Abstract
Dendritic cells (DCs) capture and internalize human immunodeficiency virus (HIV)-1 through C-type lectins, including DC-SIGN. These cells mediate efficient infection of T cells by concentrating the delivery of virus through the infectious synapse, a process dependent on the cytoplasmic domain of DC-SIGN. Here, we identify a cellular protein that binds specifically to the cytoplasmic region of DC-SIGN and directs internalized virus to the proteasome. This cellular protein, leukocyte-specific protein 1 (LSP1), was defined biochemically by immunoprecipitation and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. LSP1 is an F-actin binding protein involved in leukocyte motility and found on the cytoplasmic surface of the plasma membrane. LSP1 interacted specifically with DC-SIGN and other C-type lectins, but not the inactive mutant DC-SIGNΔ35, which lacks a cytoplasmic domain and shows altered virus transport in DCs. LSP1 diverts HIV-1 to the proteasome. Down-regulation of LSP1 with specific small interfering RNAs in human DCs enhanced HIV-1 transfer to T cells, and bone marrow DCs from lsp1−/− mice also showed an increase in transfer of HIV-1BaL to a human T cell line. Proteasome inhibitors increased retention of viral proteins in lsp1+/+ DCs, and substantial colocalization of virus to the proteasome was observed in wild-type compared with LSP1-deficient cells. Collectively, these data suggest that LSP1 protein facilitates virus transport into the proteasome after its interaction with DC-SIGN through its interaction with cytoskeletal proteins.
DCs are professional antigen-presenting cells that are positioned throughout the peripheral immune system (1–3). DCs capture antigen and present processed antigenic peptides through MHC molecules (for review see references 4–7). Immature DCs migrate from the blood into tissues where they detect foreign antigens. Upon activation and maturation, these cells enlarge and migrate further to secondary organs where interaction with T cells can occur (8). HIV-1 infects permissive cells by interacting with CD4 molecules on the target cell and the gp120 subunit on the envelope of the virus (9, 10). This interaction causes a conformational change in the gp120 subunit, allowing it now to interact with specific G protein–coupled receptors of chemokines (11–15). Primary HIV-1 infections most commonly occur at mucosal surfaces of the human body, where immature DCs reside (16–22).
C-type lectins found on the surface of DCs have been implicated in binding viruses and facilitating their uptake on mucosal surfaces (23–26). DC-SIGN, a major C-type lectin found on most but not all DCs, has been characterized as a gp120 binding protein of higher affinity than CD4 (24). DC-SIGN facilitates rapid internalization of intact HIV-1 in both immature and mature DCs that contributes to enhanced infection in trans of target cells during formation of an infectious synapse (24, 27, 28). Both the dileucine and tyrosine-based motifs in the cytoplasmic domain of the DC-SIGN molecule are critical for the internalization of HIV and other viruses (27, 29). Incoming HIV-1 particles in DCs are internalized by various DC-SIGN–dependent and –independent pathways. A fraction of HIV-1 internalized in DCs is degraded immediately in the lysosomes. Some of the virus that escapes degradation is retained in endocytic compartments within the cytoplasm and is either transmitted by recycling to permissive CD4+ lymphocytes or degraded by the proteasome (30, 31). The process by which DC-SIGN internalizes and transfers HIV-1 is thought to be mediated through classical endocytic and recycling pathways (32); however, other cellular proteins involved in this process are unknown. In this work, we describe an actin binding molecule, leukocyte-specific protein 1 (LSP1), which interacts with the cytoplasmic domain of DC-SIGN and affects the transport of HIV-1 through the DC.
RESULTS
The cytoplasmic domain of DC-SIGN required for HIV-1 internalization interacts with LSP1
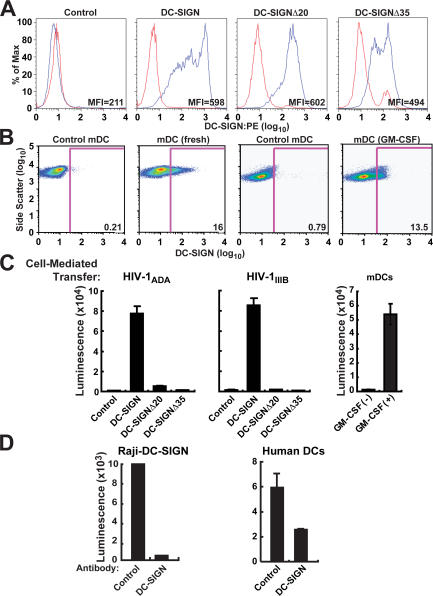
DC-SIGN, a C-type lectin on immature DCs, mediates rapid internalization of intact HIV and contributes to enhanced infection in trans of target cells that express CD4 and chemokine receptors (24, 27). Raji B cells were first used to analyze the effects of full-length DC-SIGN and DC-SIGN with cytoplasmic domain truncations, DC-SIGNΔ35, lacking both dileucine and tyrosine-based motifs, and DC-SIGNΔ20, missing the dileucine motif. These DC-SIGN forms showed comparable cell surface expression, although the DC-SIGNΔ35 mutant showed slightly lower expression (Fig. 1 A). Also, human myeloid DCs (mDCs) cultured in GM-CSF or freshly isolated mDCs showed comparable surface expression of DC-SIGN compared with control stained cells (Fig. 1 B); however, less HIV-1 transfer to T cells occurs in those cells not cultured in GM-CSF (Fig. 1 C, right). Freshly isolated mDCs had previously been reported to express low levels of DC-SIGN that increase significantly after incubation with IL-4 (33). Compared with full-length DC-SIGN, when Raji B cells expressing DC-SIGN mutants were incubated with HIV-1ADA (CCR5 tropic) or HIV-1IIIB (CXCR4 tropic) at 37°C for 2 h, washed, and incubated with A3R5 or MT2 cells, they failed to mediate enhancement of T cell infection (Fig. 1 C). We and others have previously shown that Raji cells cannot be infected by HIV-1, and so all infection in Fig. 1 C is mediated by trans-infection rather than cis-infection (22, 27). The DC-SIGN mutant data further support this conclusion, and direct capture of virus versus internalization in the Raji DC-SIGN and deletion mutants has been shown previously (27). In addition, CXCR4-tropic virus cannot infect mature or immature DCs (22). 20 μg/ml mAbs to DC-SIGN (BD Biosciences) completely inhibited HIV-1 transfer by Raji B cells to MT2 T leukemia cells and partially inhibited transfer mediated by human DCs when incubated for 2 h at 37°C before and during transfer of HIV-1ADA (Fig. 1 D). Collectively, these data suggest a role for DC-SIGN in mediating uptake and transfer of the virus by human DCs.
Figure 1.
DC-SIGN is necessary for HIV-1 uptake and transfer to T cells. (A) DC-SIGN expression on Raji B cells. Raji B cells expressing full-length DC-SIGN, DC-SIGNΔ35, and DC-SIGNΔ20 were incubated with human mAb DC-SIGN/PE for 30 min at 4°C, washed once with PBS, and analyzed by flow cytometry (MFI, mean fluorescence intensity). (B) DC-SIGN expression on human mDCs. Immature human mDCs were incubated with 2 μg of control or human mAb DC-SIGN (eBioscience) for 30 min at 4°C, washed once with PBS, and incubated with donkey anti–mouse Alexa 647–conjugated IgG for 30 min at 4°C, washed once with PBS, and analyzed by flow cytometry. (C) Uptake and transfer of CCR5- and CXCR4-tropic HIV-1 by DC-SIGN. Raji B cells expressing full-length DC-SIGN, DC-SIGNΔ35, and DC-SIGNΔ20 were pulsed with 750 ng/ml HIV-1ADA (CCR5-tropic) or HIV-1IIIB (CXCR4) at 37°C for 2 h, trypsinized, washed, and incubated with A3R5 or MT2 cells, respectively. Also, human mDCs were pulsed with ∼780 ng/ml HIV-1ADA at 37°C for 2 h, trypsinized, washed, and incubated with A3R5. All cells were lysed 72 h after incubation and analyzed for luciferase activity. (D) mAbs to DC-SIGN inhibit uptake and transfer of HIV-1 into T leukemia cells by Raji B cells or human DCs. Raji B cells (left) expressing full-length DC-SIGN and human DCs (right) were incubated with mAb to DC-SIGN for 30 min at 4°C before incubating with 750 ng/ml HIV-1ADA for 2 h at 37°C. Cells were then washed and incubated with MT2 T leukemia cells. Cells were lysed 72 h after incubation and analyzed for luciferase activity.
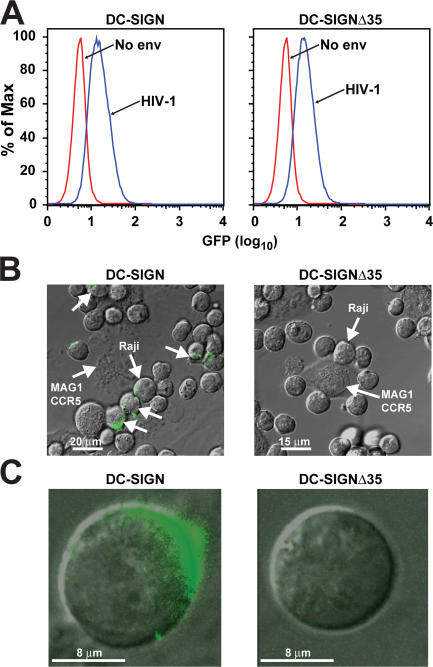
To determine whether the cytoplasmic domain of DC-SIGN was required for internalization, HIV-1–GFP-labeled virions were incubated with Raji B cell lines expressing WT DC-SIGN or DC-SIGNΔ35. HIV-1–GFP-labeled virions were incubated at 4°C for 30 min to quantify cell surface binding. To assess internalization, cells were incubated with ∼780 ng/ml of HIV-1–GFP-labeled virions at 37°C for 2 h and treated with trypsin for 5 min before being added to HeLa cells expressing CD4/CCR5 (MAGI-CCR5). Raji cells expressing DC-SIGNΔ35 showed comparable cell surface expression and were able to bind GFP-labeled HIV-1 virions (Fig. 2 A). It has been shown previously (27) that HIV-1 pseudotypes bind to DC-SIGN and the other DC-SIGN mutants. However, in contrast to cells expressing WT DC-SIGN, cells with DC-SIGNΔ35 did not internalize HIV-1 (Fig. 1, B and C, left vs. right panel). Thus, internalization appears to be critical for mediating trans-enhancement of HIV infection.
Figure 2.
The cytoplasmic domain of DC-SIGN is necessary for HIV-1 internalization. (A) Raji B cells expressing full-length DC-SIGN and cytoplasmic 35 amino acid–deleted DC-SIGNΔ35 bind HIV-1. Approximately 780 ng/ml HIV-1 GFP-labeled (blue peak) and a No-Env negative control (red peak) virions were incubated with Raji cells expressing full-length and the cytoplasmic mutant DC-SIGNΔ35 for 30 min at 4°C. Cells were then analyzed by flow cytometry for cell surface–bound virus. (B and C) Raji cells expressing full-length DC-SIGN internalize HIV-1. Raji cells were incubated with HIV-1-GFP virions at 37°C for 2 h, trypsinized, washed, and added to HeLa cells expressing CD4/CCR5 (MAGI-CCR5) to visualize not only internalization, but polarization. Unlabeled arrows indicate polarization of virus between cells. Cells were viewed using confocal microscopy (B, low magnification; C, high magnification) and analyzed using Leica software.
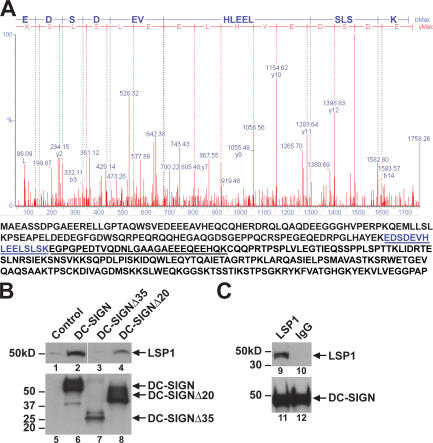
Because internalization and trans-enhancement of HIV infection are linked and mediated by the cytoplasmic region of DC-SIGN, we sought to identify cellular proteins that interacted with this region. DC-SIGN, DC-SIGNΔ35, and DC-SIGNΔ20 were immunoprecipitated from whole cell extracts of their respective Raji lines with a DC-SIGN mAb that reacted with the extracellular portion of the molecule. The proteins in the immunoprecipitates were separated by two-dimensional gel electrophoresis and visualized by Coomassie blue staining. Two cellular proteins coprecipitated with WT DC-SIGN but were absent in DC-SIGNΔ35 and DC-SIGNΔ20 control samples. These proteins, ∼45 and 50 kD, were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry as LSP1 (Fig. 3 A) and actin (not depicted). To confirm the mass spectrometry data, Raji cells expressing DC-SIGN or the nonfunctional deletion mutants DC-SIGNΔ35 and DC-SIGNΔ20 were lysed and total protein was immunoprecipitated with a monoclonal anti–DC-SIGN antibody conjugated to agarose beads. Immunoblot of these precipitates showed that endogenous LSP1 interacted with the full-length DC-SIGN and partially with the DC-SIGNΔ20 mutation; however, DC-SIGNΔ35 failed to interact with LSP1 (Fig. 3 B, lanes 1–4). The DC-SIGN immunoprecipitation experiments (Fig. 3 B) were performed with one mAb against the extracellular domain for immunoprecipitation, whereas a rabbit polyclonal antibody was used for Western blotting, and its reactivity with the mutant versus WT may differ and offer a possible explanation for the differences in expression between DC-SIGN and DC-SIGNΔ35. The interaction of DC-SIGNΔ35 with LSP1 was also not seen when more lysate was used and normalized to levels of DC-SIGN (not depicted). To determine if this interaction occurred in human DCs, monocyte-derived DCs (MDDCs) were isolated from healthy donors and cultured in RPMI, 50 ng/ml hGM-CSF, and 100 ng/ml IL-4 for 7 d before being lysed, and total protein was immunoprecipitated in the presence of protease inhibitors with a monoclonal anti–DC-SIGN antibody conjugated to agarose beads. Immunoblotting, as seen with the Raji cell line, confirmed the interaction between DC-SIGN and LSP1 (Fig. 3 C). MDDCs express higher levels of DC-SIGN when compared with human mDCs and can be isolated in greater cell numbers.
Figure 3.
LSP1 associates with the cytoplasmic domain of DC-SIGN. (A) Identification of proteins interacting with the cytoplasmic domain of DC-SIGN. Raji cells, expressing DC-SIGN, DC-SIGNΔ35, and DC-SIGNΔ20 were lysed in cell lysis buffer and 2 mg of total protein was incubated overnight with 5 μg of monoclonal anti–DC-SIGN antibody and protein G conjugated to agarose beads at 4°C. The immunoprecipitated material was washed and suspended in 2-D gel electrophoresis loading buffer. After 2-D gel electrophoresis, gels were stained with Coomassie blue. Coomassie-stained protein spots that were unique for the full-length DC-SIGN were picked, digested with trypsin, and analyzed by MALDI-TOF. A representation of the mass spectrometry data for the 15–amino acid LSP1 peptide is shown (top). Protein sequence of LSP1 is shown, and the peptides identified by matrix-assisted laser desorption/ionization time-of-flight are underlined (bottom). (B) LSP1 interacts with full-length DC-SIGN but not the cytoplasmic tail–deleted mutant. The parental cell line Raji-1 and Raji B cells expressing DC-SIGN and nonfunctional mutants DC-SIGNΔ35 and DC-SIGNΔ20 were lysed, and total protein was immunoprecipitated with monoclonal anti–DC-SIGN antibody and protein G conjugated to agarose beads overnight at 4°C. Immunoprecipitates were assayed for LSP1 (lanes 1–4) and DC-SIGN (lanes 5–8) expression using polyclonal antibodies by immunoblot. The control represents cell lysate from the parental cell line Raji-1. (C) LSP1 interacts with DC-SIGN in human DCs. Human MDDCs were isolated from healthy donors and cultured in RPMI, 50 ng/ml hGM-CSF, and 100 ng/ml IL-4 for 7 d before being lysed, and total protein was immunoprecipitated in the presence of protease inhibitors with a monoclonal anti–DC-SIGN antibody conjugated to agarose beads. Immunoprecipitates were assayed for LSP1 (lanes 9 and 10) and DC-SIGN (lanes 11 and 12) expression using polyclonal antibodies by immunoblot.
DC-SIGN binds to a distinct domain of LSP1 between amino acids 276 and 304
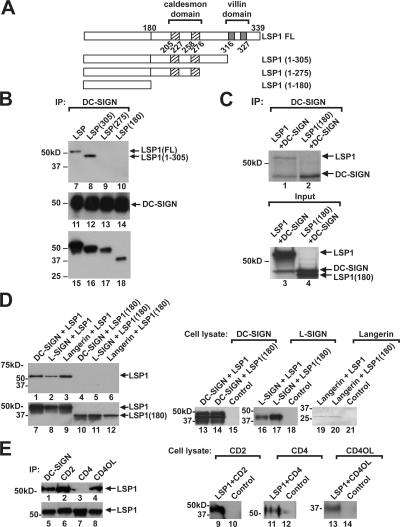
LSP1 is an F-actin binding protein involved in cell motility, cell adhesion, and IgM internalization (34–37). The caldesmon-like (CI and CII) and villin-like (VI and VII) are two domains on LSP1 that bind F-actin to facilitate neutrophil motility (38). To map the domain of LSP1 that interacted with DC-SIGN, stop codons (TAG) were introduced at amino acids 180, 275, and 305, generating several LSP1 mutants (Fig. 4 A). LSP1(1–305) lacked the villin-like domains (VI and VII) and LSP1(1–275) contained only the caldesmon-like (CI and CII) domains, whereas LSP1(1–180) lacked both the caldesmon-like (CI and CII) and villin-like (VI and VII) domains. LSP1 and the mutants were transfected with DC-SIGN in 293T cells. 72 h after transfection, cells were harvested, and cell lysates were immunoprecipitated with anti–DC-SIGN antibody. Immunoblots showed that DC-SIGN interacted with WT LSP1 and LSP1(1–305) that lacked villin-like domains but failed to interact with LSP1(1–275) that contained only the caldesmon-like domains and LSP1(1–180) that lacked both the caldesmon-like and villin-like domains. These data show that amino acids 276–304, which are independent of the F-actin binding regions, are essential for interaction between DC-SIGN and LSP1 (Fig. 4 B) and suggest that LSP1 interaction with the cellular cytoskeleton is required for its function. To determine if this interaction was direct or the product of multiple proteins, 35S-labeled in vitro cotranslated full-length LSP1 or LSP1(1–180) and DC-SIGN proteins were immunoprecipitated in the presence of protease inhibitors with a monoclonal DC-SIGN antibody conjugated to agarose beads and electrophoresed on a 10% polyacrylamide gel. The proteins were visualized by autoradiography, showing a direct interaction between the full-length LSP1, but not the mutant (Fig. 4 C).
Figure 4.
DC-SIGN associates with LSP1 through a region distinct from its actin binding domains. (A) The caldesmon-like CI and CII (hatched) and villin-like VI and VII (gray) on LSP1 that bind F-actin are indicated. The early truncation mutants generated by site-directed mutagenesis at amino acids 305, 275, and 180 are shown. (B) 293T cells (106 cells/well) in a six-well plate were cotransfected with full-length DC-SIGN and full-length LSP1 or the mutants (1–305, 275, and 180) as indicated. Cell lysates prepared 72 h after transfection were immunoprecipitated with anti–DC-SIGN antibody and assayed for LSP1 (lanes 7–10) and DC-SIGN (lanes 11–14) by immunoblot. Cell lysates of transfected cells were analyzed by immunoblot for LSP1 expression in full-length and mutant 1–305, 1–275, and 1–180 constructs (lanes 15–18). (C) 35S-labeled in vitro–cotranslated full-length LSP1 interacts directly with DC-SIGN. Full-length LSP1 or LSP1(1–180) cDNA were in vitro cotranslated with DC-SIGN in rabbit reticulocytes in the presence of 35S. Labeled proteins were immunoprecipitated in the presence of protease inhibitors with monoclonal anti–DC-SIGN conjugated to agarose beads, electrophoresed on a 10% polyacrylamide gel, and visualized by autoradiography. LSP1 and DC-SIGN (lanes 1 and 3), DC-SIGN (lanes 2 and 4), and LSP1(1–180) (lane 4) are shown. (D) 293T cells (106 cells/well) in a six-well plate were cotransfected with full-length LSP1 or LSP1(1–180) along with DC-SIGN, L-SIGN, and Langerin. Cell lysates prepared 72 h after transfection were immunoprecipitated with anti–DC-SIGN, L-SIGN, or Langerin or mAbs, and immunoblotted with polyclonal antibody to LSP1 (lanes 1–6). Expression of full-length LSP1 and LSP1(1–180) in the cell lysate (lanes 7–12) is shown. DC-SIGN (lanes 13 and 14), L-SIGN (lanes 16 and 17), and Langerin (lanes 19 and 20) expression in the cell lysates was assayed by immunoblot. The control is lysate from 293T cells transfected with a GFP plasmid. (E) LSP1 interacts with other extracellular surface molecules. 293T cells (106 cells/well) in a six-well plate were cotransfected with full-length LSP1 along with DC-SIGN, CD2, CD4, and CD40L. Cell lysates prepared 72 h after transfection were immunoprecipitated with anti–DC-SIGN, CD2, CD4, and CD40L mAbs and immunoblotted with polyclonal antibody to LSP1 (lanes 1–4), or expression of full-length LSP1 in the cell lysate was determined (lanes 5–8). CD2 (lanes 9 and 10), CD4 (lanes 11 and 12), and CD40L (lanes 13 and 14) expression in the cell lysates were assayed by immunoblotting. The control is lysate from 293T cells transfected with a GFP plasmid.
To examine the interactions of LSP1 with other C-type lectins, LSP1 and LSP1(1–180) were cotransfected with DC-SIGN, L-SIGN, and Langerin in 293T cells. Cell lysates were immunoprecipitated with the respective C-type lectin antibodies. Immunoblots revealed that all C-type lectins interacted with LSP1 (Fig. 4 D, lanes 1–3) and not LSP1(1–180) (Fig. 4 D, lanes 4–6), suggesting a role for LSP1 in other C-type lectin-dependent processes. To address the binding specificity of C-type lectins' cytoplasmic domain with LSP1, full-length LSP1 was also incubated with irrelevant molecules (CD2, CD4, and CD40L) and showed interaction with CD2 and CD40L, but not the CD4 molecule (Fig. 4 E).
LSP1 down-modulation by specific small interfering RNAs (siRNAs) in human DCs facilitates HIV-1 transfer to T cells
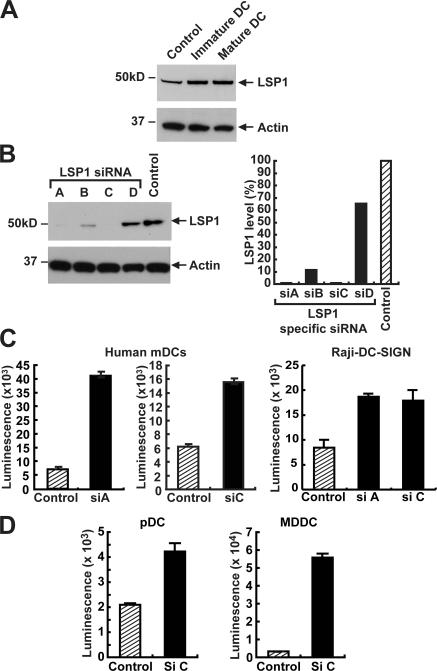
To determine whether LSP1 was detectable in immature and mature mDCs, mDCs from healthy individuals were examined before and after maturation with poly:IC. Both immature and mature mDCs expressed LSP1, and the levels did not change with maturation (Fig. 5 A), even though HIV-1 transfers more efficiently in mature mDCs (22). The physiological consequences of LSP1–DC-SIGN interactions were further studied by LSP1-specific siRNAs. Four LSP1-specific siRNAs (Fig. 5 B) were synthesized, and their effectiveness in down-regulating LSP1 was determined initially in Raji cells. Two siRNAs effectively decreased endogenous LSP1 in Raji B cells (Fig. 5 B, siRNAs in A and C), and this knockdown was most efficient at 24 h, with LSP1 levels returning to normal in 48 h. Down-regulation of LSP1 did not change expression of DC-SIGN, CD80, or MHC class II; however, it enhanced the transfer of HIV-1 to T cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061604/DC1).
Figure 5.
LSP1 down-regulation causes increased HIV-1 transfer in human DCs. (A) LSP1 expression in human DCs. Cell lysates prepared from immature or poly:IC-matured mDCs isolated from human elutriated monocytes were assayed for LSP1 expression by a polyclonal anti-LSP1 antibody. Expression was measured against control cell lysates from 293T transfected with the LSP1 gene. (B) Specific siRNAs for human LSP1 cause a down-regulation of LSP1 expression. Raji–DC-SIGN cells were transfected with the indicated LSP1, and control siRNA duplexes were introduced by nucleofection. 24 h after transfection, cell lysates (20 μg of total protein) were assayed for LSP1 expression by immunoblot (left). The film images were digitized using an Epson scanner and quantified using Bio-Rad Quantity One software. The numbers obtained for LSP1 were corrected using the numbers obtained from actin and plotted relative to control in a Microsoft Excel graph as indicated (right). (C and D) Down-regulation of human LSP1 in human DCs and Raji DC-SIGN cell line causes an increase in HIV-1 transfer to T cells. Mature human DCs were transiently transfected by GeneSilencer with siRNA for LSP1 (siA and siC) or scrambled siRNA (control). To label transfected cells, unrelated Cy5 siRNA was mixed with both control siRNA and LSP1 siRNAs at a ratio of 4:1. 8 h after the siRNA transfection, the cells were sorted for the Cy5 label and plated onto 96-well plates. 24 h after transfection, cells were pulsed with luciferase expressing 780 ng/ml HIV-1ADA for 2 h at 37°C, washed five times, and incubated with A3R5 T cells. Cells were lysed 72 h after transfection and analyzed for luciferase activity. These experiments were performed in three independent HIV-1− donors in triplicate samples, and two such experiments are represented in the figure.
To determine the effect of HIV-1 internalization and transfer to T cells, human mDCs (incubated with poly:IC), MDDCs, and plasmacytoid DCs (pDCs) isolated from HIV-1− donors were transfected with the GeneSilencer reagent containing siRNAs for LSP1 (siC or siA) or a negative control (siRNA scramble). In transfected cells, siRNAs were mixed with an unrelated fluorescent Cy5-labeled scrambled siRNA at a ratio of 4:1 and identified by flow cytometry after 8 h. Cells were sorted for the Cy5 label, plated onto a 96-well tissue culture plate, and pulsed 24 h after transfection with a single round replication-competent virus expressing a luciferase reporter (HIV-1ADA) for 2 h at 37°C, washed, and incubated with A3R5 (CCR5-tropic) T leukemia cells. Cells were assayed for luciferase activity 72 h after transduction. The results demonstrate that LSP1 siRNA-treated Raji cells and various populations of human DCs facilitated greater transfer of HIV-1 to T cells compared with control (Fig. 5, C and D). These data suggest that LSP1 interacts with DC-SIGN and/or other C-type lectins to direct the virus away from pathways mediating trans-infection.
Increased HIV-1 transfer to human T cells by lsp1−/− DCs
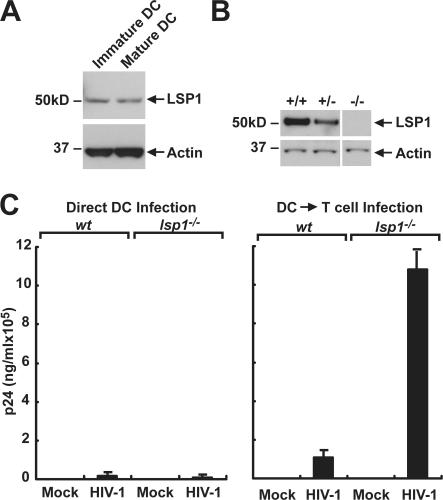
To investigate the role of LSP1 in HIV-1 trafficking through DCs, we first determined whether DCs from LSP1 knockout mice conferred the same enhancement of infection to T cells observed in human DCs. To test this hypothesis, bone marrow–derived DCs (BMDCs) were isolated from lsp1+/+ and lsp1−/− mice (34). DCs were incubated with CpG oligonucleotide for 24–48 h to induce maturation. Total protein from immature and mature BMDCs was assayed for LSP1 expression. As with human DCs, expression levels were not affected by maturation (Fig. 6 A), and lsp1−/− DCs were null for LSP1 (Fig. 6 B). To determine the effect of LSP1 in murine DCs, mature BMDCs from lsp1+/+ and lsp1−/− mice were incubated with live HIV-1BaL (∼780 ng/ml) for 2 h at 37°C, washed extensively, and incubated alone or with A3R5 T cells. p24 levels were assayed in supernatants 72 h after infection. Similar to knockdown in human DCs, murine DCs that lacked LSP1 increased transfer of virus to T cells (Fig. 6 C). Because the murine DCs mimic the effect seen in human DCs, they were used to further understand the mechanisms of LSP1-mediated HIV-1 internalization and the infection of T cells.
Figure 6.
Murine DCs from lsp1−/− mice increase HIV-1 uptake and transfer to T cells. (A) LSP1 expression in murine DCs remains constant after maturation. Cell lysates prepared from immature or CpG-matured bone marrow–derived murine DCs were assayed for LSP1 expression by a polyclonal anti-LSP1 antibody. (B) LSP1 expression in mature BMDCs isolated from lsp1−/−, lsp1+/−, and lsp1+/+ littermates. Cell lysates prepared from CpG-matured bone marrow–derived murine DCs were assayed for LSP1 expression by a polyclonal anti-LSP1 antibody. (C) LSP1-null BMDCs enhance HIV-1 trans-infection of T cells. Mature BMDCs (105/well) from WT (wt) or lsp1−/− mice were pulsed with HIV-1BaL (∼780 ng/ml of p24/ml) or RPMI alone for 2 h at 37°C. Cells were washed extensively to remove free virus and replaced with fresh media, and A3R5 T cells were added to one set of mock or HIV-1–infected BMDCs followed by incubation for another 72 h. At the appropriate time, cell supernatants were collected, and p24 ELISA was performed as instructed by the manufacturer (Coulter). The data are representative of duplicate experiments.
DC LSP1 traffics HIV-1 to the proteasome for degradation
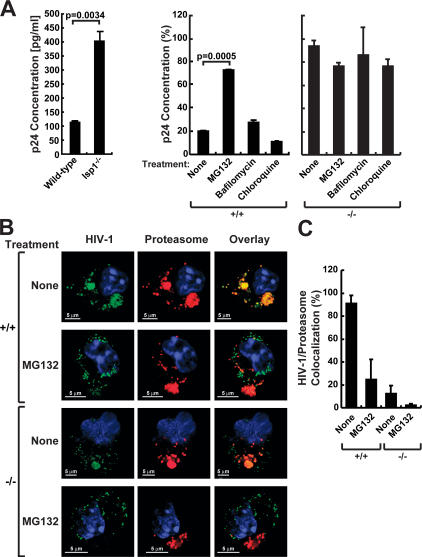
Incoming HIV-1 particles in DCs are bound and internalized by various DC-SIGN–dependent and –independent pathways. Immediately after internalization, most of the virions are degraded in an acidic lysosomal compartment. A fraction of the virus that escapes degradation is retained in endocytic compartments and is either transmitted to permissive CD4+ lymphocytes or degraded by the proteasome (30, 31). To investigate the role of LSP1 in DC processing and transport of HIV-1, HIV-1–GFP was incubated with DCs in the presence or absence of proteasome or lysosome inhibitors. Because of the inability to confirm the complete silencing of LSP1 and the limited window of knockdown in human mDCs, the LSP1 knockout mice provide the best model system to study the degradative pathway in the total absence of LSP1. Differentiated BMDCs were incubated with the lysosomal inhibitors chloroquine (100 μM), the endosome acidification inhibitor bafilomycin A1 (10 μg/ml), or the proteasomal inhibitor MG132 in RPMI (5 μg/ml) or RPMI alone for 1 h before transduction with concentrated HIV-1–GFP for 2 h. Cells were washed extensively and incubated with the respective inhibitors. Before lysis, cells were treated with trypsin and washed to remove virus bound to the cell surface. Total protein isolated at various time points after the 2-h transduction (0 h, 1 h, and 3 h) was assayed by ELISA for p24 Gag. Results at 1 h showed that more retention of HIV-1 occurred in lsp1−/− BMDCs when compared with lsp1+/+ controls (Fig. 7 A, left). The p24 values at other times showed a similar trend as the 1-h time point, but the effect was maximal at 1 h. In lsp1+/+ control cells treated with the proteasome inhibitor MG132, HIV-1 retention was higher compared with chloroquine-, bafilomycin-, or vehicle-treated lsp1+/+ cells (Fig. 7, middle) and were similar to lsp1−/− BMDCs when treated with the same inhibitors (Fig. 7, right), suggesting that LSP1 may be involved in the shuttling of virus to the proteasome for degradation.
Figure 7.
LSP1 traffics HIV-1 to the proteasome. (A) HIV-1 degradation is decreased in lsp1−/− DCs, and proteasome inhibitors block HIV-1 degradation in the presence of LSP1. Mature BMDCs from lsp1−/− and wt mice were pulsed with HIV-1–GFP for 2 h at 37°C, washed with PBS, trypsinized, and lysed with p24 lysis buffer reagent (left). Mature BMDCs from wt (lsp1+/+) were pretreated for 1 h with MG132 (proteasome inhibitor), chloroquine, or bafilomycin A-1 (lysosomal inhibitors) in RPMI or RPMI alone in a 96-well tissue culture dish (5 × 104 cells). Cells were then pulsed with HIV-1–GFP for 2 h at 37°C in the presence of inhibitors, washed with PBS, replaced with media containing the respective inhibitors, and incubated for an additional 1 h. Cells were then trypsinized, washed, and lysed with p24 lysis buffer reagent (right). p24 Gag concentrations were assayed by ELISA. These data are representative of the 1-h time point from multiple experiments performed in triplicate. The percentage values were calculated by setting the amount of p24 measured immediately after removing the virus from the cells, the 0-h time point, to 100%. The differences in p24 after several hours were then measured and compared with the 0-h time point. (B and C) HIV-1 shows greater colocalization with the proteasome in DCs expressing LSP1. (B) Mature BMDCs from lsp1−/− (−/−) and wt (+/+) mice were pulsed with HIV-1–GFP (green) for 30 min at 37°C. For proteasome inhibitor studies, BMDCs from lsp1−/− and wt mice were incubated with the proteasomal inhibitor MG132 in 5 μg/ml RPMI or RPMI alone for 1 h before infection by HIV-1–GFP (green) for 30 min. Cells were washed, fixed, and stained with a mAb to the 20S proteasome subunit a-4 (red) and viewed using confocal microscopy. HIV-1 that colocalizes with proteasomes appears yellow (overlay). (C) HIV-1 colocalization with proteasomes is representative of a percentage of HIV-1 that colocalizes with a percentage of proteasomes above a measurable threshold. Percentage colocalization was calculated using the Leica confocal software. Three images/sample were used, and error bars were assigned accordingly.
To determine the colocalization of HIV-1 to the proteasome in DCs, mature BMDCs from lsp1−/− and wt mice were pulsed with HIV-1–GFP for 30 min at 37°C. For proteasome inhibitor studies, BMDCs from lsp1−/− and wt mice were incubated with the proteasomal inhibitor MG132 in RPMI (5 μg/ml) or RPMI alone for 1 h before infection by concentrated HIV-1–GFP for 30 min. Cells were washed extensively and stained with a mAb to the 20S proteasome subunit a-4. BMDCs from lsp1−/− mice showed more HIV-1 retention and less colocalization to the proteasome (Fig. 7 B). In contrast, those cells from wt mice showed greater colocalization of HIV-1 to the proteasome (Fig. 7 B, yellow). Once DCs were treated with the proteasome inhibitor MG132, colocalization was markedly diminished in both lsp1−/− and wt mice (Fig. 7 C). Collectively, these results are consistent with the model that LSP1 shuttles the internalized pool of HIV-1 to the proteasome and that lack of LSP1 facilitates HIV-1 transfer.
DISCUSSION
In this study, we have demonstrated that DC-SIGN, a C-type lectin on DCs that mediates HIV-1 uptake and transfer to T cells, interacts with LSP1, an actin-binding cytoskeletal protein. HIV-1 uptake is dependent on the cytoplasmic domain of the DC-SIGN molecule (Fig. 1). By using immunoprecipitation for biochemical purification and identification by mass spectrometry, we showed that LSP1 interacts specifically with this cytoplasmic region. Our results also show that LSP1 interacts with other C-type lectins, L-SIGN and Langerin, which are present on the human DC. Because these C-type lectins have been shown to mediate HIV-1 transfer independently of DC-SIGN (39), LSP1 may be involved with these C-type lectins' interaction with HIV-1. Langerin, unlike DC-SIGN, contains a proline-rich region that is likely responsible for binding and rapid internalization of pathogens. CD2 and CD40L both contain this proline-rich region, possibly providing the region necessary for interaction with LSP1. Down-regulation of LSP1 siRNAs in human DCs or murine lsp1−/− BMDCs showed a dramatic increase in the amount of virus transferred from the DC to the susceptible T cell, as LSP1 was shown to facilitate proteasomal degradation of HIV-1.
A leukocytic protein, LSP1 (also known as WP34, pp52, and leufactin) is a 52-kD F-actin binding phosphoprotein expressed in all human leukocytes and leukocytic cell lines (40–42). The basic C-terminal domain contains amino acid sequences homologous to two known F-actin binding proteins, caldesmon and the villin headpiece (36, 37). Although LSP1 is an F-actin binding protein, it is also a very important regulator of microfilamentous cytoskeleton dynamics (34). After HIV-1 uptake in the DCs, it is internalized into a specialized viral endosome, which is distinct of early and late endosomal vesicles (43), where a fraction of virus remains undigested and polarizes to the infectious synapse between the targeted T cells (24, 27, 28). HIV-1 virus that does not polarize is subjected to lysosomal processing and MHC II antigen presentation, or it is degraded by the proteasome (30, 31). Because LSP1 interacts specifically with full-length DC-SIGN and not a truncated cytoplasmic domain mutant, this finding suggests that it is involved with trafficking HIV through the DC. LSP1 has proven important in polarizing the actin cytoskeleton and aiding in motility of the cell. Using proteasome inhibitors and confocal microscopy, we show that LSP1 helps to shuttle the HIV-1 virus into the proteasome, promoting its degradation, a process independent of its interaction with DC-SIGN. In the absence of LSP1, HIV-1 degradation decreases and more virus is able to recycle to the surface, promoting transfer to T cells. We do not know why the proteasomes in murine DCs were not susceptible to bafilomycin as they are in human DCs. Because they are isolated differently from the human cells and are grown in cytokines, it is possibly a difference in the patterns of gene expression in these cells, although we cannot exclude a species effect. Proteasomal inhibitors can affect ubiquitin levels in the cells, which could explain the decrease in HIV-1 degradation; however, in the absence of LSP1, there was less colocalization of HIV-1 to the proteasome and no significant difference in HIV-1 degradation in the lsp1−/− BMDCs compared with wt cells treated with proteasomal inhibitors, suggesting that this effect was independent of ubiquitin effects. This experiment confirmed the role of LSP1 in this degradative process.
Our study reveals new insights into HIV-1 trafficking through DCs leading to the enhancement of T cell infection by DC-SIGN–internalized virus. The role of DC-SIGN in trans-infection is not completely understood, in part because other C-type lectins may be involved in the process in some DC populations, and blocking of DC-SIGN with mAbs does not always completely inhibit HIV-1 transfer. Although one previous study contradicted the report of Kwon et al. (27) that point mutants in the tyrosine and dileucine motifs of the cytoplasmic domain of DC-SIGN do not affect gp120 binding, it is important to recognize that the latter study analyzed internalization with gp120 protein rather than virus (44), and the significance of this assay for virus internalization and transfer is questionable. Nonetheless, consistent with the present work, a mutant with a deletion of the cytoplasmic domain in that study showed the same loss of function as seen here examined by transmission of the lentiviral vector.
The discovery that LSP1, an actin-binding molecule, interacts with DC-SIGN has implications for understanding the trans-enhancement of T cell infection by DCs, possibly leading to ways of blocking transfer. Sequestering actin and the cytoskeleton may lead to decreased transfer of HIV-1, but not without possible serious effects on DC viability. Antigen internalized by DCs has been shown to lead to classical MHC II processing, peptide loading, and surface presentation (45). We find that increased transfer of HIV-1 to T cells in the absence of LSP1 is due to decreased HIV-1 degradation in the proteasome; however, insights into the effect of LSP1 on peptide processing and antigen presentation have yet to be investigated. Collectively, these data suggest a role for LSP1 trafficking of HIV-1 to the proteasome for viral degradation. Continued elucidation of HIV-1 trafficking in DCs provides us with a greater understanding of how C-type lectins, such as DC-SIGN, mediate viral uptake and transfer to susceptible target cells.
MATERIALS AND METHODS
Plasmids and siRNA.
The DC-SIGN CITE-GFP and DC SIGNΔ35 CITE-GFP were expressed under a CMV promoter/enhancer. The cDNA-encoding LSP1 (accession no. BI911034) was expressed in pCMV-SPORT6 (Invitrogen). Mutagenesis of LSP1 was performed using a Stratagene Quick Change Site-Directed Mutagenesis kit according to the manufacturer's directions. To introduce a stop codon to generate LSP1 (1–305, 275, and 180) mutants, the following primers were used: LSP1(1–305): 5′ primer: GGGAGGCTCCAAGACCTCATAATCTAGATCAACAATTAAGAGCACCCC; LSP1(1–275): 5′ primer: GGTGAGGTACAGGCTCAGTCTTAATCTAGAGCGGCCAAGACTCCGTCC; and LSP1(1–180): 5′ primer: CCCAGCCCCTTGGTCTTGTAATCTAGAGAGGGGACCATCGAACAGAGC. Only the sense strands of the mutagenesis primers are shown. The PCR products were digested with DpnI at 37°C for 1 h per the manufacturer's protocol and transformed into TOP10 (Invitrogen) cells. A pool of siRNAs specific for human LSP1 was designed based on the cDNA gene sequence. LSP1 siRNA duplexes siRNA LSP1-a (5′-CAGGAGGAGCACCAGAAAU-3′), siRNA LSP1-b (5′-GUCCACCUGGAGGAGUUGA-3′), LSP1-c (5′-UGGAGACAUGAGCAAGAAAUU-3′), LSP1-d (5′-CCUGAGCCCUACCACCAAAUU-3′), and negative control (5′-UUCUCCGAACGUGUCACGUdTdT-3′) siRNAs were manufactured by QIAGEN. Cy5 siRNA was made by Dharmacon Inc.
Virus production, entry, transduction, and infection assays.
Pseudotyped HIV-1ADA lentivirus-expressing luciferase was prepared by transient cotransfection of 293T cells using calcium phosphate (Promega). In brief, the packaging vector pMD 8.2, pHR-luciferase, and the envelope expressing vector pSVIII-HIVADA or pRSV-HIVIIIB were transiently transfected into 293T cells. Supernatants were harvested 48 and 72 h after transfection, filtered, and stored at −80°C. Virus concentration was determined by an ELISA assay for the p24 antigen (Beckman Coulter) (16–22).
GFP-Vpr–labeled HIV-1 lentivirus (HIV-1–GFP) was produced by transfection of 293 T cells with the pLAI provirus and the plasmid pEGFP- C3 (CLONTECH Laboratories, Inc.) containing the entire Vpr coding region fused to the carboxyterminus of eGFP (GFP-Vpr). Cells were washed at 16–20 h after transfection and replenished with fresh media. 48 h later, supernatants were harvested, filtered through a 0.45-μm syringe filter, and concentrated. In brief, 32 ml of supernatant was layered on 5 ml of Optiprep (Iodoxinal) medium (Invitrogen) and centrifuged at 50,000 g for 1.5 h with a Surespin 630 rotor (Sorvall). The last 3 ml of supernatant remaining above the Optiprep interface was collected and frozen at −80°C in 500-μl aliquots (27, 29). Concentrated HIV-1BaL (MOI, ∼1; 7.8 μg/ml p24) was prepared in PBMCs and provided by J. Mascola and M. Louder (Vaccine Research Center, NIAID, NIH).
24 h after transfection of siRNAs, HIV-1ADA infection was performed in a 96-well flat-bottomed luciferase culture plate by removing 200 μl RPMI culture media and adding 200 μl of virus stock. After incubation for 2 h at 37°C, cells were washed twice and incubated with 1.5 × 105 A3R5 T cells for 48 h, lysed, and assayed for luciferase activity with a commercially available kit (Promega). Similarly, BMDCs from LSP1 transgenic mice were infected with WT HIV-1BaL (MOI, ∼1; μg/ml 7.8 p24) for 2 h and washed five times with RPMI. All animal experiments were reviewed and approved by the Animal Care and Use Committee, Vaccine Research Center (VRC), NIAID, and performed in accordance with all relevant federal and NIH guidelines and regulations. The DCs were incubated with A3R5 T cells for 48 h and assayed for p24.
Lsp1+/− and C57BL/6 wt mouse BMDCs were isolated and incubated in 20 ng/ml RPMI plus GM-CSF in a 96-well tissue culture dish (5 × 104). The cells were matured in 5 μg/ml of ODN CpG (1829) for 24–48 h. Cells were pretreated with 10 μg/ml bafilomycin, 5 μg/ml MG132, 100 μM chloroquine, or alone in RPMI for 1 h. Media were then removed, and DCs were pulsed with HIV-1–GFP for 2 h. Cells were washed once with PBS and lysed in 1X p24 cell lysis buffer at time points 0 h, 1 h, and 3 h and stored at −30°C. Lysates were assayed for p24 activity by ELISA (Beckman Coulter HIV-1 p24 Antigen EIA) and read at 450/570 nm dual wavelength on a SPECTRAmax Plus 384 (16–22).
Cells and transfections.
Parental control Raji-1 cells and Raji-1 cells stably transfected with human DC-SIGN (Raji DC-SIGN) or DC-SIGN with a cytoplasmic truncation that lacks both the dileucine motif and the tyrosine-based motif in the cytoplasmic tail (Raji DC-SIGNΔ35 and DC-SIGNΔ20) were provided by D. Littman (New York University School of Medicine, New York, NY) and maintained in RPMI media at 37°C and 5% CO2. A3R5 T cells were provided by J. Mascola (Vaccine Research Center, NIAID, NIH) and cultured in RPMI and geneticin (G418).
Human mDCs and pDCs were purified from elutriated monocytes from healthy adult donors by a two-step procedure consisting of automated leukapheresis and counterflow centrifugal elutriation at the Transfusion Medicine Department of the Warren Grant Magnuson Clinical Center, NIH. Because the cells only are used and are without identifiers, this work with the cells is exempt from IRB review. mDCs and pDCs were isolated from the elutriated monocyte fraction with negative selection by removing cells expressing BDCA-4 and CD19 with microbeads (Miltenyi Biotec), followed by positive selection using antibodies to CD1c (Miltenyi Biotec). mDCs and pDCs were then cultured in a medium containing 10 ng/ml GM-CSF (PeproTech) for 18–24 h before any experiments. mDCs were also induced to mature using 50 μg/ml poly:IC (Sigma-Aldrich) for 48 h. Human MDDCs were generated from monocytic cells cultured in RPMI, hGM-CSF, and IL-4 for 7 d before phenotyping cells for CD11c to ensure purity.
Lsp1 −/−, lsp1+/−, and C57BL/6 mice (The Jackson Laboratory) were killed by cervical dislocation to ensure that the animal would not revive. Femurs were then removed, excising the muscle and fat from the bone. Once the bone was exposed, the upper and lower knobs of the bone were removed, exposing the shaft. The shaft was then flushed with PBS plus 2X penicillin streptomycin by a 27-gauge needle and syringe. Cells were harvested, centrifuged, and resuspended in 1 ml RPMI plus GM-CSF (20 ng/ml). The cells were then cultured for 7 d, adding 30 ml of RMPI plus GM-CSF (20 ng/ml) every 3 d. The total cell volume was then harvested and resuspended in RPMI plus GM-CSF. BMDCs were matured using 5 μg/ml ODN CpG (1829) for 24–48 h. All animal experiments were approved by IACUC and conducted in compliance with all relevant federal and NIH policies.
Matured human DCs were transfected with a control of LSP1 siRNA (QIAGEN). For each transfection, 225 nM siRNA (180 nM unlabeled siRNA and 45 nMCy5-labeled siRNA) was used. Transfections were performed in a 4:1 mixture of unlabeled siRNA and Cy5. To tranfect the mature human DCs with siRNA, 106 cells were resuspended in 1 ml of serum-free RPMI. The siRNA mixture was combined with 9 μl of GeneSilencer (Genlantis) and added to the cell suspension according to the manufacturer's protocol. The cells were washed 8 h after transfection and sorted by a fluorescence-activated cell sorter (Becton Dickinson) to identify Cy5-labeled siRNA-transfected cells. Sorted cells were seeded in a 96-well plate at 5 × 104 cells/well.
Immunoprecipitation, immunoblot, and mass spectrometry.
Raji cells expressing DC-SIGN or a nonfunctional mutant, DC-SIGNΔ35 or DC-SIGNΔ20, were lysed in 1X cell lysis buffer (Cell Signaling). 1 or 2 mg of total protein was incubated with monoclonal anti–DC-SIGN antibody for 2 h. Protein G (Invitrogen) conjugated to agarose beads was then added and incubated overnight at 4°C. The mixture was washed three times with 1X cell lysis buffer and loaded onto a polyacrylamide 4–12% gel for 1-D or 2-D electrophoresis. Gels were stained with Coomassie blue or proteins were transferred to a PVDF membrane (Invitrogen) and assayed for LSP1 and DC-SIGN by immunoblot using polyclonal antibodies.
Protein identification of 1-D or 2-D gel-separated proteins was performed on reduced and alkylated, trypsin-digested samples prepared by standard mass spectrometry protocols. Tryptic digests were chromatographed (1 μl/min), and peptides were separated using a Zorbax C18SBW reverse phase column (0.15 mm ID × 100 mm). The mobile phase consisted of a gradient prepared from solvent A (0.2% formic acid) and solvent B (99.8% acetonitrile, 0.2% formic acid) at room temperature. For these fractionated digests, capillary LC-tandem MS (LC-MS/MS) was performed with a CapLC and a quadruple-time of flight mass spectrometer (Qtof-2; Waters Micromass). Computer-controlled data-dependent automated switching to MS/MS provided peptide sequence information. MassLynx and Global Server software were used for data acquisition and processing. Data processing and databank searching were performed with Mascot software (Matrix Science). The NCBInr protein database from The National Center for Biotechnology Information, NLM/NIH, was used for the search analysis.
35S-labeled in vitro transcription translation.
Full-length LSP1 or LSP1(1–180) cDNAs were in vitro cotranslated with DC-SIGN in rabbit reticulocytes in the presence of 35S (TNT-sport6; Promega). Labeled proteins were immunoprecipitated in the presence of protease inhibitors with monoclonal anti–DC-SIGN conjugated to agarose beads electrophoresed on a 10% polyacrylamide gel. The gel was fixed and dried using a slab gel dryer (Savant SGD 2000) at 80°C for 5 h. Labeled proteins were visualized by autoradiography after 9 h.
Confocal microscopy.
105 Raji B cells expressing full-length DC-SIGN and cytoplasmic 35 amino acid–deleted DC-SIGNΔ35 were incubated with 100 μl of a GFP-labeled HIV-1 pseudolentivirus for 2 h. Cells were treated with trypsin-EDTA, washed, and added to MAGI-CCR5 HeLa cells (104 cells/well) plated onto eight-well coverslip slides (Nunc). Sequential images of live cells were recorded every 3 min by confocal microscopy (SP2-AOBS; Leica Microsystems), and uptake, polarization, and transfer were assessed with representative cells.
105 mature BMDCs from lsp1−/− and wt mice were pulsed with 100 μl HIV-1–GFP for 30 min at 37°C. For proteasome inhibitor studies, BMDCs from lsp1−/− and wt mice were incubated with the proteasomal inhibitor MG132 in 5 μg/ml RPMI or RPMI alone for 1 h before infection by concentrated HIV-1–GFP for 30 min. Cells were washed once with PBS plus 2% FCS, fixed, permeablized (BD Biosciences), and stained with a mAb (15 μg/ml) to the 20S proteasome subunit a-4 (BioMol). Cells were viewed using confocal microscopy and analyzed using Leica software.
Online supplemental material.
Fig. S1 demonstrates that LSP1 down-regulation causes increased HIV-1 transfer in Raji B cells that does not affect normal surface receptor expression. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20061604/DC1.
Supplemental Material
Acknowledgments
We dedicate this paper to Lakshmanan Ganesh, M.D., Ph.D., co-first author, valued colleague, mentor, and friend, whose most promising career was tragically cut short by illness while this paper was in submission. We thank Ati Tislerics for assistance with manuscript preparation; Toni Garrison and Brenda Hartman for figure preparation; Mary-Ann Robinson and Raynaldo Martin for 2-D gel and mass spectrophotometry assistance; Owen Schwartz, Juraj Kabat, and Meggan Czapiga for confocal imaging assistance; and members of the Nabel lab for helpful discussions and advice. We also thank Paul Kubes for help in acquiring the transgenic mice for this study.
This research was supported by the Intramural Research Program of the U.S. NIH, Vaccine Research Center, NIAID, and the National Cancer Institute of Canada.
The authors have no conflicting financial interests.
Abbreviations used: BMDC, bone marrow–derived DC; LSP1, leukocyte-specific protein 1; mDC, myeloid DC; MDDC, monocyte-derived DC; pDC, plasmacytoid DC; siRNA, small interfering RNA.
A. Smith and L. Ganesh contributed equally to this work.
Dr. Ganesh died on 14 October 2006.
References
- 1.Steinman, R.M., and K. Inaba. 1999. Myeloid dendritic cells. J. Leukoc. Biol. 66:205–208. [DOI] [PubMed] [Google Scholar]
- 2.Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, R.M., and M.C. Nussenzweig. 2002. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 99:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 5.Chesnut, R.W., and H.M. Grey. 1985. Antigen presenting cells and mechanisms of antigen presentation. Crit. Rev. Immunol. 5:263–316. [PubMed] [Google Scholar]
- 6.Thery, C., and S. Amigorena. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45–51. [DOI] [PubMed] [Google Scholar]
- 7.Trombetta, E.S., and I. Mellman. 2005. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23:975–1028. [DOI] [PubMed] [Google Scholar]
- 8.Robinson, S.P., K. Saraya, and C.D. Reid. 1998. Developmental aspects of dendritic cells in vitro and in vivo. Leuk. Lymphoma. 29:477–490. [DOI] [PubMed] [Google Scholar]
- 9.Lasky, L.A., G. Nakamura, D.H. Smith, C. Fennie, C. Shimasaki, E. Patzer, P. Berman, T. Gregory, and D.J. Capon. 1987. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 50:975–985. [DOI] [PubMed] [Google Scholar]
- 10.Dalgleish, A.G., P.C. Beverley, P.R. Clapham, D.H. Crawford, M.F. Greaves, and R.A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 312:763–767. [DOI] [PubMed] [Google Scholar]
- 11.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. DiMarzio, S. Marmon, R.E. Sutton, C.M. Hill, et al. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 381:661–666. [DOI] [PubMed] [Google Scholar]
- 12.Dragic, T., V. Litwin, G.P. Allaway, S.R. Martin, Y. Huang, K.A. Nagashima, C. Cayanan, P.J. Maddon, R.A. Koup, J.P. Moore, and W.A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 381:667–673. [DOI] [PubMed] [Google Scholar]
- 13.Alkhatib, G., C. Combadiere, C.C. Broder, Y. Feng, P.E. Kennedy, P.M. Murphy, and E.A. Berger. 1996. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 272:1955–1958. [DOI] [PubMed] [Google Scholar]
- 14.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P.D. Ponath, L. Wu, C.R. Mackay, G. LaRosa, W. Newman, et al. 1996. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 85:1135–1148. [DOI] [PubMed] [Google Scholar]
- 15.Doranz, B.J., J. Rucker, Y. Yi, R.J. Smyth, M. Samson, S.C. Peiper, M. Parmentier, R.G. Collman, and R.W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 85:1149–1158. [DOI] [PubMed] [Google Scholar]
- 16.Weissman, D., Y. Li, J. Ananworanich, L.J. Zhou, J. Adelsberger, T.F. Tedder, M. Baseler, and A.S. Fauci. 1995. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 92:826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blauvelt, A., H. Asada, M.W. Saville, V. Klaus-Kovtun, D.J. Altman, R. Yarchoan, and S.I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Invest. 100:2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granelli-Piperno, A., V. Finkel, E. Delgado, and R.M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9:21–29. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R.M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehner, T., L. Hussain, J. Wilson, and M. Chapman. 1991. Mucosal transmission of HIV. Nature. 353:709. [DOI] [PubMed] [Google Scholar]
- 21.Veazey, R., and A. Lackner. 2003. The mucosal immune system and HIV-1 infection. AIDS Rev. 5:245–252. [PubMed] [Google Scholar]
- 22.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R.A. Koup, and G.J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis, B.M., S. Scharnowske, and A.J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA. 89:8356–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geijtenbeek, T.B., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597. [DOI] [PubMed] [Google Scholar]
- 25.Turville, S.G., J. Arthos, K.M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A.L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood. 98:2482–2488. [DOI] [PubMed] [Google Scholar]
- 26.Turville, S.G., P.U. Cameron, A. Handley, G. Lin, S. Pohlmann, R.W. Doms, and A.L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. [DOI] [PubMed] [Google Scholar]
- 27.Kwon, D.S., G. Gregorio, N. Bitton, W.A. Hendrickson, and D.R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 16:135–144. [DOI] [PubMed] [Google Scholar]
- 28.McDonald, D., L. Wu, S.M. Bohks, V.N. KewalRamani, D. Unutmaz, and T.J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 300:1295–1297. [DOI] [PubMed] [Google Scholar]
- 29.Yang, Z.-Y., Y. Huang, L. Ganesh, K. Leung, W.-P. Kong, O. Schwartz, K. Subbarao, and G.J. Nabel. 2004. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 78:5642–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turville, S.G., J.J. Santos, I. Frank, P.U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak Jr., et al. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 103:2170–2179. [DOI] [PubMed] [Google Scholar]
- 31.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J.P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood. 103:2648–2654. [DOI] [PubMed] [Google Scholar]
- 32.Engering, A., T.B. Geijtenbeek, S.J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C.G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118–2126. [DOI] [PubMed] [Google Scholar]
- 33.Granelli-Piperno, A., I. Shimeliovich, M. Pack, C. Trumpfheller, and R.M. Steinman. 2006. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J. Immunol. 176:991–998. [DOI] [PubMed] [Google Scholar]
- 34.Jongstra-Bilen, J., V.L. Misener, C. Wang, H. Ginzberg, A. Auerbach, A.L. Joyner, G.P. Downey, and J. Jongstra. 2000. LSP1 modulates leukocyte populations in resting and inflamed peritoneum. Blood. 96:1827–1835. [PubMed] [Google Scholar]
- 35.Wang, C., H. Hayashi, R. Harrison, B. Chiu, J.R. Chan, H.L. Ostergaard, R.D. Inman, J. Jongstra, M.I. Cybulsky, and J. Jongstra-Bilen. 2002. Modulation of Mac-1 (CD11b/CD18)-mediated adhesion by the leukocyte-specific protein 1 is key to its role in neutrophil polarization and chemotaxis. J. Immunol. 169:415–423. [DOI] [PubMed] [Google Scholar]
- 36.Jongstra-Bilen, J., P.A. Janmey, J.H. Hartwig, S. Galea, and J. Jongstra. 1992. The lymphocyte-specific protein LSP1 binds to F-actin and to the cytoskeleton through its COOH-terminal basic domain. J. Cell Biol. 118:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong, M.J., I.A. Malapitan, B.A. Sikorski, and J. Jongstra. 2003. A cell-free binding assay maps the LSP1 cytoskeletal binding site to the COOH-terminal 30 amino acids. Biochim. Biophys. Acta. 1642:17–24. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Q., Y. Li, and T.H. Howard. 2000. Human lymphocyte-specific protein 1, the protein overexpressed in neutrophil actin dysfunction with 47-kDa and 89-kDa protein abnormalities (NAD 47/89), has multiple F-actin binding domains. J. Immunol. 165:2052–2058. [DOI] [PubMed] [Google Scholar]
- 39.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865–12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein, D.P., J. Jongstra-Bilen, K. Ogryzlo, R. Chong, and J. Jongstra. 1989. Lymphocyte-specific Ca2+-binding protein LSP1 is associated with the cytoplasmic face of the plasma membrane. Mol. Cell. Biol. 9:3043–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jongstra, J., M.E. Ittel, N.N. Iscove, and G. Brady. 1994. The LSP1 gene is expressed in cultured normal and transformed mouse macrophages. Mol. Immunol. 31:1125–1131. [DOI] [PubMed] [Google Scholar]
- 42.Pulford, K., M. Jones, A.H. Banham, E. Haralambieva, and D.Y. Mason. 1999. Lymphocyte-specific protein 1: a specific marker of human leucocytes. Immunology. 96:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J.F. Arrighi, G. Blot, F. Leuba, J.M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 6:488–501. [DOI] [PubMed] [Google Scholar]
- 44.Burleigh, L., P.Y. Lozach, C. Schiffer, I. Staropoli, V. Pezo, F. Porrot, B. Canque, J.L. Virelizier, F. Arenzana-Seisdedos, and A. Amara. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J. Virol. 80:2949–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turley, S.J., K. Inaba, W.S. Garrett, M. Ebersold, J. Unternaehrer, R.M. Steinman, and I. Mellman. 2000. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 288:522–527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.