Abstract
Nonhealing forms of leishmaniasis in humans are commonly associated with elevated levels of the deactivating cytokine IL-10, and in the mouse, normally chronic infections can be cleared in the absence of IL-10. Using a Leishmania major strain that produces nonhealing dermal lesions in a T helper type 1 (Th1) cell–polarized setting, we have analyzed the cellular sources of IL-10 and their relative contribution to immune suppression. IL-10 was produced by innate cells, as well as CD4+CD25+Foxp3+ and CD4+CD25−Foxp3− T cells in the chronic lesion. Nonetheless, only IL-10 production by antigen-specific CD4+CD25−Foxp3− T cells, the majority of which also produced IFN-γ, was necessary for suppression of acquired immunity in Rag−/− reconstituted mice. Surprisingly, Rag−/− mice reconstituted with naive CD4+ T cells depleted of natural T regulatory cells developed more severe infections, associated with elevated levels of IL-10 and, especially, Th2 cytokines in the site. The data demonstrate that IL-10–producing Th1 cells, activated early in a strong inflammatory setting as a mechanism of feedback control, are the principal mediators of T cell–derived IL-10–dependent immune suppression in a chronic intracellular infection.
The intracellular protozoan Leishmania can produce a spectrum of clinical diseases, ranging from a single cutaneous ulcer that spontaneously heals, to chronic cutaneous or mucocutaneous lesions that are nonhealing or slow to resolve, to visceral disease that is generally fatal in the absence of treatment. Mammalian host protection against Leishmania is dependent on the development of a Th1 response, which arms infected macrophages for enhanced leishmanicidal capacity. Based largely on the extensive data pertaining to L. major infection in BALB/c mice, the immunologic mechanisms underlying nonhealing forms of leishmaniasis in mice and humans have conventionally been investigated in the context of a parasite-driven, Th2-polarized response in which IL-4 is especially dominant (for review see reference 1). Th2 cell dominance, however, has failed to adequately explain nonhealing or reactivated forms of cutaneous or visceral leishmaniasis in humans. Analysis of chronic, localized lesions in humans has revealed increased expression of proinflammatory cytokines and high levels of IL-10 but low or undetectable amounts of Th2-associated cytokines (2, 3). Lesions in patients with reactivating post-kala-azar dermal leishmaniasis contained mainly CD3+ cells, with IL-10 and IFN-γ the predominant cytokines (4, 5). In human visceral leishmaniasis, elevated levels of IFN-γ mRNA have been found in target organs, such as the spleen and bone marrow, accompanied by increased levels of IL-10 (6–8).
Accumulating evidence from other mouse models of nonhealing or disseminating forms of leishmaniasis have reinforced pathogenetic mechanisms that take into account the presence of parasite-driven Th1 responses that are suppressed either in magnitude or function by IL-10. IL-10 is crucial for suppressing the healing response in mice with cutaneous lesions caused by L. mexicana and in preventing clearance of L. donovani from liver and spleen (9–11). Even in the L. major–BALB/c infection model, Th2 cell immune polarization appears to be superimposed on IL-10–mediated suppressive pathways to account for the hypersusceptibility of this mouse strain, as BALB/c IL-4Rα–deficient mice are not fully resistant until IL-10 function is also impaired (12). We have recently introduced a model of nonhealing L. major in conventionally resistant C57BL/6 mice, in which IL-10 functions in a Th1 cell–polarized setting to prevent clinical cure, and have argued that this model better reflects the conditions underlying nonhealing forms of clinical disease (13).
IL-10 has pleiotropic, primarily antiinflammatory properties that include suppression of DC functions and rendering macrophages unresponsive to activation signals (for review see reference 14). IL-10 can be produced by many cell types, including B cells, macrophages, DCs, Th2 cells, and distinct populations of T reg cells. Importantly, the sources of IL-10 in chronic forms of leishmanial disease in mice or humans remains poorly defined. Macrophages have been shown to produce IL-10 in response to LPS plus FcR signaling by IgG-opsonized amastigotes in vitro (15); although there is no direct evidence that macrophages are a critical source of infection-induced IL-10 in vivo, antigen–antibody complexes enhanced susceptibility via an IL-10–dependent pathway (11, 16), as did transgenic overexpression of IL-10 by macrophages and other APCs (17). CD4+ T cells have also been implicated as an important source of IL-10 in BALB/c mice, insofar as treatment of IL-4Rα−/− BALB/c mice with a CD4-depleting antibody, or an anti–IL-10 receptor antibody, in each case promoted strong resistance against L. major (12). Furthermore, IL-10 production by CD25 Foxp3− CD4+ T cells was a strong correlate of disease progression in L. donovani–infected mice (18). Finally, naturally occurring CD4+CD25+Foxp3+ T reg cells have been identified in the healed lesions of C57BL/6 mice and have been shown to function in an IL-10–dependent manner to prevent sterile cure (19).
The possibility that an imbalance in the number or activities of natural T reg cells might explain nonhealing forms of leishmaniasis seems especially strong given the many examples of chronic viral, bacterial, and parasitic infections in humans and mice where depletion of CD4+CD25+ T cells from PBMCs or lymphoid tissue was shown to increase antigen-specific proliferative or IFN-γ responses (for review see reference 20). Although naturally occurring T reg cells originate in the thymus and are controlled by the activity of the transcription factor Foxp3 (for review see reference 21), CD4+ T cells with regulatory activities can also be generated from conventional naive T cells after antigen encounter in the periphery (for review see references 22, 23). Antigen-induced, IL-10–producing CD4+ T cells—arising from CD4+CD25−Foxp3− cells and variably referred to as Tr1 cells, adaptive T reg cells, or inducible T reg cells—have been found to regulate colitis induced by Heliobacter hepaticus infections and to suppress protective Th1 responses to Bordetella pertussis in mice (24, 25). In humans, IL-10–producing, inducible T reg cells may limit immune responses to Mycobacterium tuberculosis antigens and to helminth antigens in patients with onchocerciasis (26, 27). IL-10–secreting CD8+ T cells that can suppress certain autoimmune responses represent another distinct lineage of inducible T reg cells (28), though their role in infectious diseases has not yet been demonstrated. Thus, multiple T and non–T cell sources of IL-10 can potentially contribute to suppression of curative responses in chronic infections, and IL-10–producing CD4+ T cells are themselves heterogeneous with respect to their origin, activation requirements, and mechanism of action.
The present studies were designed to clarify the relative contribution of innate and T cell–derived sources of IL-10 to nonhealing infection with L. major, emphasizing the respective roles of IL-10–producing CD4+ T cell subsets that function locally to control Th1 cell–mediated immunity and pathology in the skin.
RESULTS
T cell–derived IL-10 is produced by Foxp3+ and Foxp3− CD4+ cells
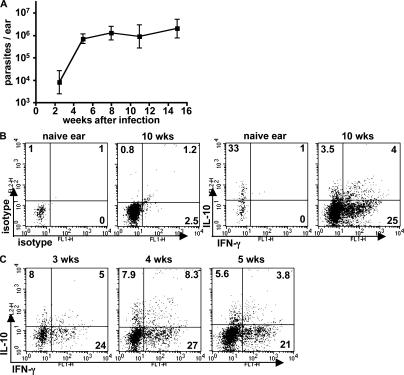
We previously reported on an isolate of L. major that produces nonhealing lesions containing persistently high parasite burdens after low dose, intradermal inoculation in C57BL/6 mice (13). The immune response in the site of the chronic lesion is predominantly composed of IFN-γ–producing CD4+ T cells, with IL-4 and IL-13 detectable in only very low amounts. IL-10 was found to play an essential role in the evolution of the chronic lesion, as the parasites were effectively controlled in IL-10–deficient mice and in WT mice treated during the chronic phase with anti–IL-10 receptor antibody. The key features of the model are reinforced by new data presented in Fig. 1: after intradermal inoculation of 1,000 metacyclic promastigotes of L. major NIH/Sd, parasite numbers increased in the ear dermis during the first 4–5 wk, followed by containment of parasite growth but an inability to reduce the parasite burden or resolve the cutaneous lesion (not depicted) for up to 16 wk. The chronic lesion contained a 15-fold increase, relative to naive ears, in the number of CD4+ T cells that accumulated in the site. Ex vivo restimulation with PMA/ionomycin was used to evaluate the cytokine-producing capacity of the CD4+ T cell subsets recovered from the ear dermis (Fig. 1 B). No IFN-γ or IFN-γ/IL-10 double producers were observed from CD4+ T cells recovered from naive ears, although a high proportion of the low numbers of the CD4+ T cells recovered from the steady-state dermis produced IL-10. In contrast, 25% of the CD4+ T cells in the chronic lesion (10 wk) produced IFN-γ, accompanied by ∼7.5% of CD4+ T cells that produced IL-10, of which approximately half produced both cytokines. A kinetic analysis during the early development of the effector response revealed the presence of IFN-γ+/IL-10+ CD4+ T cells by 3 wk after infection (Fig. 1 C).
Figure 1.
CD4+ T lymphocytes producing both IFN-γ and IL-10 are associated with nonhealing lesions in C57BL/6 mice infected with L. major NIH/Sd. Mice were inoculated intradermally with 1,000 metacyclic-stage parasites in each ear. (A) Parasite burdens in the ear lesions were monitored throughout the infection. Values represent the mean ± SD of six mouse ears at each time point during one experiment and are representative of at least three independent experiments. (B) 10 wk after infection, ear lesion cells or naive control ear cells were stimulated in vitro with PMA and ionomycin for 4 h and analyzed for intracellular cytokine production. (C) Ear lesion cells were analyzed for cytokine production at the indicated time points after infection. All cells shown are TCRβ+CD4+ lymphocytes, and quadrant values are the percentage of total gated CD4+ T cells. The plots are from six pooled ears per time point from one experiment and are representative of at least three.
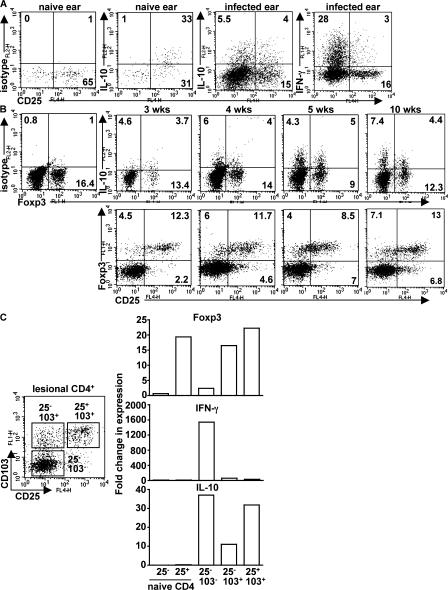
PMA/ionomycin stimulation of naive dermal CD4+ T cells indicated that all of the IL-10 production was confined to the resident CD25+ cells (Fig. 2 A). In contrast, in the cells recovered from the chronic lesion (10 wk), IL-10 was produced by approximately equivalent numbers of CD25− and CD25+ cells, whereas the vast majority of the IFN-γ–producing cells were CD25−. Foxp3 staining, which provides a more stringent criterion for identification of natural T reg cells, indicated that at 3 wk after infection, ∼15% of the lesional CD4+ cells expressed Foxp3, and this frequency was more or less maintained throughout the chronic phase of disease (Fig. 2 B). Of note, a substantial number of the Foxp3+ cells were CD25−. IL-10 production by the CD25− cells was not confined to the Foxp3+ population, however, because CD4+Foxp3+ and CD4+Foxp3− cells were each a source of IL-10 in the lesion. Kinetic analysis of IL-10 production by CD4+Foxp3− and CD4+Foxp3+ cells showed that both populations were present during the early phase of the effector response (3 wk after infection), suggesting that these cells are not a consequence of chronic antigen stimulation in the inflammatory site but arise early and may be causally related to the evolution of the noncure phenotype.
Figure 2.
CD4+ T cells producing IL-10 in response to infection with L. major NIH/Sd comprise multiple subsets. (A) Naive ear cells or cells from the chronic ear lesion were stimulated in vitro with PMA and ionomycin for 4 h and analyzed for intracellular cytokine production. (B) A kinetic analysis of IL-10 production and Foxp3 expression was performed on in vitro–restimulated lesional cells at the indicated time points. The data shown are TCRβ+CD4+ cells from six pooled ears from one experiment and are representative of at least three independent experiments. Quadrant values are the percentage of total gated CD4+ T cells. (C) 10 wk after infection, TCRβ+CD4+ ear lesion cells were purified by cell sorting into three subsets according to cell surface expression of CD25 and CD103. Real-time PCR analysis was performed to quantify gene expression of Foxp3, IFN-γ, and IL-10. Data are expressed as the fold change relative to naive CD4+CD25− lymph node cells. Two independent experiments were performed with similar results.
The CD4+ T cell subsets in the chronic ear lesions were further characterized by ex vivo analysis of cytokine mRNA. The CD4+ cells were sort purified based on expression of CD25 and the integrin CD103, as the latter has been shown to be highly expressed in natural T reg cells accumulating in the dermal lesions of L. major–infected mice (29). As shown in Fig. 2 C, CD25+CD103+ cells were highly enriched for both Foxp3 and IL-10 expression but not IFN-γ. CD25−CD103+ cells also expressed Foxp3, intermediate levels of IL-10 message, and again no IFN-γ. Importantly, CD25−CD103− cells expressed high levels of IFN-γ and IL-10 but only low levels of Foxp3. Collectively, the data clearly show that infection with L. major NIH/Sd drives local IL-10 production by both CD4+CD25+CD103+Foxp3+ and CD4+CD25−CD103−Foxp3− T cells and suggest that as the later cells are the exclusive source of IFN-γ, they are also the source of the cells capable of production of both cytokines that can be recovered from the inflammatory site.
The contribution of innate and T cell sources of IL-10 to suppression
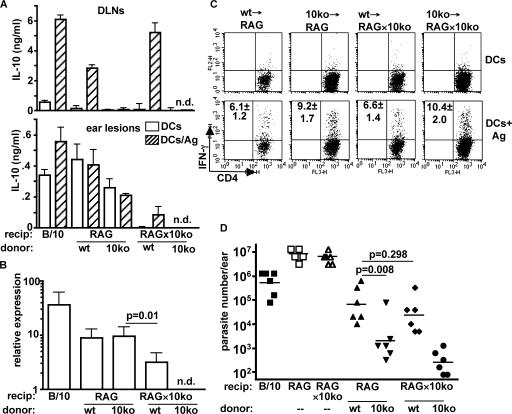
Because cell types in addition to CD4+ T cells may produce IL-10 and because macrophages, in particular, have been implicated in IL-10 production in other nonhealing models of cutaneous leishmaniasis (11, 16, 30), experiments were designed to evaluate the relative contribution made by CD4+ T cells versus innate cells to the IL-10–mediated suppression of immunity in L. major NIH/Sd–infected mice. Unfractionated naive T cells from C57BL/10 or IL-10−/− mice were adoptively transferred into RAG−/− or RAG−/− × IL-10−/− recipients, thereby restricting the source of IL-10 to either T cell or non–T cell compartments. 11 wk after transfer and infection, IL-10 was detected by ELISA in draining lymph node cells from RAG−/− and RAG−/− × IL-10−/− recipients of T cells from WT mice and was dependent on the addition of exogenous parasite antigen (Fig. 3 A). IL-10 was not detected in the lymph nodes of recipients of IL-10−/− T cells. In contrast, IL-10 was detectable in the ear lesions from mice that received WT or IL-10−/− T cells and was produced in the absence of exogenous antigen, indicating that there is a non–T cell source of IL-10 in the inflammatory site. Ex vivo analysis by real-time PCR also indicated that IL-10 was being produced in the lesions of mice reconstituted with IL-10−/− T cells (Fig. 3 B). Furthermore, comparison of IL-10 production at the protein and message level suggests that the major source of IL-10 in the ear lesion was innate cells.
Figure 3.
T cells are the dominant source of IL-10–mediated immune suppression in L. major NIH/Sd infection. 2 × 106 T lymphocytes from lymph nodes of naive C57BL/10 or IL-10−/− mice were adoptively transferred into RAG−/− or RAG−/− × IL-10−/− recipients, followed by intradermal inoculation of L. major NIH/Sd 1 d later. 11 wk after infection, mice were killed for analysis. (A) Draining lymph node cells or ear lesion cells were stimulated in vitro with BMDCs in the absence or presence of L. major antigen for 72 h, followed by IL-10 measurement by ELISA. Groups are labeled with the source of donor T cells specified below either RAG−/− or RAG−/− × IL-10−/− recipient mice. Data are the mean ± SD of triplicate samples from one experiment. The experiment was performed three times with similar results. (B) Real-time PCR was performed on ear lesion tissue for quantification of IL-10. The values are the fold change in gene expression relative to naive controls. Data are the mean ± SD of four ears from one experiment and are representative of three experiments. (C) Ear lesion cells were stimulated in vitro with BMDCs or BMDCs infected with L. major amastigotes. After overnight incubation, monensin was added in the final 4 h, followed by measurement of intracellular IFN-γ. The plots shown are from one experiment, and the values in the upper left quadrants are the total number of IFN-γ+CD4+ cells/ ear × 10−3 and are the mean ± SD of three independent experiments. (D) Parasite burdens in the ear lesions were enumerated by limiting dilution. Unreconstituted RAG−/− and RAG−/− × IL-10−/− mice are also shown as controls. The data points are from six mice from one experiment and are representative of three independent experiments. Horizontal lines represent geometric means.
To determine what effect the source of IL-10 had on the Th1 effector response, the number of Leishmania antigen-specific IFN-γ–producing cells was measured by flow cytometry (Fig. 3 C). The mice that had received IL-10−/− T cells had significantly increased numbers of lesional IFN-γ–producing CD4+ T cells (9.2 ± 1.7 × 103 cells in RAG−/− mice and 10.4 ± 2 × 103 cells in RAG−/− × IL-10−/− mice) compared with the mice receiving IL-10–sufficient T cells (6.1 ± 1.2 × 103 cells in RAG−/− mice and 6.6 ± 1.4 × 103 cells in RAG−/− × IL-10−/− mice), suggesting that the T cell–derived IL-10 had a greater influence on the effector response than the IL-10 derived from non–T cell sources. Most importantly, T cell–derived IL-10 exerted the greatest suppressive effect on the ability of the recipient mice to control infection with L. major NIH/Sd (Fig. 3 D). At 11 wk, RAG−/− mice that received IL-10−/− T cells had significantly lower parasite burdens than RAG−/− × IL-10−/− recipients of WT T cells, demonstrating that the presence of IL-10 from T cells alone is sufficient to suppress killing. When IL-10 production was absent only from the innate compartment, parasite burdens were no different from the group containing both sources of IL-10, demonstrating that IL-10 from non–T cells, despite its relative abundance within the inflammatory site, contributes little to the suppression of immunity. The reconstituted animals with generalized IL-10 deficiency displayed the greatest resistance to infection, although their parasite burdens were not significantly different (P > 0.05) than the mice deficient in T cell–derived IL-10 only. Of note, the T cell–reconstituted mice in each case controlled the infection far better than the unreconstituted mice, demonstrating that the acquired resistance is only partially compromised by IL-10. Because these experiments involved transfers of T cells alone, the potential contribution from antibody-mediated IL-10 induction through FcR signaling on macrophages would be absent. The transfers into RAG−/− and RAG−/− × IL-10−/− recipient mice were therefore performed using total splenocytes from WT or IL-10−/− mice. The results again showed that T cells were the dominant source of IL-10–mediated suppression of immunity in the reconstituted mice (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061886/DC1), with no significant difference observed between the RAG−/− and RAG−/− × IL-10−/− recipient groups. Collectively, the data indicate that IL-10 is locally produced by both T cells and innate cells but that antigen-specific, T cell–derived IL-10 is both necessary and sufficient for suppression of acquired resistance to infection with L. major NIH/Sd.
CD4+CD25−Foxp3− cells provide the major contribution to IL-10–mediated suppression by T cells
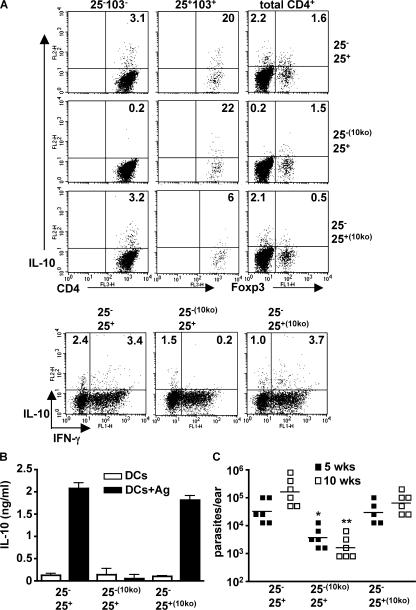
To evaluate the relative contribution of the CD4+IL-10+ subsets to the suppression of protective immunity in L. major NIH/Sd–infected mice, CD4+ cells from naive WT or IL-10−/− mice were sort purified into CD25− and CD25+ subsets and cotransferred into RAG−/− mice in the following combinations: CD25−WTCD25+WT, CD25−10KOCD25+WT, and CD25−WTCD25+10KO, followed by infection 1 d later. The infections were allowed to proceed to the chronic phase for the analysis of IL-10 production and its correlation with parasite burdens in the ear. When IL-10–sufficient cells of each subset were cotransferred, the cell numbers and cytokine profiles in the lesion reflected those observed in intact mice (Fig. 2 A): CD4+ T cells able to produce IL-10 were found within both the CD25+CD103+/Foxp3+ and CD25−CD103−/Foxp3− populations, with a high proportion of the CD4+IL-10+ cells also positive for IFN-γ (Fig. 4 A). In contrast, in mice that received IL-10−/− CD4+CD25− cells, the IL-10–producing cells were confined to the CD25+CD103+/Foxp3+ populations, and importantly, no IFN-γ/IL-10 double producers were observed. Mice that received IL-10−/− CD4+CD25+ cells showed residual IL-10 production from CD25+CD103+/Foxp3+ cells, suggesting that the cotransferred WT CD25− cells contained contaminating natural T reg cells, or there was conversion of low numbers of Foxp3− to Foxp3+ cells during infection (31). More importantly, these mice maintained normal IL-10 production from CD4+CD25−CD103− and CD4+Foxp3− cells, suggesting that antigen-specific IL-10 production by CD4+ T cells during infection does not appear to be influenced by IL-10 production by natural T reg cells. These recipients also revealed that when IL-10 production was primarily confined to the CD25−/Foxp3− population, a clear majority (78%) of the CD4+ T cells recovered from the lesion also produced IFN-γ. ELISA measurement of IL-10 (Fig. 4 B) and IFN-γ (not depicted) in the draining lymph nodes reinforced the ear lesion data, demonstrating that antigen-specific cytokine secretion was dependent on the transfer of IL-10–sufficient CD25− cells. Finally, the ability of IL-10 to suppress the development of protective immunity in the reconstituted mice was dependent on its production by CD25− Foxp3− cells, as mice receiving IL-10−/− CD25− cells were able to control the infection and displayed a 100-fold reduction in mean parasite burden at 10 wk, regardless of whether the cotransferred natural T reg cells were IL-10 competent or not (Fig. 4 C). Thus, IL-10 from CD4+CD25−Foxp3− T cells, the majority of which also produce IFN-γ, plays the dominant role in the pathogenesis of the chronic, noncuring infection in these mice.
Figure 4.
IL-10 produced by antigen-induced CD4+CD25−Foxp3− T cells is necessary for the suppression of immunity. CD4+ lymph node T cells from naive WT or IL-10−/− mice were separated into CD25− and CD25+ fractions and adoptively cotransferred in a 10:1 ratio in the indicated combinations into RAG−/− recipients, followed by intradermal inoculation of 1,000 L. major NIH/Sd metacyclic promastigotes 1 d later. (A) 10 wk after infection, mice were killed, and lesion cells were stimulated with PMA and ionomycin for 4 h for measurement of intracellular IL-10 and IFN-γ. CD4+ cells were gated on CD25−CD103− and CD25+CD103+ for identification of subsets of IL-10–producing cells. Foxp3 expression and IFN-γ/IL-10 was measured from total CD4+ cells. (B) Draining lymph node cells were stimulated in vitro with BMDCs in the absence or presence of L. major antigen for 72 h, followed by measurement of IL-10 by ELISA. Values shown are the mean ± SEM. (C) Parasite burdens in ear lesions were measured at 5 and 10 wk. The data points are from six mice in each group. The experiments were performed twice with similar results. Horizontal lines represent geometric means. *, P = 0.0005; **, P < 0.0001 in comparison to mice receiving CD25+/− T cells from IL-10–sufficient mice. Ag, antigen.
A caveat of the adoptive cotransfers is that CD25, though a reliable marker of natural T reg cells in populations of naive cells, may not be suitable to track the fate of the original CD25+ and CD25− subsets after their homeostatic proliferation and L. major–driven activation during 10 wk in vivo. To address this concern, congenic mice were used as a source of the naive CD25+ and CD25− cells at the time of cotransfer. When lesional CD4 cells were analyzed 10 wk later for CD45.1 expression in relation to CD25, ∼90% of the cells were found to have maintained their original phenotype in each case (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061886/DC1), thereby justifying the use of CD25 as a marker of natural T reg cells in the analysis of the cotransfer experiments depicted in Fig. 4. Furthermore, the RAG−/− reconstituted mice maintained relative frequencies of CD25− and CD25+ cells in the lesions that approximated those observed in intact mice (Fig. 2).
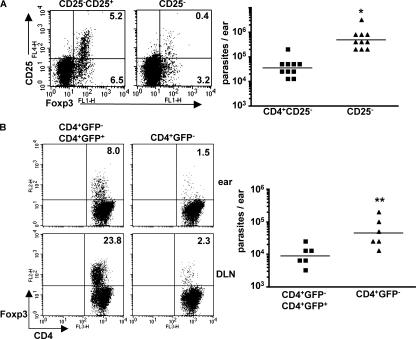
Removal of natural T reg cells exacerbates infection
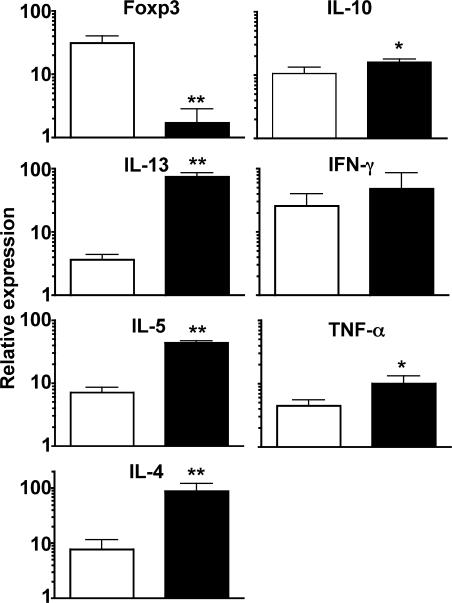
Although IL-10 derived from natural T reg cells does not appear to contribute greatly to the suppression of immunity in C57BL/6 mice infected with L. major NIH/Sd, these cells might still play a critical role in suppression by production of other regulatory cytokines (e.g., TGF-β) or by cell contact–dependent mechanisms. To determine what effect, if any, the presence of natural T reg cells had on the development of the immune response in this infection, CD4+ T cells from the lymph nodes of naive C57BL/10 mice were depleted or not of CD25+ cells by sort purification and adoptively transferred into RAG−/− recipients. 10 wk after transfer and infection, the cell composition and the parasite burdens in the lesions were measured. Although Foxp3+ cells still accumulated in the ear lesions of mice reconstituted with CD25+-depleted cells (3.6% of CD4+ T cells), their frequency was significantly lower than in the lesions of mice receiving the total CD4+ cells (11.7% of CD4+ T cells; Fig. 5 A). Nonetheless, the mice receiving the CD25+-depleted cells had higher parasite burdens, suggesting that, if anything, the natural T reg cells appeared to play a role in promoting host resistance (Fig. 5 A). Because a percentage of the sort purified CD4+CD25− cells are Foxp3+ (Fig. 2 C) and because homeostatic proliferation of the transferred CD4+CD25− cells may result in an outgrowth of the Foxp3+ lineage (32), we used bicistronic mice with an enhanced GFP reporter inserted into the Foxp3 locus as a source of CD4+ T cells from which natural T reg cells might be more faithfully removed (33). 6 wk after adoptive transfer and infection, mice that had received CD4+GFP− cells had few Foxp3+ cells in the draining lymph nodes (2.3%) and at the site of infection (1.5%) compared with the group that received unselected CD4+ cells (8 and 23.8%, respectively; Fig. 5 B). In agreement with the experiment involving transfer of CD25+-depleted cells, the absence of Foxp3−GFP+ cells resulted in a significant increase in parasite burdens in the ear. The termination of the experiment at 6 wk after infection as opposed to a more chronic stage of infection was conditioned by the mice becoming moribund, demonstrating weight loss and signs of colitis that were confirmed on necropsy (unpublished data). The development of colitis in T cell–reconstituted RAG −/− mice is taken as additional evidence for the functional depletion of natural T reg cells in the these recipients (34, 35). The frequency of IL-10+Foxp3− cells, the majority of which also produced IFN-γ, was slightly greater than in the mice transferred with total CD4+ cells (unpublished data). These results were confirmed by the real-time PCR analysis of lesional cytokine mRNA, which revealed slight increases in IL-10, IFN-γ, and TNF mRNA levels in mice reconstituted with the Foxp3-depleted cells (Fig. 6). Interestingly, the lesional cells in these mice also showed an increase in IL-4, IL-5, and IL-13 mRNA expression, indicating that natural T reg cells functioned to suppress the development of a Th2 response. Thus, naive CD4+Foxp3− T cells alone were capable of being activated by the L. major NIH/Sd strain to produce IFN-γ and/or IL-10 and of being recruited to the site of infection in the skin, where they established the conditions necessary for the development of contained, but chronic, nonhealing lesions. The influence of natural T reg cells in this process appeared to be a global suppression of the effector response, including IL-10–producing Th1 cells and, especially, Th2 cells, so that on balance their activities may actually have promoted host immunity in the inflammatory site.
Figure 5.
The absence of natural T reg cells exacerbates the infection. (A) 2 × 106 naive CD4+CD25− T cells alone or in combination with 2 × 105 CD4+CD25+ cells were adoptively transferred into RAG−/− recipients, followed by intradermal inoculation with L. major NIH/Sd 1 d later. 10 wk after infection, mice were killed for parasite burden and immune analysis. Surface CD25 and nuclear Foxp3 expression were measured in lesional cells, and the quadrant values represent the percentage of total CD4+ cells in the ear. For parasite burdens, data points are from 10 ears (from five mice) from one experiment and are representative of at least three independent experiments. Horizontal lines represent geometric means. *, P = 0.008. (B) 2 × 106 CD4+GFP− cells from naive Foxp3-GFP knock-in mice were adoptively transferred alone or in combination with 2 × 105 CD4+GFP+ cells into RAG−/− recipients. 6 wk after infection, mice were killed, and lesions were analyzed for parasite burdens and cell composition. Foxp3 expression was measured in draining lymph nodes and ear lesions. Parasite burden data points are from six mice. The experiment was performed three times with similar results. Horizontal lines represent geometric means. **, P = 0.014.
Figure 6.
Natural T reg cells suppress Th2 cell development. 2 × 106 CD4+GFP− cells from naive Foxp3-GFP knock-in mice were adoptively transferred alone (shaded bars) or in combination with 2 × 105 CD4+GFP+ cells (open bars), followed by infection 1 d later. 6 wk after infection, mice were killed, and real-time PCR was performed on RNA isolated from ear lesions. Values shown are the mean ± SEM and are relative gene expression normalized to 18S rRNA. Data were generated from cells pooled from six mice in each group and are representative of three experiments. *, P < 0.05; **, P < 0.01.
DISCUSSION
It is well established that endogenous IL-10 is a central mediator of immune homeostasis, necessary to keep in check the strong inflammatory reactions that can accompany the expression of antimicrobial immunity in local tissues. A consequence of the balance struck between host immunity and pathology can be chronic or persistent infection, and the absence of IL-10 has been shown to result in more efficient clearance of a variety of pathogens (for review see reference 23). In leishmaniasis, aside from the Th2 cell–polarizing conditions that underlie the extreme susceptibility of BALB/c mice to cutaneous strains of Leishmania, chronic forms of cutaneous or visceral disease in humans and in other mouse models are better explained by the presence of ongoing Th1 responses that are compromised in intensity or function by IL-10 (for review see reference 36). As many innate cells and lymphocyte subsets can produce IL-10, the relative contribution of these cells to the antiinflammatory/immunosuppressive cascade in sites of chronic infection has not been carefully defined. The present study addresses the dominant source of IL-10–dependent immune suppression induced by a strain of L. major that produces nonhealing dermal lesions in the face of a strong Th1 response in C57BL/6 mice. The infection induced localized recruitment and production of IL-10 from innate cells, as well as from CD4+CD25+Foxp3+ natural T reg cells and CD4+CD25−Foxp3− T cells. The latter cells appear to be closely linked to the Th1 effector response, in that the majority also produced high amounts of IFN-γ. In Rag−/− reconstituted, infected mice, the IL-10–producing Th1 cells were generated in the absence of either natural T reg cells or IL-10 from innate sources and, most importantly, were found to play a necessary role in the suppression of protective immunity in the site. The data are consistent with a regulatory pathway that relies not on a committed lineage of cells or on suboptimal conditions of immune activation, but on a strong proinflammatory environment that drives Th1 cells through a program of development that includes IL-10 secretion as a mechanism of feedback control.
The studies have documented IL-10 production associated with both CD4+CD25+Foxp3+ and CD4+CD25−Foxp3− T cells that accumulate in the chronic lesion, and by using RAG−/− mice reconstituted with naive CD4+ T cells that were IL-10–deficient in either the CD25+Foxp3+ or CD25−Foxp3− subset, they have clarified the possible interdependence of these cells and their respective contributions to the suppression of protective immunity. IL-10 produced by CD25−Foxp3− cells was necessary for the evolution of the nonhealing phenotype, because mice receiving naive CD25−Foxp3− cells from IL-10–deficient mice developed strong resistance, even when cotransferred with CD25+Foxp3+ cells from IL-10–sufficient mice. Although we have found it difficult to stain for intracellular IL-10 in antigen-stimulated cells recovered from the ear dermis, the antigen specificity of the IL-10 response was substantiated using draining lymph node cells and was only detected in mice receiving CD25−Foxp3− cells from IL-10–sufficient mice.
Because natural T reg cells might suppress immunity by IL-10–independent mechanisms, their contribution was further evaluated in RAG−/− mice reconstituted with naive CD4+ T cells depleted or not of CD25+ cells. The influence of natural T reg cells was, surprisingly, to promote host resistance. Thus, transfer of FACS-purified naive CD4+CD25− T cells to RAG−/− mice (Fig. 5 A) resulted in significantly greater parasite burdens 10 wk after infection compared with mice receiving total CD4+ T cells, despite the accumulation of fewer numbers of CD25+Foxp3+ cells in the chronic lesion. Transfer of CD4+ T cells from bicistronic reporter mice that were more efficiently depleted of Foxp3+ cells produced similar results and also induced colitis, confirming the functional depletion of the natural T reg cells. The present work suggests that in the absence of Foxp3+ cells, a mechanism of suppression was removed that promoted expansion of antigen-induced CD4+ T cells regardless of effector type, including IL-10–producing Th1 and Th2 cells, so that on balance there were more disease-exacerbating T cells in the inflammatory site. The effects on Th2 cytokines were especially interesting as early, albeit transient, Th2 responses have been observed in L. major–infected, resistant mice (37, 38). The data suggest a role for natural T reg cells in extinguishing this early response in intact mice. Similar effects of CD25+ T reg cell depletions at the time of challenge in intact mice have been reported in other nonhealing models of cutaneous leishmanaisis, including L. amazonensis–infected C57BL/6 mice (39) and L. major–infected BALB/c mice (40, 41), which in each case resulted in increased IL-4 production and more rapid development of early lesions. Although unregulated Th2 cell development might have contributed to the enhancement of L. major NIH/Sd infection in Rag−/− mice reconstituted with CD4+ T cells depleted of CD25+ or Foxp3+ cells, it should be emphasized that, in intact mice, the IL-10–dependent, nonhealing phenotype develops in the absence of a sustained Th2 response and proceeds normally in C57BL/6 IL-4−/− mice (13). The reconstitution experiments using defined and carefully monitored CD4+ T cell subsets demonstrate that the L. major NIH/Sd strain drives IL-10 production by CD4+ CD25−Foxp3− T cells independent of natural T reg cells, and that this response is necessary and sufficient for the evolution of noncure infections.
Although natural T reg cells appear to modulate the development of the effector response at the outset of infection and play an important role in preventing the complete elimination of L. major in the skin after clinical cure (19), in chronic inflammatory lesions natural T reg cells contend with IL-10–producing Th1 cells as a subordinate component of the homeostatic suppressor response. The relative roles that these CD4+ T cell subsets play in infection outcomes involving healing versus nonhealing strains of L. major is consistent with the functions postulated for natural and antigen-inducible T reg cell subsets within different immunologic settings involving high- versus low-level inflammation, respectively (23, 42).
What is the relationship of the IL-10–producing Th1 cells that arise in response to L. major NIH/Sd infection to other antigen-inducible T reg cell populations that have been described in mouse and human infections involving, for example, B. pertusis, H. hepaticus, Onchocerca volvulus, Plasmodium falciparum, and M. tuberculosis (24–27, 43)? In particular, what is their relationship to Tr1 cells that are often cited as the relevant model of extrathymically induced, IL-10–producing CD4+ T cells? Tr1 cells are generated under tolerizing conditions, requiring high concentrations of IL-10, immunosuppressive drugs, and/or immature DCs for their differentiation (44–47). In contrast, the L. major–induced, IL-10–producing CD4+ T cells appear to be generated under conditions that drive strong Th1 responses, and their generation after transfer of naive CD4+ T cells into Rag−/− × IL-10−/− mice provides direct evidence that they are not dependent on innate sources of IL-10 for their differentiation or suppressive function. More importantly, the cytokine profiles of the cells are clearly distinct, as the majority of the L. major–induced, IL-10–producing CD4+ T cells also produced IFN-γ, whereas Tr1 cells, particularly in the mouse, produce high levels of IL-10 with or without TGF-β, but little or no IFN-γ (for review see reference 48). Although IL-10 single producers were found to accumulate in the chronic lesions of intact mice, the reconstitution experiments revealed that when natural T reg cells were eliminated as a source of IL-10, the vast majority of the IL-10–producing CD4+CD25−Foxp3− T cells also produced IFN-γ.
The production of IL-10 from T cells participating in a Th1 response has been a common finding in patients with chronic infections (for review see reference 49). They have been observed, for example, in CD4+ T cell clones from bronchoalveolar lavage of active pulmonary tuberculosis patients, T cell lines from patients with Lyme disease and peripheral blood CD4+ T cells from patients with acute P. falciparum malaria (50–52). In mice, a high frequency of Th1 cells generated in response to immunization with killed Brucella abortus– or T. gondii–irradiated tachyzoites, or infection with influenza virus, were found to coexpress IL-10 (53–55). It is possible that the antigen-specific, IL-10–producing CD4+ T cells described in other chronic infections (for review see reference 22) might, on closer examination, be found to simultaneously produce IFN-γ. The fact that IL-12 instructs both IFN-γ and IL-10 secretion by anti-CD3– or PHA–activated human T cells (56, 57) suggests that Th1 responses undergo a normal autoregulatory program of development. In recent experiments, IL-12 induction of IFN-γ was shown to provide an obligate signal for local reactivation of IL-10 production by effector Th1 cells in T. gondii–infected mice, providing further evidence that conditions promoting Th1 cell immunity also establish a negative feedback loop to minimize tissue injury in the inflammatory site (58). Recent experiments indicate that an IL-10 secretion by IFN-γ+T-bet+Foxp3− cells recovered from T. gondii–infected mice is less stable and requires more sustained activation compared with their production of IFN-γ, and have clearly established the duality of purpose of these cells for maintaining strong antimicrobial immunity and limiting collateral damage in the host (see Jankovic et al. [59] on p. 273 of this issue).
Our studies are the first, so far as we are aware, to formally demonstrate a role for IL-10–producing Th1 cells in the pathogenesis of chronic infection and suggest a similar role for these cells in the chronic human infections for which T cells producing both IFN-γ and IL-10 have been described. In addition, the observations may be relevant to other noncuring forms of leishmaniasis, in particular visceral disease, for which IL-10 production by CD25−Foxp3−CD4+ T cells in spleens from L. donovani–infected mice (60), and in splenic aspirates from patients with active disease (Nylen, S., personal communication), has recently been described. The results begin to inform the paradoxical observations previously reported that the nonhealing L. major strain induces an earlier and stronger Th1 response than a healing strain (13). It remains to be determined how the L. major NIH/Sd strain survives the initial onslaught of activating cytokines. The appearance of the IFN-γ+IL-10+ Foxp3−CD4+ T cells in the inoculation site as early as 3 wk after infection might suggests that the sequential commitment of Th1 cells to local IL-10 secretion (61) is sufficiently contracted, so as to prevent effective parasite elimination before the onset of homeostatic control.
MATERIALS AND METHODS
Mice and reagents.
The following mice were used in this study and were purchased from Taconic Farms: C57BL/6J, C57BL/10SgSnAi, B6.SJL Cd45a(Ly5a)/NAi, C57BL/10SgSnAi-[ko]IL10N10, C57BL/10SgSnAi-[ko]RAG2, and C57BL/10SgSnAi-[ko]IL10-[ko]RAG2N12. Foxp3-GFP knock-in mice were generated by Bettelli et al. (33) and were kindly supplied to us by Y. Belkaid (National Institutes of Health, Bethesda, MD). All mice were maintained in the NIAID animal care facility under specific pathogen-free conditions and were used under a study protocol approved by the NIAID Animal Care and Use Committee.
Parasite preparation and intradermal inoculation.
L. major strain NIH/Sd (MHOM/SN/74/SD) was cultured in medium 199 with 20% heat-inactivated FCS (Gemini Bio-Products), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 40 mM Hepes, 0.1 mM adenine (in 50 mM Hepes), 5 mg/ml hemin (in 50% triethanolamine), and 1 mg/ml 6-biotin (M199/S). Infective-stage metacyclic promastigotes of L. major were isolated from stationary cultures by density gradient centrifugation, as previously described (62). 1,000 metacyclic promastigotes were inoculated into the ear dermis using a 30-gauge needle in a volume of ∼5–10 μl. The development of the lesion was monitored by measuring the diameter of the ear lesion with a direct-reading vernier caliper (Thomas Scientific).
Processing of ear tissue and estimation of parasite load.
Parasite titrations were performed as previously described (37). The two sheets of infected ear dermis were separated, deposited in DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.2 mg/ml Liberase CI purified enzyme blend (Roche Diagnostics Corp.), and incubated for 2 h at 37°C. Digested tissue was placed in a grinder and processed in a tissue homogenizer (Medimachine; Becton Dickenson). Tissue homogenates were filtered through a 70-μm cell strainer (Falcon Products) and serially diluted in 96-well flat-bottom microtiter plates containing biphasic medium prepared using 50 μl of NNN medium containing 30% defibrinated rabbit blood overlaid with 50 μl M199/S. The number of viable parasites in each sample was determined from the highest dilution at which promastigotes could be detected after 7–14 d of incubation at 25°C.
Restimulation of leukocytes for cytokine analysis.
To characterize leukocytes in the inoculation site, the ears were collected, and the ventral and dorsal dermal sheets were prepared as described in the previous section. After preparation, cells were analyzed for surface phenotype by flow cytometry. For in vitro restimulation, unfractionated cervical draining lymph node cells were incubated for 72 h at 37°C, 5% CO2 at a concentration of 2 × 106 cells in 200 μl RPMI 1640 containing 10% FCS, 10 mM Hepes, l-glutamine, and penicillin/streptomycin in round-bottom 96-well plates in the presence of 2 × 105 bone marrow–derived DCs (BMDCs) with or without 50 μg/ml of freeze-thaw Leishmania antigen prepared from NIH/Sd stationary phase promastigotes. For restimulation of dermal lymphocytes, cells were stimulated with BMDCs or amastigote-infected BMDCs overnight, with monensin (Golgistop; BD Biosciences) added during the last 4 h. For measurement of intracellular IL-10, dermal leukocytes were incubated with 10 ng/ml PMA and 500 ng/ml ionomycin for 4 h in the presence of monensin (Golgistop). The cells were analyzed for surface markers and intracytoplasmic staining for cytokines.
Immunolabeling and flow cytometry.
The following antibodies used for immunophenotyping were purchased from BD Biosciences: APC anti–mouse TCRβ chain (H57-597), PE-Cy5 anti–mouse CD4(L3T4) (RM4-5), PE or APC anti–mouse CD25(PC61), FITC anti–mouse CD103(M290), FITC anti–mouse IFN-γ (XMG-1.2), and PE anti–mouse IL-10 (JES5-16E3). Foxp3 staining was performed using eBioscience reagents according to manufacturer's protocol. The isotype controls used (all obtained from BD Biosciences) were rat IgG2b (A95-1), rat IgG2a (R35-95), and hamster IgG, group 2 (Ha4/8). Before staining, lymph node or dermal cells were incubated with an anti-Fc III/II receptor mAb (2-4G.1) in PBS containing 0.1% BSA and 0.01% NaN3. The staining of surface and intracytoplasmic markers was performed sequentially: the cells were stained first for their surface markers, followed by a permeabilization step and staining for IFN-γ and IL-10. For each sample, 250,000 cells were acquired for analysis. The data were collected and analyzed using CellQuest Pro software (BD Biosciences) and a flow cytometer (FACSCalibur; BD Biosciences). The lymphocytes from ear cells were identified by characteristic size (forward light scatter) and granularity (side light scatter) and by lymphocyte surface phenotype.
Cell purification for adoptive transfers.
Whole CD4+ T cells were purified from spleens and lymph nodes of mice by negative selection (Miltenyi Biotec). CD4+CD25+ and CD4+CD25− subsets were sort purified using a sorter (FACSVantage; BD Biosciences), after which >99.9% were pure. Foxp3 expression was measured on sorted cells by flow cytometry and real-time PCR to confirm natural T reg cell and naive cell populations, respectively. For adoptive transfer, recipients received 2 × 106 whole CD4+ cells intravenously or, where indicated in the figures, 2 × 106 CD4+CD25− cells with or without 2 × 105 CD4+CD25+ cells. In some cases, splenocytes were depleted of erythrocytes with ACK lysis buffer (Cambrex) and used for adoptive transfer into RAG−/− recipients. For Foxp3-GFP knock-in mice, purified CD4+ T cells were sorted into GFP− and GFP+ fractions and transferred intravenously into recipient mice in the same relative numbers.
Real-time PCR.
For analysis of gene expression, ears were removed and immediately placed in RNAlater (QIAGEN). Ear tissue was mechanically disrupted using liquid nitrogen and a mortar and pestle. Homogenates were passed through Qiashredder columns, and RNA was purified using an RNAeasy mini kit (QIAGEN), according to the manufacturer's protocol. Reverse transcription was performed using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). Real-time PCR was performed on a sequence detection system (ABI Prism 7900; Applied Biosystems). PCR primer probe sets were predeveloped gene expression assays designed by Applied Biosystems, and the quantities of products were determined by the comparative threshold cycle method using the equation 2−ΔΔCt to determine the fold increase in product. Each gene of interest was normalized to the 18S rRNA endogenous control, and the fold change in expression is displayed as relative to naive controls.
Statistical analysis.
Statistical significance between groups was determined by the unpaired, two-tailed student's t test using Prism software (GraphPad).
Online supplemental material.
Fig. S1 shows parasite burdens in recipient mice of whole splenocytes. Fig. S2 uses the congenic marker CD45.1 to follow the fate of transferred CD25+ cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061886/DC1.
Supplemental Material
Acknowledgments
We thank Kim Beacht for expert technical assistance, Yasmine Belkaid for helpful discussions and for providing the Foxp3-GFP knock-in mice, and Dragana Jankovic and Alan Sher for critical review of the manuscript.
The authors have no conflicting financial interests.
Abbreviation used: BMDC, bone marrow–derived DC.
References
- 1.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845–858. [DOI] [PubMed] [Google Scholar]
- 2.Melby, P.C., F.J. Andrade-Narvaez, B.J. Darnell, G. Valencia-Pacheco, V.V. Tryon, and A. Palomo-Cetina. 1994. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 62:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louzir, H., P.C. Melby, A. Ben Salah, H. Marrakchi, K. Aoun, R. Ben Ismail, and K. Dellagi. 1998. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J. Infect. Dis. 177:1687–1695. [DOI] [PubMed] [Google Scholar]
- 4.Gasim, S., A.M. Elhassan, E.A. Khalil, A. Ismail, A.M. Kadaru, A. Kharazmi, and T.G. Theander. 1998. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin. Exp. Immunol. 111:64–69. (published erratum appears in Clin. Exp. Immunol. 1998. 112:547) [DOI] [PMC free article] [PubMed]
- 5.Ismail, A., A.M. El Hassan, K. Kemp, S. Gasim, A.E. Kadaru, T. Moller, A. Kharazmi, and T.G. Theander. 1999. Immunopathology of post kala-azar dermal leishmaniasis (PKDL): T-cell phenotypes and cytokine profile. J. Pathol. 189:615–622. [DOI] [PubMed] [Google Scholar]
- 6.Ghalib, H.W., M.R. Piuvezam, Y.A. Skeiky, M. Siddig, F.A. Hashim, A.M. el-Hassan, D.M. Russo, and S.G. Reed. 1993. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J. Clin. Invest. 92:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karp, C.L., S.H. el-Safi, T.A. Wynn, M.M. Satti, A.M. Kordofani, F.A. Hashim, M. Hag-Ali, F.A. Neva, T.B. Nutman, and D.L. Sacks. 1993. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J. Clin. Invest. 91:1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenney, R.T., D.L. Sacks, A.A. Gam, H.W. Murray, and S. Sundar. 1998. Splenic cytokine responses in Indian kala-azar before and after treatment. J. Infect. Dis. 177:815–818. [DOI] [PubMed] [Google Scholar]
- 9.Murray, H.W., C.M. Lu, S. Mauze, S. Freeman, A.L. Moreira, G. Kaplan, and R.L. Coffman. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 70:6284–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy, M.L., U. Wille, E.N. Villegas, C.A. Hunter, and J.P. Farrell. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31:2848–2856. [DOI] [PubMed] [Google Scholar]
- 11.Buxbaum, L.U., and P. Scott. 2005. Interleukin 10- and Fcgamma receptor-deficient mice resolve Leishmania mexicana lesions. Infect. Immun. 73:2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noben-Trauth, N., R. Lira, H. Nagase, W.E. Paul, and D.L. Sacks. 2003. The relative contribution of IL-4 receptor signaling and IL-10 to susceptibility to Leishmania major. J. Immunol. 170:5152–5158. [DOI] [PubMed] [Google Scholar]
- 13.Anderson, C.F., S. Mendez, and D.L. Sacks. 2005. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 174:2934–2941. [DOI] [PubMed] [Google Scholar]
- 14.Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. [DOI] [PubMed] [Google Scholar]
- 15.Kane, M.M., and D.M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141–1147. [DOI] [PubMed] [Google Scholar]
- 16.Miles, S.A., S.M. Conrad, R.G. Alves, S.M. Jeronimo, and D.M. Mosser. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 201:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groux, H., F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M.G. Roncarolo, and R.L. Coffman. 1999. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162:1723–1729. [PubMed] [Google Scholar]
- 18.Stager, S., A. Maroof, S. Zubairi, S.L. Sanos, M. Kopf, and P.M. Kaye. 2006. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10(+) CD4(+) T cells. Eur. J. Immunol. 36:1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 20.Belkaid, Y., and B.T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353–360. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. [DOI] [PubMed] [Google Scholar]
- 22.Mills, K.H., and P. McGuirk. 2004. Antigen-specific regulatory T cells–their induction and role in infection. Semin. Immunol. 16:107–117. [DOI] [PubMed] [Google Scholar]
- 23.O'Garra, A., P.L. Vieira, P. Vieira, and A.E. Goldfeld. 2004. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J. Clin. Invest. 114:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kullberg, M.C., D. Jankovic, P.L. Gorelick, P. Caspar, J.J. Letterio, A.W. Cheever, and A. Sher. 2002. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus–induced colitis. J. Exp. Med. 196:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuirk, P., C. McCann, and K.H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boussiotis, V.A., E.Y. Tsai, E.J. Yunis, S. Thim, J.C. Delgado, C.C. Dascher, A. Berezovskaya, D. Rousset, J.M. Reynes, and A.E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Invest. 105:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoguina, J., M. Mempel, J. Larbi, M. Badusche, C. Loliger, O. Adjei, G. Gachelin, B. Fleischer, and A. Hoerauf. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291–1300. [DOI] [PubMed] [Google Scholar]
- 28.Noble, A., A. Giorgini, and J.A. Leggat. 2006. Cytokine-induced IL-10- secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 107:4475–4483. [DOI] [PubMed] [Google Scholar]
- 29.Suffia, I., S.K. Reckling, G. Salay, and Y. Belkaid. 2005. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 174:5444–5455. [DOI] [PubMed] [Google Scholar]
- 30.Padigel, U.M., and J.P. Farrell. 2005. Control of infection with Leishmania major in susceptible BALB/c mice lacking the common gamma-chain for FcR is associated with reduced production of IL-10 and TGF-beta by parasitized cells. J. Immunol. 174:6340–6345. [DOI] [PubMed] [Google Scholar]
- 31.Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 32.Zelenay, S., T. Lopes-Carvalho, I. Caramalho, M.F. Moraes-Fontes, M. Rebelo, and J. Demengeot. 2005. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc. Natl. Acad. Sci. USA. 102:4091–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 34.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, H., B. Hu, D. Xu, and F.Y. Liew. 2003. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J. Immunol. 171:5012–5017. [DOI] [PubMed] [Google Scholar]
- 36.Sacks, D., and C. Anderson. 2004. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol. Rev. 201:225–238. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977. [DOI] [PubMed] [Google Scholar]
- 38.Reiner, S.L., S. Zheng, Z.E. Wang, L. Stowring, and R.M. Locksley. 1994. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 179:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji, J., J. Masterson, J. Sun, and L. Soong. 2005. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J. Immunol. 174:7147–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aseffa, A., A. Gumy, P. Launois, H.R. MacDonald, J.A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 169:3232–3241. [DOI] [PubMed] [Google Scholar]
- 41.Xu, D., H. Liu, M. Komai-Koma, C. Campbell, C. McSharry, J. Alexander, and F.Y. Liew. 2003. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 170:394–399. [DOI] [PubMed] [Google Scholar]
- 42.Bluestone, J.A., and A.K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253–257. [DOI] [PubMed] [Google Scholar]
- 43.Plebanski, M., K.L. Flanagan, E.A. Lee, W.H. Reece, K. Hart, C. Gelder, G. Gillespie, M. Pinder, and A.V. Hill. 1999. Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity. 10:651–660. [DOI] [PubMed] [Google Scholar]
- 44.Barrat, F.J., D.J. Cua, A. Boonstra, D.F. Richards, C. Crain, H.F. Savelkoul, R. de Waal-Malefyt, R.L. Coffman, C.M. Hawrylowicz, and A. O'Garra. 2002. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)– and Th2-inducing cytokines. J. Exp. Med. 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen- specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 46.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levings, M.K., S. Gregori, E. Tresoldi, S. Cazzaniga, C. Bonini, and M.G. Roncarolo. 2005. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 105:1162–1169. [DOI] [PubMed] [Google Scholar]
- 48.Battaglia, M., S. Gregori, R. Bacchetta, and M.G. Roncarolo. 2006. Tr1 cells: from discovery to their clinical application. Semin. Immunol. 18:120–127. [DOI] [PubMed] [Google Scholar]
- 49.Trinchieri, G. 2001. Regulatory role of T cells producing both interferon γ and interleukin 10 in persistent infection. J. Exp. Med. 194:F53–F57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerosa, F., C. Nisii, S. Righetti, R. Micciolo, M. Marchesini, A. Cazzadori, and G. Trinchieri. 1999. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 92:224–234. [DOI] [PubMed] [Google Scholar]
- 51.Pohl-Koppe, A., K.E. Balashov, A.C. Steere, E.L. Logigian, and D.A. Hafler. 1998. Identification of a T cell subset capable of both IFN-gamma and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 160:1804–1810. [PubMed] [Google Scholar]
- 52.Winkler, S., M. Willheim, K. Baier, D. Schmid, A. Aichelburg, W. Graninger, and P.G. Kremsner. 1998. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect. Immun. 66:6040–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 54.Sarawar, S.R., and P.C. Doherty. 1994. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J. Virol. 68:3112–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svetic, A., Y.C. Jian, P. Lu, F.D. Finkelman, and W.C. Gause. 1993. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-gamma in CD4+ T cells. Int. Immunol. 5:877–883. [DOI] [PubMed] [Google Scholar]
- 56.Gerosa, F., C. Paganin, D. Peritt, F. Paiola, M.T. Scupoli, M. Aste-Amezaga, I. Frank, and G. Trinchieri. 1996. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 183:2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyaard, L., E. Hovenkamp, S.A. Otto, and F. Miedema. 1996. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J. Immunol. 156:2776–2782. [PubMed] [Google Scholar]
- 58.Shaw, M.H., G.J. Freeman, M.F. Scott, B.A. Fox, D.J. Bzik, Y. Belkaid, and G.S. Yap. 2006. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J. Immunol. 176:7263–7271. [DOI] [PubMed] [Google Scholar]
- 59.Jankovic, D., M.C. Kullberg, C.G. Feng, R.S. Goldszmid, C.M. Collazo, M. Wilson, T.A. Wynn, M. Kamanaka, R.A. Flavell, and A. Sher. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stager, S., A. Maroof, S. Zubairi, S.L. Sanos, M. Kopf, and P.M. Kaye. 2006. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. 36:1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Assenmacher, M., M. Lohning, A. Scheffold, R.A. Manz, J. Schmitz, and A. Radbruch. 1998. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur. J. Immunol. 28:1534–1543. [DOI] [PubMed] [Google Scholar]
- 62.Spath, G.F., and S.M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.