Abstract
Interleukin (IL)-10 is a cytokine that modulates both innate and adaptive immunity, primarily by exerting antiinflammatory effects. IL-10 was originally thought to be produced only by T helper (Th)2 cells, but is now known to be made by a variety of cell types. During many infections, CD4+ T cells produce both interferon (IFN)-γ, the signature Th1 cytokine, and IL-10. New data now show that the IL-10 produced by effector Th1 cells helps limit the collateral damage caused by exaggerated inflammation. But this control may also limit the effectiveness of the immune response, resulting in a failure to fully eliminate pathogens.
IL-10 suppresses many functions of natural killer (NK) cells and T cells, primarily by preventing antigen-presenting cells (APCs) from producing proinflammatory cytokines, such as IL-12, and from up-regulating molecules involved in antigen presentation and lymphocyte activation (1). IL-10 prevents the up-regulation of many genes in phagocytic cells and dendritic cells (DCs) that are normally induced by stimulation via Toll-like receptors (TLRs) or other pattern recognition receptors. Although most of IL-10's effects are suppressive, this cytokine also exerts some immune-stimulating effects, such as promoting the generation of cytotoxic T lymphocytes, activating B cells (1), and up-regulating a small number of genes in TLR-activated phagocytic cells and DCs (2).
Upon binding to its two-chain receptor, IL-10 acts primarily by preventing gene transcription through a mechanism that requires STAT3 and de novo protein synthesis but is otherwise poorly understood (3, 4). Many cell types can produce IL-10, including phagocytic cells, conventional DCs, T cells, B cells, and NK cells (1). IL-10 was originally described as a cytokine produced specifically by CD4+ Th2 cells, but later studies showed that it was secreted by both Th1 and Th2 cells. IL-10 is also made by CD4+ Foxp3+ CD25+ “natural” regulatory T (T reg) cells and IL-10–induced CD4+ T reg cells (Tr1 cells) (1). In this issue, two papers (Jankovic et al. [5] p. 273 and Anderson et al. [6] p. 285) investigate the cellular origin of IL-10 in Toxoplasma gondii– and Leishmania major–infected animals. These new data show that although most of the cell types mentioned above produce IL-10 during infections, the IL-10 responsible for limiting inflammatory pathology during T. gondii infection and for maintaining a chronic nonresolving infection with L. major is produced by CD4+ Foxp3− CD25− antigen-specific Th1 cells (which also produced IFN-γ), rather than by Th2 cells, T reg cells, or innate cells.
How IL-10 helps protect
Many mechanisms are in place to prevent exaggerated inflammatory and immune responses and thus protect the host from immune-mediated damage. But in many cases, IL-10 has proven to be an essential factor in this protection. For example, animals which are genetically deficient for IL-10 or are treated with antibodies that neutralize IL-10 functions die rapidly when infected with pathogens such as T. gondii or Trypanosoma cruzi. In these models, death is caused by the overproduction of proinflammatory cytokines, despite the fact that the infection is controlled as well as or better than in wild-type animals (7, 8). During infection with viruses, such as lymphocyte choriomeningitis virus, the production of IL-10 maintains a chronic, nonhealing infection, and neutralization of IL-10 allows the animals to clear the virus (9, 10). In tumor-bearing animals, the profound anergy and unresponsiveness of tumor-infiltrating DCs and macrophages is rapidly reversed by neutralization of IL-10 (11). Injection of TLR ligands into the tumors of these anti–IL-10–treated animals induces a rapid hemorrhagic necrosis that may result in the complete elimination of the tumors (12).
Although IL-10 is a secreted cytokine that can have systemic effects, its cellular origin has been shown to profoundly affect the resulting immune regulation. Mice with a T cell–specific inactivation of the IL-10 gene, for example, succumb to severe immunopathology upon infection with T. gondii, similar to mice with complete IL-10 deficiency, but have normal innate responses to lipopolysaccharide (LPS) (13). Because the effects of IL-10 on T cells are primarily indirect, the cells that respond to the T cell–produced IL-10 in this model are probably the accessory cells or APCs with which the T cells interact. Conversely, mice in which IL-10 is inactivated in phagocytic cells (macrophages and neutrophils) are hyperresponsive to LPS but not to CpG-containing oligonucleotides, suggesting that CpG induce IL-10 production by TLR9+ cells other than macrophages, such as B cells or DCs (14). These data suggest that the identity of both the IL-10–producing cell and the IL-10–reponding cell affects the resulting response.
Regulation of IL-10 production in T cells
In many chronic infections in human and experimental animals, CD4+ T cells can produce high levels of both IL-10 and IFN-γ (for review see reference 15). For example, both cytokines are produced by short term CD4+ T cell clones expanded from bronchoalveolar lavage fluid, but not from peripheral blood, of patients with active tuberculosis (16). Although the nature of these cells has not been fully characterized, the production of high levels of both cytokines in the absence of significant levels of other Th2 cytokines, suggests that these cells are Th1 cells, rather than Th2 cells or T reg cells (15). In vitro, human CD4+ and CD8+ T cell clones that produce high levels of both IFN-γ and IL-10 could be generated by expanding the cells with polyclonal stimulation in the presence of IL-12 (17). In that study, IL-12 was required within the first 3 days after proliferation was induced, and for at least 2 weeks thereafter, for the cells to be stably primed for the production of both cytokines upon restimulation. This priming was maintained even if the cells had been expanded for several weeks in the absence of IL-12. The presence of IL-4 during Th1 priming completely prevented subsequent IL-10 production but only modestly affected subsequent IFN-γ production. Conversely, IL-4 was needed for IL-10 production by T cells primed under Th2 conditions. Similar to the effect of IL-12 in priming IFN-γ–IL-10 coproducing Th1 cells, type I IFN has been shown to induce naive cord blood CD4+ T cells to differentiate into Tr1 cells that produce both IL-10 and IFN-γ (18). Because both IL-12 and type I IFN activate STAT4 in human T cells, it is possible that this transcription factor is partly responsible for the priming of both the IL10 and the IFNγ genes in human T cells. Overall, the data in both humans and mice suggests that both Th1 and Th2 cells can produce IL-10 but that different regulatory mechanisms are at play.
Interestingly, the chromatin organization of the IL10 gene in Th1 and Th2 cells is different from that of the IL4 and IFNγ genes. The IL10 promoter and some putative regulatory elements are silenced in Th1 cells (19, 20). The remainder of the IL10 locus in both Th1 and Th2 cells, however, contains hypersensitivity sites not present in naive T cells (19–22). The IL4 promoter is also silenced in Th1 cells but, unlike the IL10 locus, contains no hypersensitivity sites other than those present in naive T cells (23). The IFNγ locus is similarly silenced in Th2 cells (23). Thus, it has been suggested that the IL10 locus may be in a reversible histone deacetylase–responsive state in Th1 cells (20), which may permit its reactivation in situations such as continuous stimulation by chronic infection or maximal in vitro activation in the presence IL-12 (17). The regulation of IL-10 production has been characterized in many cell types, such as Th2 cells, macrophages, and DCs, and involves both common and cell type–specific regulatory elements (21, 22, 24). It remains to be established whether unique mechanisms of IL-10 regulation are present in Th1 cells.
IL-10 production during acute and chronic infections
The two papers in this issue address which subset of CD4+ T cells produces the IL-10 that prevents T. gondii–induced mortality and suppresses inflammation during chronic cutaneous infections with L. major (5, 6).
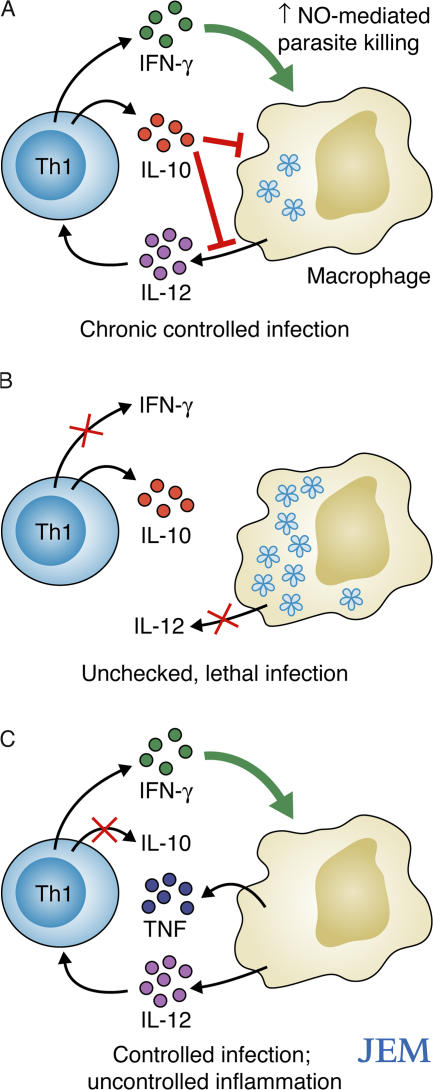
A previous study had identified the CD4+ T cells that produce both IL-10 and IFN-γ during T. gondii infection, and showed that cytokine induction in these cells did not require IL-12, IL-18, IL-23, STAT4, IL-4, STAT6, or IFN-γ (25 and unpublished data). IL-10 production by these T cells was required to prevent early mortality caused by excessive inflammation. Here, Jankovic et al. (5) show that T cell–produced IL-10 is required for survival during both the acute and chronic phases of T. gondii infection (Fig. 1, A–C). The authors clearly identified the IL-10–producing T cells as activated, T-bet+ Th1 cells that are distinct from Th2 cells, natural Foxp3+ T reg cells, and other subsets of induced T reg cells. These Th1 T cells were fully functional effector Th1 cells, as demonstrated by their ability to induce nitric oxide–mediated parasite destruction by macrophages, which was comparable to that of Th1 cells producing IFN-γ only. The production of IL-10 was transient, observed in only a fraction of the IFN-γ–producing cells, and was induced more rapidly from recently activated than from resting cells (5). The instability of IL-10 synthesis, which was observed only when the Th1 cells were fully activated, likely helps prevent sustained IL-10–mediated suppression of effector functions, which might hamper parasite clearance.
Figure 1.
T. gondii infection in C57BL/6 mice. (A) In intact animals, IFN-γ–producing Th1 cells efficiently control the infection. Some of these Th1 cells also produce IL-10, which helps limit the Th1 response and limit inflammation. (B) Blocking IFN-γ or IL-12 allows the infection to proceed unchecked, causing the rapid death of the animals. (C) Blocking the production of IL-10 by Th1 cells triggers an overproduction of proinflammatory cytokines by T cells and innate cells, resulting in the death of the infected animals, despite parasite clearance.
Cutaneous infections with L. major (Friedlin strain) can be clinically cured by a Th1-mediated mechanism in resistant C57BL/6 mice. A small number of parasites can persist, however, and a sterile cure can be achieved only if IL-10 is neutralized (26). IL-10 is produced mostly by CD4+ T cells in this model, and all of the IL-10–producing CD4+ cells at the site of infection, and half of those in the draining lymph node (LN), also produce IFN-γ (26). Other studies have shown that during the chronic phase of L. major infection, approximately half of the CD4+ T cells at the site of infection are antigen-specific CD25+ Foxp3+ T reg cells, which inhibit the response of CD25− effector T cells via both IL-10–dependent and –independent mechanisms (27, 28). These T reg cells produce most of the IL-10 responsible for the maintenance of chronic infection, whereas CD25− T cells produce most of the IFN-γ (27).
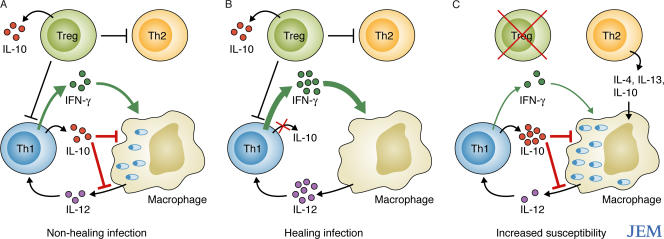
Anderson et al. now characterize the source of IL-10 in C57BL/6 mice infected intradermally with a clinical isolate of L. major (NIH/Sd) that produces heavily infected, nonhealing lesions, even in the presence of a vigorous Th1 response (6). This experimental model is reminiscent of clinical leishmaniasis, which is characterized by a Th1-type response associated with IL-10 production that, in some cases, fails to induce healing or to prevent visceral spreading of the infection. In mice infected with L. major NIH/Sd, the IL-10 produced by T cells, but not by innate cells, was required for the suppression of the healing response, although most of the IL-10 at the lesion site was produced by innate cells (Fig. 2 A). The majority of IL-10 in the draining LNs, on the other hand, was produced by T cells, including both CD25+ Foxp3+ T reg cells and CD4+ CD25− Foxp3− T cells. A majority of the latter cells also produced IFN-γ (6).
Figure 2.
L. major NIH/Sd infection in C57BL/6 mice. (A) In intact animals, IFN-γ–producing Th1 cells only partially control the infection, and nonhealing lesions are established. (B) Blocking the production of IL-10 by Foxp3− Th1 cells increases the Th1 effector response, which helps clear the infection. (C) Depleting Foxp3+ T reg cells increases the parasite burden in parallel with the production of IL-10 and Th2 cytokines, resulting in increased susceptibility to infection.
Adoptive transfer experiments using these two IL-10–producing cell subsets showed that, surprisingly, only the IL-10 produced by the CD4+ CD25− Foxp3− T cells suppressed the healing response, whereas IL-10 from the CD25+ Foxp3+ T reg cells was ineffective (6) (Fig. 2 B). Depletion of the T reg cells did, however, result in an increased parasite burden, suggesting that these cells promoted host resistance against this strain of L. major, possibly by suppressing a deleterious Th2 response and/or by decreasing IL-10 production by the Th1 cells (Fig. 2 C).
These results indicate that the ability of IL-10 to suppress the in vivo immune response depends not only on whether the cytokine is produced by innate cells or immune CD4+ T cells, but also on the specific type of CD4+ T cell. These findings are difficult to interpret and could be explained either by a differential ability of regulatory and effector CD4+ T cells to migrate into the LNs or to localize in different regions of the LNs. It is also possible that IL-10 may need to be produced at the site of cell–cell contact during the cognate interaction between the effector CD4+ T cells and the APCs. However, it is also possible that the IL-10 produced by T reg cells is efficient in antagonizing the Th1 response, but that this effect is functionally negated by the concurrent removal of the parasite-friendly Th2 response.
Concluding remarks
These data suggest that activated effector Th1 cells produce IL-10 as a means of preventing collateral immune damage—a mechanism of self control. But this mechanism can also be used by pathogens to prevent their elimination. Although IL-12 is not necessary for the generation of IL-10–producing Th1 cells in T. gondii–infected animals, it is interesting that in cultures of human T cells, extreme Th1 polarization in the presence of high concentration of IL-12 induces apparent irreversible priming for the production of high levels of both IL-10 and IFN-γ (17). IL-12–induced IFN-γ was also recently shown to provide a signal for the reactivation of IL-10 in Th1 cells from T. gondii–infected mice (29). In that study, IL-10 reactivation was mediated by the IFN-γ–driven expression of inducible costimulator ligand (ICOS-L) on non–T cells (29). Interestingly, plasmacytoid DCs, which themselves do not produce IL-10, have been shown to promote IL-10 secretion from CD4+ and CD8+ T cells via ICOS-L expression induced during DC maturation (30). Indeed, plasmacytoid DCs are emerging as essential APCs for the induction of antigen-specific tolerance in several experimental models (31, 32).
The challenge is now to characterize the specific molecular mechanisms that lead to priming of the IL10 gene in Th-1 and Th2 cells and to IL-10 reactivation and expression in Th1-committed cells. It will also be important to identify the specific role of different subsets and maturation stages of APCs and DCs in regulating the differentiation of effector T cells and T reg cells, and in controlling feedback mechanisms, and to determine the role of the different costimulatory pathways in regulating these responses. Knowledge of these mechanisms may allow us to identify new targets of intervention for the treatment and prevention of infectious diseases. Some of these regulatory mechanisms might also be involved in the immunosuppressive environment seen in tumors, and their study could provide new mechanistic insights into cancer immunology.
Acknowledgments
I would like to thank Dr. Yasmine Belkaid for advice and discussions.
G.T. is at Cancer and Inflammation Program, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702.
References
- 1.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 2.Lang, R., D. Patel, J.J. Morris, R.L. Rutschman, and P.J. Murray. 2002. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169:2253–2263. [DOI] [PubMed] [Google Scholar]
- 3.Amezaga, M.A., F. Bazzoni, C. Sorio, F. Rossi, and M.A. Cassatella. 1992. Evidence for the involvement of distinct signal transduction pathways in the regulation of constitutive and interferon g-dependent gene expression of NADPH oxidase components (gp91-phox, p47-phox, and p22-phox) and high-affinity receptor for IgG (FcgR-I) in human polymorphonuclear leukocytes. Blood. 79:735–744. [PubMed] [Google Scholar]
- 4.Murray, P.J. 2005. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. USA. 102:8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankovic, D., M.C. Kullberg, C.G. Feng, R.S. Goldszmid, C.M. Collazo, M. Wilson, T.A. Wynn, M. Kamanaka, R.A. Flavell, and A. Sher. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson, C.F., M. Oukka, V.J. Kuchroo, and D. Sacks. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzinelli, R.T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF- alpha. J. Immunol. 157:798–805. [PubMed] [Google Scholar]
- 8.Hunter, C.A., L.A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F.G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311–3316. [PubMed] [Google Scholar]
- 9.Brooks, D.G., M.J. Trifilo, K.H. Edelmann, L. Teyton, D.B. McGavern, and M.B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejrnaes, M., C.M. Filippi, M.M. Martinic, E.M. Ling, L.M. Togher, S. Crotty, and M.G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicari, A.P., C. Chiodoni, C. Vaure, S. Ait-Yahia, C. Dercamp, F. Matsos, O. Reynard, C. Taverne, P. Merle, M.P. Colombo, et al. 2002. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiducci, C., A.P. Vicari, S. Sangaletti, G. Trinchieri, and M.P. Colombo. 2005. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65:3437–3446. [DOI] [PubMed] [Google Scholar]
- 13.Roers, A., L. Siewe, E. Strittmatter, M. Deckert, D. Schluter, W. Stenzel, A.D. Gruber, T. Krieg, K. Rajewsky, and W. Muller. 2004. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 200:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siewe, L., M. Bollati-Fogolin, C. Wickenhauser, T. Krieg, W. Muller, and A. Roers. 2006. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur. J. Immunol. 36:3248–3255. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri, G. 2001. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J. Exp. Med. 194:F53–F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerosa, F., C. Nisii, S. Righetti, R. Micciolo, M. Marchesini, A. Cazzadori, and G. Trinchieri. 1999. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 92:224–234. [DOI] [PubMed] [Google Scholar]
- 17.Gerosa, F., C. Paganin, D. Peritt, F. Paiola, M.T. Scupoli, M. Aste-Amezaga, I. Frank, and G. Trinchieri. 1996. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 183:2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levings, M.K., R. Sangregorio, F. Galbiati, S. Squadrone, R. de Waal Malefyt, and M.G. Roncarolo. 2001. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166:5530–5539. [DOI] [PubMed] [Google Scholar]
- 19.Jones, E.A., and R.A. Flavell. 2005. Distal enhancer elements transcribe intergenic RNA in the IL-10 family gene cluster. J. Immunol. 175:7437–7446. [DOI] [PubMed] [Google Scholar]
- 20.Im, S.H., A. Hueber, S. Monticelli, K.H. Kang, and A. Rao. 2004. Chromatin-level regulation of the IL10 gene in T cells. J. Biol. Chem. 279:46818–46825. [DOI] [PubMed] [Google Scholar]
- 21.Wang, Z.Y., H. Sato, S. Kusam, S. Sehra, L.M. Toney, and A.L. Dent. 2005. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J. Immunol. 174:2098–2105. [DOI] [PubMed] [Google Scholar]
- 22.Saraiva, M., J.R. Christensen, A.V. Tsytsykova, A.E. Goldfeld, S.C. Ley, D. Kioussis, and A. O'Garra. 2005. Identification of a macrophage-specific chromatin signature in the IL-10 locus. J. Immunol. 175:1041–1046. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal, S., O. Avni, and A. Rao. 2000. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 12:643–652. [DOI] [PubMed] [Google Scholar]
- 24.Shoemaker, J., M. Saraiva, and A. O'Garra. 2006. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J. Immunol. 176:3470–3479. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 26.Belkaid, Y., K.F. Hoffmann, S. Mendez, S. Kamhawi, M.C. Udey, T.A. Wynn, and D.L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 28.Suffia, I.J., S.K. Reckling, C.A. Piccirillo, R.S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw, M.H., G.J. Freeman, M.F. Scott, B.A. Fox, D.J. Bzik, Y. Belkaid, and G.S. Yap. 2006. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J. Immunol. 176:7263–7271. [DOI] [PubMed] [Google Scholar]
- 30.Ito, T., M. Yang, Y.H. Wang, R. Lande, J. Gregorio, O.A. Perng, X.F. Qin, Y.J. Liu, and M. Gilliet. 2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- 32.Ochando, J.C., C. Homma, Y. Yang, A. Hidalgo, A. Garin, F. Tacke, V. Angeli, Y. Li, P. Boros, Y. Ding, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7:652–662. [DOI] [PubMed] [Google Scholar]