R. Expression of PRL-1 nuclear PTPase is associated with proliferation in liver but with differentiation in intestine. Am J Physiol. 1996;271:G121–G129. doi: 10.1152/ajpgi.1996.271.1.G121. [DOI] [PubMed] [Google Scholar]
- 15.Carter DA. Expression of a novel rat protein tyrosine phosphatase gene. Biochim Biophys Acta. 1998;1442:405–408. doi: 10.1016/s0167-4781(98)00173-0. [DOI] [PubMed] [Google Scholar]
- 16.Rundle CH, Kappen C. Developmental expression of the murine Prl-1 protein tyrosine phosphatase gene. J Exp Zool. 1999;283:612–617. doi: 10.1002/(sici)1097-010x(19990501)283:6<612::aid-jez14>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Kirby CE, Herbst R. The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J Biol Chem. 2002;277:46659–46668. doi: 10.1074/jbc.M206407200. [DOI] [PubMed] [Google Scholar]
- 18.Peters CS, Liang X, Li S, Kannan S, Peng Y, Taub R, Diamond RH. ATF-7, a novel bZIP protein, interacts with the PRL-1 protein-tyrosine phosphatase. J Biol Chem. 2001;276:13718–13726. doi: 10.1074/jbc.M011562200. [DOI] [PubMed] [Google Scholar]
- 19.Kessler-Becker D, Krieg T, Eckes B. Expression of pro-inflammatory markers byhuman dermal fibroblasts in a three-dimensional culture model is mediated by an autocrine interleukin-1 loop. Biochem J. 2004;379:351–358. doi: 10.1042/BJ20031371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daoud SS, Munson PJ, Reinhold W, Young L, Prabhu VV, Yu Q, LaRose J, Kohn KW, Weinstein JN, Pommier Y. Impact of p53 knockout and topotecan treatment on gene expression profiles in human colon carcinoma cells: a pharmacogenomic study. Cancer Res. 2003;63:2782–2793. [PubMed] [Google Scholar]
- 21.Peng Y, Du K, Ramirez S, Diamond RH, Taub R. Mitogenic up-regulation of the PRL-1 protein-tyrosine phosphatase gene by Egr-1. Egr-1 activation is an early event in liver regeneration. J Biol Chem. 1999;274:4513–4520. doi: 10.1074/jbc.274.8.4513. [DOI] [PubMed] [Google Scholar]
- 22.Cho SH, Lee CH, Ahn Y, Kim H, Kim H, Ahn CY, Yang KS, Lee SR. Redox regulation of PTEN and protein tyrosine phosphatases in H(2)O(2) mediated cell signaling. FEBS Lett. 2004;560:7–13. doi: 10.1016/s0014-5793(04)00112-7. [DOI] [PubMed] [Google Scholar]
- 23.den Hertog J, Groen A, van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys. 2005;434:11–15. doi: 10.1016/j.abb.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Forman HJ, Fukuto JM, Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 25.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 26.Rudolph J. Catalytic mechanism of Cdc25. Biochemistry. 2002;41:14613–14623. doi: 10.1021/bi0263513. [DOI] [PubMed] [Google Scholar]
- 27.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenylamide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 28.Groen A, Lemeer S, van der Wijk T, Overvoorde J, Heck AJ, Ostman A, Barford D, Slijper M, den Hertog J. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 29.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 30.Cideciyan AV, Zhao X, Nielsen L, Khani SC, Jacobson SG, Palczewski K. Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc Natl Acad Sci U S A. 1998;95:328–333. doi: 10.1073/pnas.95.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomany SC, Cruickshanks KJ, Klein R, Klein BE, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2004;122:750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CL, Rickman CB, Shaw SJ, Ebright JN, Kelly U, Sancar A, Rickman DW. Expression of the blue-light receptor cryptochrome in the human retina. Invest Ophthalmol Vis Sci. 2003;44:4515–4521. doi: 10.1167/iovs.03-0303. [DOI] [PubMed] [Google Scholar]
- 34.Sun JP, Wang WQ, Yang H, Liu S, Liang F, Fedorov AA, Almo SC, Zhang ZY. Structure and Biochemical Properties of PRL-1, a Phosphatase Implicated in Cell Growth, Differentiation, and Tumor Invasion(,) Biochemistry. 2005;44:12009–12021. doi: 10.1021/bi0509191. [DOI] [PubMed] [Google Scholar]
- 35.Welte S, Baringhaus KH, Schmider W, Muller G, Petry S, Tennagels N. 6,8-Difluoro-4-methylumbiliferyl phosphate: a fluorogenic substrate for protein tyrosine phosphatases. Anal Biochem. 2005;338:32–38. doi: 10.1016/j.ab.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 36.Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci U S A. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohn J, Rudolph J. Catalytic and chemical competence of regulation of cdc25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- 38.Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 40.Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell TR, Jr., Gong J, Abasi H, Blumberg J, Taylor A. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 41.Ebright JN, Rickman C Bowes. Rapid, Reproducible Real-Time Quantitative RT-PCR Using the iCycler iQ Real-Time PCR Detection System and iQ Supermix. BioRadiations. 2003;110:32–35. [Google Scholar]
- 42.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZY. Protein-tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit Rev Biochem Mol Biol. 1998;33:1–52. doi: 10.1080/10409239891204161. [DOI] [PubMed] [Google Scholar]
- 45.Denu JM, Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 46.Denu JM, Stuckey JA, Saper MA, Dixon JE. Form and function in protein dephosphorylation. Cell. 1996;87:361–364. doi: 10.1016/s0092-8674(00)81356-2. [DOI] [PubMed] [Google Scholar]
- 47.Barford D, Flint AJ, Tonks NK. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994;263:1397–1404. [PubMed] [Google Scholar]
- 48.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 49.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanito M, Nishiyama A, Tanaka T, Masutani H, Nakamura H, Yodoi J, Ohira A. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- 51.Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 52.Clairborne A, Miller H, Parsonage D, Ross RP. Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. Faseb J. 1993;7:1483–1490. doi: 10.1096/fasebj.7.15.8262333. [DOI] [PubMed] [Google Scholar]
- 53.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 54.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 55.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 56.Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005;88:269–281. doi: 10.1111/j.1444-0938.2005.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 57.Penn JS, Naash MI, Anderson RE. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987;44:779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- 58.Wenzel A, Grimm C, Marti A, Kueng-Hitz N, Hafezi F, Niemeyer G, Reme CE. c-fos controls the "private pathway" of light-induced apoptosis of retinal photoreceptors. J Neurosci. 2000;20:81–88. doi: 10.1523/JNEUROSCI.20-01-00081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]


