Abstract
Although interferon γ (IFN-γ) secretion is essential for control of most intracellular pathogens, host survival often also depends on the expression of interleukin 10 (IL-10), a cytokine known to counteract IFN-γ effector functions. We analyzed the source of regulatory IL-10 in mice infected with the protozoan parasite Toxoplasma gondii. Unexpectedly, IFN-γ–secreting T-bet+Foxp3− T helper type 1 (Th1) cells were found to be the major producers of IL-10 in these animals. Further analysis revealed that the same IL-10+IFN-γγ population displayed potent effector function against the parasite while, paradoxically, also inducing profound suppression of IL-12 production by antigen-presenting cells. Although at any given time point only a fraction of the cells appeared to simultaneously produce IL-10 and IFN-γ, IL-10 production could be stimulated in IL-10−IFN-γ+ cells by further activation in vitro. In addition, experiments with T. gondii–specific IL-10+IFN-γ+ CD4 clones revealed that although IFN-γ expression is imprinted and triggered with similar kinetics regardless of the state of Th1 cell activation, IL-10 secretion is induced more rapidly from recently activated than from resting cells. These findings indicate that IL-10 production by CD4+ T lymphocytes need not involve a distinct regulatory Th cell subset but can be generated in Th1 cells as part of the effector response to intracellular pathogens.
In addition to their effector functions, CD4 T lymphocytes play a major role in regulating the magnitude of both innate and adaptive immune responses. This regulatory activity has been traditionally attributed to distinct CD4+ T cells subsets. Thus, Th1 cells are known to down-modulate Th2 cell function through their production of IFN-γ, whereas IL-10 secretion by Th2 cells governs the extent of Th1 responses (1–3). In addition to this cross-regulation between Th cell subsets, a second, more recently discovered form of CD4-dependent suppression is that mediated by CD25+Foxp3+ T reg cells, which are both naturally occurring and/or induced as a consequence of antigen (Ag) stimulation (4–6).
CD4+ T cell–dependent regulation plays a major function in determining the outcome of infection. In addition to governing effector responses against pathogens themselves, CD4 T lymphocytes serve a critical role in limiting these responses so that they do not become detrimental to the host. A key cytokine involved in both activities is IL-10. This mediator potently down-regulates APC functions (7–9) and is produced by Th2 lymphocytes (2), Tr1 cells (10), CD25+ (11, 12) and CD25− (13, 14) T reg cells, and Th1 cells (15), as well as B lymphocytes (16) and APCs themselves (17, 18). Consistent with the known suppression of Th1 effector responses by IL-10, mice deficient in this cytokine have been shown to be more resistant to several different intracellular pathogens (19–21). However, in the case of several infectious agents, IL-10 KO animals actually display increased susceptibility (22–25).
An important example of the latter phenomenon occurs in IL-10–deficient mice infected with Toxoplasma gondii (22), an intracellular protozoan parasite that in IL-10–sufficient animals triggers a potent Th1 response, which rapidly shuts down parasite growth. When infected with T. gondii, IL-10 KO mice undergo acute mortality, which is paradoxically accompanied by enhanced parasite clearance. Instead, the death of these animals appears to stem from uncontrolled production of proinflammatory cytokines and IFN-γ, leading to severe tissue pathology. Importantly, CD4+ T cells have been shown to play a critical role in the fatal outcome of T. gondii infection in IL-10 KO mice. Thus, their mortality can be prevented by treatments that decrease the magnitude of the CD4 T cell response, such as administration of anti-CD4 mAb (22), simultaneous blockade of CD28/CD80 and CD40/CD40L (26), or blockade of CD28/CD80 with concurrent IL-12 neutralization (27).
Interestingly, in addition to being the major mediators of immunopathology in T. gondii–exposed IL-10 KO mice, CD4 T lymphocytes appear to be the major source of the cytokine responsible for the prevention of disease in infected IL-10–sufficient animals. Thus, mice with a targeted deletion of IL-10 in CD4+ T cells display the same phenotype as conventional IL-10 KO animals after T. gondii infection (28). Because Th2 cells do not arise in WT mice exposed to T. gondii under the conditions of systemic inoculation used in these experiments (29), this finding suggested that the IL-10 that protects against the immunopathologic consequences of toxoplasma infection is derived from either T reg or Th1 CD4+ T cells.
In the current study, we have formally investigated the source of the CD4 T lymphocyte–derived IL-10 that prevents T. gondii–induced acute mortality. Unexpectedly, we found that essentially all of the cytokine derives from conventional T-bet+Foxp3− Th1 lymphocytes, the same cell population that displays effector function against the parasite. Importantly, IL-10 expression by these cells is not a stable property and is preferentially induced after recent Ag exposure. Collectively, our findings indicate that Th1 lymphocytes themselves are a critical source of biologically active IL-10 and do not represent a specialized T cell subset endowed with regulatory function.
RESULTS
IL-10 signaling is required for host survival during both the acute and chronic phases of T. gondii infection
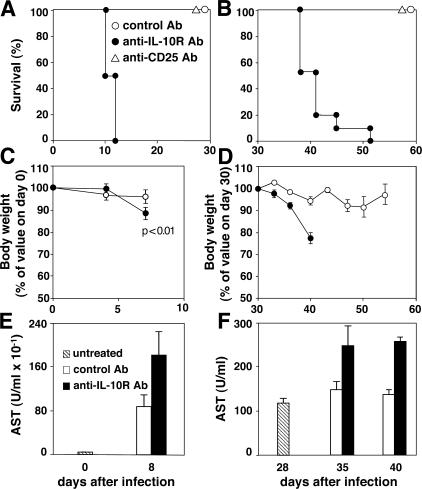
In contrast to WT animals, IL-10 KO mice infected with avirulent strains of T. gondii display acute tissue pathology and mortality associated with overproduction of proinflammatory cytokines (22, 26, 27, 30). To determine whether this phenotype results solely from the absence of active IL-10–mediated suppression as opposed to indirect developmental effects caused by the lack of this cytokine gene, we first asked if IL-10–dependent signaling is also necessary for survival of infected WT animals and, if yes, whether this requirement additionally extends to chronically infected hosts. To do so, we treated WT mice beginning either 2 d before or 28 d after infection with a blocking anti–IL-10R mAb and monitored their survival. In contrast to animals injected with control mAb, which survived for the entire period of the experiment, mice treated with anti–IL-10R mAb at the time of infection rapidly succumbed, recapitulating the phenotype originally observed in infected IL-10 KO animals (Fig. 1 A). The same kinetics of mortality were observed when mice were treated with anti–IL-10R mAb during the chronic phase, demonstrating a protective role of the cytokine once latent infection has been established (Fig. 1 B). In both situations, the decreased survival of the anti–IL-10R mAb-treated mice was preceded by significant weight loss (Fig. 1, C and D) and hepatic dysfunction as indicated by increased levels of serum aspartate aminotransferase (AST; Fig. 1, E and F), consistent with previous observations in infected IL-10 KO animals (22).
Figure 1.
IL-10R signaling is required for host survival during both acute and chronic infection with an avirulent strain of T. gondii. WT mice inoculated i.p. with 20 ME-49 cysts were treated with control (open circles; n = 10), anti–IL-10R (closed circles; n = 10), or anti-CD25 (open triangles; n = 10) mAbs either at days −2 and +2 (A, C, and E) or at days +28, 30, and 33 (B, D, and F). Survival (A and B) and body weight (C and D) of the infected mice was then monitored. In addition, levels of hepatic ASTs (E and F) were measured in sera of individual mice before (hatched bars) or after treatment with the control (open bars) or anti–IL-10R (shaded bars) mAbs. The results shown represent the mean ± SD for each group. Comparable results were obtained in two additional experiments.
In the same experiments, parallel groups of animals were injected with depleting anti-CD25 mAb to assess the possible contribution of naturally occurring CD25+ T reg cells as a source of IL-10. The resulting CD25 depletion failed to affect host survival when initiated either during acute or chronic infection (Fig. 1, A and B).
CD4+ T cells are a major source of host-protective IL-10 and negatively regulate IL-12 production in acutely infected mice
As a consequence of T. gondii exposure, serum levels of IL-12p40 and IFN-γ transiently increase and peak on day 7 after infection (21). Treatment with anti–IL-10R, but not anti-CD25 (not depicted), mAb resulted in further elevation of these two cytokines (Fig. 2). Anti–IL-10R mAb administration also dramatically augmented levels of IL-10, probably as a consequence of blockade of cytokine consumption. To assess the contribution of CD4 T lymphocytes in the observed serum cytokine responses, groups of infected mice were treated in parallel with either anti–IL-10R plus anti-CD4 mAb or anti-CD4 mAb alone. Simultaneous depletion of CD4+ T cells completely abrogated the increases in IFN-γ and IL-10 resulting from anti–IL-10R mAb treatment, suggesting that Th lymphocytes serve as a major source of these two cytokines. In contrast, ablation of CD4+ T lymphocytes caused only a minor decrease in serum IL-12 levels in the same anti–IL-10R mAb–treated animals. Unexpectedly, anti-CD4 mAb depletion induced a sharp increase in IL-12 in infected mice in the absence of concurrent anti–IL-10R mAb administration (Fig. 2). The latter findings suggested that during T. gondii infection CD4+ T cells suppress rather than promote IL-12 production.
Figure 2.
Serum cytokine levels in T. gondii–infected mice treated with anti–IL-10R mAb in the presence or absence of concurrent CD4 T cell depletion. Uninfected or infected mice treated on days −2 and +2 relative to the time of infection with control mAb, anti–IL-10R mAb, anti–IL-10R plus anti-CD4 mAb, or anti-CD4 mAb only were bled on day 8 after infection. Serum concentrations of IL-12, IFN-γ, and IL-10 were measured by ELISA. Bars represent the mean ± SEM of the cytokine levels in three to five mice per group from one representative of two experiments performed. *, P < 0.05; **, P < 0.01; NS, P = 0.2.
To directly test this hypothesis, we reconstituted RAG × IL-10 double KO mice with either WT or IL-10–deficient CD4+ T cells from naive donors and infected them with T. gondii 7 d later. When serum cytokine levels were measured 1 wk later, the recipients of WT, but not IL-10 KO, CD4+ T cells showed decreased IL-12 levels relative to the nonreconstituted controls animals (Fig. 3 A). Although the WT CD4+ T cell–reconstituted mice displayed significantly increased IFN-γ production relative to nonreconstituted animals, the levels observed were threefold lower than those measured in the recipients of IL-10 KO CD4+ T cells, probably as a consequence of the decreased IL-12 production in the former mice. The uncontrolled IFN-γ production observed in the RAG × IL-10 KO mice reconstituted with IL-10 KO CD4+ cells correlated with a reduction in survival relative to the infected nonreconstituted animals (Fig. 3 B). In contrast, RAG × IL-10 KO recipients of WT CD4+ T cells were protected against T. gondii–induced lethality.
Figure 3.
Transfer of IL-10+/+, but not IL-10−/−, CD4+ cells down-regulates IL-12 production in vivo and promotes survival of T. gondii–infected RAG × IL-10 KO mice. 15 × 106 MACS-purified CD4 cells from either WT or IL-10 KO naive mice were adoptively transferred into RAG × IL-10 KO recipients. 7 d later, reconstituted (n = 5) and nonreconstituted (n = 4) RAG × IL-10 KO animals were inoculated i.p. with 20 pepsin-treated ME-49 cysts. (A) Mice were bled on day 8 after infection, and serum concentrations of IL-12 and IFN-γ were measured by ELISA. (B) Cumulative survival of each group of animals. The data shown are the pooled results from two independent experiments. *, P < 0.002; **, P < 0.001; NS, P = 0.3.
The IL-10–producing CD4+ T cells in T. gondii infection simultaneously secrete IFN-γ and are CD25−/Foxp3−
To further analyze the dynamics and cellular basis of IFN-γ and IL-10 secretion during T. gondii infection, splenocytes were restimulated with soluble tachyzoite Ag (STAg) at different time points after parasite inoculation, and both cytokines were measured in culture supernatants 72 h later. IFN-γ and IL-10 were detected together at each time point examined and appeared with similar kinetics during the course of infection (Fig. 4 A).
Figure 4.
Phenotypic analysis of IL-10– and IFN-γ–producing CD4+ T lymphocytes derived from mice chronically infected with T. gondii. Mice were inoculated with T. gondii cysts, and spleen cells were recovered at different time points after infection. (A) IFN-γ and IL-10 production by bulk splenocytes 72 h after restimulation with STAg. The values shown are the means ± SD of the cytokine concentration in culture supernatants from three mice per group. (B) Proliferative and IFN-γ/IL-10 responses of FACS-sorted CD4+ T lymphocytes from week 6–infected mice. Total CD4+ T cells or the indicated cell subpopulations were purified and restimulated with STAg in the presence of irradiated APCs, and thymidine incorporation and cytokine production were measured at 48 and 72 h, respectively. The values shown are the means ± SD of duplicate assays performed on cells pooled from three donors. (C) IL-10 and IFN-γ intracellular staining profiles of the same total CD4+ and CD4+CD25− subpopulations shown in B after additional restimulation with PMA/ionomycin. The dot plots shown were gated on CD4+ cells. The results shown in each panel are representative of three experiments performed.
To formally identify the cells secreting the two cytokines, we FACS-purified total CD4+, CD4+CD25+, CD4+CD25−, CD4+CD25−CD45RBhigh, and CD4+CD25−CD45RBlow populations from spleens of week 6–infected animals and analyzed their proliferative responses to STAg, as well as their secretion of IFN-γ and IL-10. In terms of all three parameters, the entire CD4 T cell response was contained in the CD25− subpopulation. The CD25+ fraction at this stage of infection was found to consist largely of Foxp3+ CD4 T lymphocytes (not depicted) and is likely to represent parasite Ag nonresponsive, naturally occurring T reg cells. Within the CD25− population, responsiveness was detected only in the Ag-experienced CD25−CD45RBlow CD4 T lymphocyte fraction (Fig. 4 B). We next performed intracellular cytokine staining (ICS) on these STAg-restimulated CD4 T lymphocyte cultures. Unexpectedly, only a minor percentage of the IL-10–producing cells were found to secrete this cytokine alone. Instead, IL-10+IFN-γ+ double-positive cells represented the major IL-10+ population present, constituting on average 15–20% of the total IFN-γ+ CD4+ T cells in the cultures examined (Fig. 4 C).
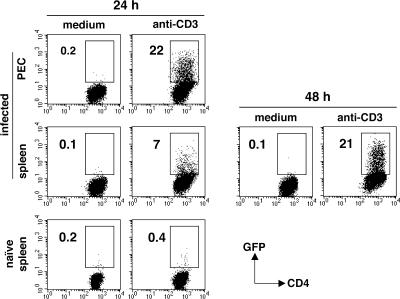
The above experiments involved cytokine measurements on lymphocytes restimulated in vitro with parasite Ag. To address whether IL-10+IFN-γ+ CD4+ T cells are also the major source of IL-10 in vivo, we performed ICS on CD4 T lymphocytes from acutely infected mice ex vivo, where the frequency of activated CD44highCD62Llow CD4+ T cells exceeds 70 and 50% in the peritoneum and spleen, respectively (unpublished data). As shown in Fig. 5 A, IL-10+ IFN-γ+ double-producing cells represented the dominant IL-10+ population present in peritoneum and spleen at day 7 after infection and constituted on average 10% of the total IFN-γ+ CD4+ T cells. This population was also found to predominate in the splenic CD4 T cell response to a second intracellular protozoan parasite, Trypanosoma cruzi (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20062175/DC1).
Figure 5.
Phenotypic analysis of IL-10– and IFN-γ–producing CD4+ T lymphocytes freshly isolated from mice with acute T. gondii infection. Peritoneal and spleen lymphocytes were recovered from day 7–infected and uninfected control mice. (A) IL-10 and IFN-γ ICS of these populations (pooled from three animals) after restimulation for 5 h with anti-CD3 mAb. The dot plots shown were gated on CD4+ cells and are representative of five experiments performed. (B) Frequency of Foxp3+ or T-bet+ CD4+ T lymphocytes in the spleen and peritoneum of naive versus day 7–infected mice. Bars represent the mean ± SD of assays on the individual animals (n = 3–5). (C) T-bet versus IL-10 expression in peritoneal IFN-γ+ CD4+ T cells recovered from infected mice and restimulated as in A. (D) Frequency of peritoneal IL-10+ CD4+ T lymphocytes within the IFN-γ+T-bet+ and IFN-γ−T-bet− populations in naive versus day 7–infected mice. Bars represent the mean ± SD of assays on the individual animals (n = 3). The data shown in C and D are representative of three experiments performed.
Because during the acute phase of T. gondii infection the CD25 marker, expressed on naturally occurring T reg cells, is also up-regulated on activated Th cell effectors, we analyzed CD4+ T cells for their expression of the transcription factor Foxp3, a specific marker of the former population. In acutely infected mice, Foxp3+CD4 T lymphocytes were barely detectable in the peritoneum, and their frequency in the spleen was actually decreased when compared with naive animals (Fig. 5 B). The absence of a substantial increase in the Foxp3+CD4+ T population after T. gondii infection was confirmed in an experiment using Foxp3gfp reporter mice (not depicted).
IL-10+IFN-γ+ CD4+ T lymphocytes exert both Th1 cell effector and regulatory activity on T. gondii–infected macrophages
The observation that the major IL-10–producing CD4 T lymphocytes in T. gondii–infected mice are also IFN-γ+ suggested that they represent a subpopulation of conventional Th1 lymphocytes. Indeed, T. gondii inoculation was found to result in a dramatic increase in the frequency of T-bet+ CD4+ T cells both in the spleen and peritoneum at day 7 after infection (Fig. 5 B). Importantly, essentially all of the IL-10+ CD4 T lymphocytes present in these preparations were found to be T-bet+ (Fig. 5, C and D), suggesting that they arise from the same precursors as classical Th1 cells.
To determine whether the IL-10+IFN-γ+ CD4 T lymphocyte subpopulation can also express effector functions characteristic of Th1 cells, we tested their ability to control parasite growth when added to T. gondii–infected IL-10–deficient macrophages. To do so, we used IL-10gfp reporter mice, designated as tiger mice (31), in which an internal ribosome entry site GFP element was inserted into the 3′ region of the IL-10 gene, thus allowing us to isolate IL-10–producing cells ex vivo without subsequent manipulation (Fig. 6 A). As shown in Fig. 6 B, the CD4+CD44high GFP+ cells (which secreted IFN-γ and IL-10) were found to stimulate even better control of intracellular parasite proliferation than the CD4+CD44high GFP− fraction (which secreted IFN-γ alone). The restriction of parasite growth induced by either cell population correlated with the level of nitrite (NO) produced in the cultures (Fig. 6 B) and was inhibited by anti–IFN-γ mAb treatment (not depicted).
Figure 6.
Dual effector and regulatory functions of T. gondii–induced IL-10+IFN-γ+ CD4 T lymphocytes. CD4+CD44+ GFP− and CD4+CD44+ GFP+ lymphocytes were purified by FACS from spleens of day 7–infected IL-10 GFP knock-in tiger mice (A) and cultured at the indicated numbers with T. gondii–infected IL-10–deficient peritoneal macrophages. (B) At 24 h, supernatants were removed, [5,6-3H]Uracil was added for an additional 18 h, and isotope incorporation was measured as an index of parasite growth. Levels of IFN-γ and IL-10 were measured in culture supernatants by specific ELISA and NO quantitated by the Griess reaction. (C) In one set of cultures, control or anti–IL-10R mAb was added at the initiation of the experiment, and levels of IL-12p40 were measured in 24-h culture supernatants. Each data point is the mean of measurements performed on duplicate cultures (SD < 10%). The exp eriment shown in B is representative of three performed; the experiment in C is representative of two performed.
Although displaying potent effector function, the IL-10+IFN-γ+ CD4+ subpopulation clearly differed from the IL-10−IFN-γ+ CD4+ fraction in its regulatory activity. Thus, when IL-12 production was measured in the T. gondii–infected IL-10–deficient macrophage cultures, secretion of the former cytokine was dramatically reduced in the presence of GFP/IL-10+ but not GFP/IL-10− CD4 T lymphocytes. This inhibition of IL-12 production was dependent on IL-10R signaling, because it was reversed in the presence of anti–IL-10R mAb (Fig. 6 C).
IL-10+IFN-γ+ CD4+ T cells from infected mice acquire an IL-10+ phenotype after further stimulation in vitro
We next asked whether the IL-10+IFN-γ+ and IL-10−IFN-γ+ CD4 T cells induced by T. gondii infection are discrete, phenotypically stable subsets or represent different steps in the Th1 cell differentiation pathway. To do so, we isolated GFP/IL-10+ and GFP/IL-10− CD4+CD44high lymphocytes from acutely infected tiger mice (as described in Fig. 6 A) and restimulated both populations in vitro with plate-bound anti-CD3 mAb. When measured by FACS, GFP expression was gradually acquired in the cultures initially containing GFP/IL-10− CD4+ T cells, reaching ∼20% of the population by 24 and 48 h for the peritoneal and splenic populations, respectively (Fig. 7). The appearance of GFP+ cells correlated with the induction of IL-10 secretion as measured by ELISA, and parallel analysis of splenic GFP/IL-10− CD4+ T cells by ICS both confirmed the appearance of IL-10+ lymphocytes and demonstrated that these cells coexpress IFN-γ (not depicted). In control experiments, no induction of GFP expression was observed in anti-CD3–stimulated CD4+ cells from uninfected animals, confirming the requirement for in vivo priming (Fig. 7). Furthermore, no changes in GFP expression were observed when the GFP/IL-10+ CD4+CD44high population was subjected to the same in vitro stimulation (not depicted).
Figure 7.
In vitro induction of IL-10 expression in IL-10− CD4+ T cells from infected mice. CD4+CD44+ GFP− lymphocytes were purified by FACS from peritoneum and spleen of day 7–infected IL-10 GFP knock-in tiger mice (n = 2–3) and cultured in medium alone or in the presence of anti-CD3 mAb. Control cultures consisted of anti-CD3–stimulated CD4+ T cells isolated from uninfected tiger mice. Induction of GFP expression was examined at 24 h after initiation of cultures and at 48 h for splenic population from infected animals. The results shown are representative of two experiments performed.
IL-10 production by T. gondii–specific IFN-γ+ CD4+ T cell clones is dependent on their state of activation
To better understand the regulation of IL-10 production in T. gondii Ag-induced Th1 cells, we followed the expression of the cytokine in a panel of established IL-10+IFN-γ+ CD4+ T cell clones derived from mice multiply immunized with STAg (32). Preliminary experiments had shown that IL-10 secretion was higher in cultures freshly restimulated with Ag. Indeed, when kinetic experiments were performed on PMA/ionomycin-stimulated cultures measuring IL-10 expression by ICS, the cytokine was found to be barely detectable in resting clones (28 d after prior Ag/APC stimulation) but was rapidly induced in the same recently activated cells (3 d after Ag/APC restimulation; Fig. 8 A). By day 7, however, IL-10 expression had markedly declined. In contrast, IFN-γ expression was comparable in clones under both resting and reactivated conditions.
Figure 8.
Distinct kinetics of IL-10 and IFN-γ secretion by T. gondii–specific CD4+ T cell clones. IL-10+IFN-γ+ T. gondii Ag-specific clones (D 36, B 71, or Clone 2; 2 × 106 clones/ml) either in a rested state (28 d after last exposure to Ag) or recently activated (3 or 7 d after exposure to Ag/APC) were stimulated with PMA/inonomycin, and their production of IL-10 and IFN-γ was monitored. (A) ICS was performed after 4.5 h of PMA/inonomycin stimulation. The dot plots shown were gated on CD4+ T cells. (B) Levels of IL-10 and IFN-γ were measured in the culture supernatants at 6, 12, 24, and 48 h after PMA/inonomycin stimulation. No further increase in either cytokine was detected at 72 h. The amount of cytokine detected at each time point is expressed as the percentage of the amount detected at the 48-h plateau time point (resting cells, IFN-γ = 211 ± 9.6 ng/ml and IL-10 = 22.6 ± 0.6 ng/ml; reactivated cells, IFN-γ = 911 ± 17.9 ng/ml and IL-10 = 115.4 ± 0.9 ng/ml). The results shown in each panel are representative of two experiments performed.
To further examine the kinetics of IL-10 secretion in resting versus activated CD4+ T clones, we followed the accumulation of both IL-10 and IFN-γ in PMA and ionomycin-stimulated cultures containing either resting or recently activated (day 3) cell populations. In agreement with the ICS data from Fig. 8 A, IL-10 production was accelerated in the activated cells, reaching maximum levels by 6 h (Fig. 8 B). In sharp contrast, IL-10 secretion by resting cells was delayed, requiring a full 48 h to achieve peak values. Interestingly, no difference between resting and activated cells was observed when the kinetics of IFN-γ production were analyzed in the same cultures. These findings suggested that the capacity of Th1 lymphocytes to synthesize IL-10 is dependent on their prior state of activation. In contrast, IFN-γ inducibility appears to be a stable property of this CD4 subset.
DISCUSSION
In the present study, we show that the IL-10 that prevents immunopatholgy and mortality in mice infected with T. gondii derives from conventional Th1 cells that acquire the ability to produce this cytokine as a consequence of the normal immune response to the parasite. Interestingly, the CD4 T lymphocytes that provide this regulatory function can simultaneously serve as effector cells for NO-dependent control of parasite growth and do not appear to represent a distinct lineage or novel CD4 subset. We hypothesize that such Ag-driven IL-10+IFN-γ+ CD4+ T cells play a major role in immune regulation during infection with intracellular pathogens by governing the extent of APC activation and are thus functionally distinct from Foxp3+CD25+ T reg cells, as well as other previously described CD4+ T reg cell populations.
Previous reports have indicated that T. gondii–infected IL-10 KO mice display markedly enhanced APC function, as indicated by increased proinflammatory cytokine production, which is likely to underlie their augmented Th1 response and, ultimately, their acute mortality (22, 26, 27, 30). We show in this paper that these sequelae can be recapitulated in WT animals by blockade of IL-10R signaling, thus emphasizing the direct role of IL-10 in the prevention of parasite-induced pathology. Moreover, we demonstrate that this IL-10–mediated protection is critical in both acute and chronic phases of T. gondii infection despite the presence of other down-regulatory mechanisms (33–35).
The results presented in this paper support the previous observations of Roers et al. (28) that CD4+ T lymphocytes are the major source of host-protective IL-10 in T. gondii infection and, furthermore, establish that IL-12p40 production is dramatically up-regulated in the absence of these cells. The latter observation was somewhat unexpected given the well-established paradigm that CD4-dependent CD40/CD40L co-stimulation of APCs (36, 37) augments rather than suppresses IL-12 production. However, in most of the situations in which this positive interaction was observed, the CD4+ T cells involved were not secreting IL-10. Thus, our findings suggest that, in the appropriate setting, IL-10 production by CD4+ T cells can override the enhancing effect of co-stimulation on IL-12 synthesis.
Systemic T. gondii infection typically induces a highly polarized Th1 cell environment in which it is difficult to detect CD4+ lymphocytes with an IL-4–producing Th2 cell phenotype (29, 32). Instead, all of the CD4-derived IL-10 measured in our experiments was associated with IFN-γ–producing cells. CD25+ T reg cells have been described to secrete both IL-10 and IFN-γ (38, 12). Nevertheless, the T. gondii–induced IL-10–producing CD4 cells studied were found to be both Foxp3− and, when characterized in the chronic phase, CD25−, and therefore are clearly distinct from naturally occurring T reg cells. Moreover, they can be distinguished from the Foxp3− IL-10–producing CD4+ T reg cells characterized by Vieira et al. (14), because the latter population does not produce IFN-γ. The additional possibility that these T. gondii–induced IL-10+ CD4 T lymphocytes represent Tr1 cells, an IL-10+ regulatory CD4+ subset with a heterogeneous cytokine production profile (10), is also unlikely, based on their developmental requirements. Thus, although Tr1 cells require IL-10 as well as culture with weakly activated DCs for their differentiation (39, 40), the IL-10+ CD4+ lymphocytes studied in this paper develop even when IL-10R signaling is blocked (not depicted) and under the conditions of strong DC activation associated with T. gondii infection (41) and, unlike Tr1 cells, are not anergic to signaling through the T cell receptor (Fig. 4). Instead, our results are most consistent with these cells representing conventional Th1 cells, a hypothesis further supported by their T-bet+ phenotype and in vitro effector function.
IL-10+ CD4+ regulatory cells with a Th1 cell phenotype have been described in other settings. Stock et al. (42) used heat-killed Listeria to induce a population of IL-10+IFN-γ+ CD4+ lymphocytes, which upon transfer suppressed Th2 cell–mediated airway hypersensitivity. However, this regulatory population was characterized as Foxp3+, and its activity in suppressing Th1 responses was not examined. Interestingly, IL-10+Foxp3− CD4+ regulatory cells with a Th1 cell phenotype recently have been demonstrated to be induced during the exposure of mice to a nonhealing strain of Leishmania major and to play a major role in determining host susceptibility (see Anderson et al. [43] on p. 283 of this issue). Whether these cells, as well as a similar population described in an L. donovani infection model (44), are functionally identical to the T. gondii–induced cells studied in this paper remains to be determined. In this regard, it should be noted that we identified a similar IL-10+ CD4+ population in mice acutely infected with T. cruzi (Fig. S1), a related protozoan pathogen, and CD4+ T cells coexpressing IL-10+IFN-γ+ have been described in several other infections, including murine Mycobacterium avium (32) and Brucella abortus (45), as well as in short-term T cell clones derived from patients with M. tuberculosis (46) or tuberculin-positive individuals (15). That such cells have not been more widely recognized and characterized may stem from their unstable IL-10–producing phenotype in vitro.
Perhaps the most unexpected finding of the present study is that the IL-10–producing Th1 lymphocytes arising in T. gondii infection simultaneously display effector function in addition to their regulatory activity. Thus, these cells are able to stimulate infected macrophages to release NO and mediate intracellular killing of the parasite. The latter observation is consistent with the finding that the IL-10+ subpopulation of Th1 lymphocytes is the same fraction that in ICS displays the brightest staining for IFN-γ. One interpretation is that this population consists of recently activated cells producing maximal levels of both cytokines but that the amounts of IL-10 secreted are insufficient to counteract the IFN-γ–mediated effector activity induced. Interestingly, preexposure to IFN-γ has recently been shown to reprogram several suppressive functions triggered by IL-10 signaling in macrophages (47). Thus, an alternative explanation of our findings is that IFN-γ production in IL-10+ Th1 cells selectively blocks the suppressive activity of IL-10 on parasite killing. Regardless of the mechanism by which Th1 cell–synthesized IL-10 fails to compromise microbicidal responses, our data strongly suggest that the major regulatory function of the cytokine in this situation is to suppress the production of IL-12, and perhaps other proinflammatory cytokines, by APCs. This in turn would act as a brake on IFN-γ synthesis by CD4+ T cells (48–50), thereby preventing the pathological consequences of an uncontrolled Th1 response (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20062175/DC1).
A critical question raised by our findings, as well as those from other studies (15, 32, 45, 46, 51) documenting IL-10 production by Th1 cells, concerns the mechanism regulating the expression of the cytokine in this population. Our previous results (32), as well as findings from preliminary experiments (not depicted), indicate that the production of this cytokine by T. gondii–induced Th1 cells does not require IL-12, IL-18, IL-23, STAT4, IL-4, STAT6, or IFN-γ. Although the precise signaling pathway involved remains to be determined, our findings strongly suggest that, under the conditions of pathogen priming, IL-10 synthesis in Th1 lymphocytes represents a step in the differentiation program of these cells in which expression of the cytokine is delayed until complete activation is achieved. In direct contrast to IFN-γ, secretion of IL-10 is transient, and we speculate that this property is the result of its dependence on the state of cellular activation. These conclusions are both consistent with and extend the findings of Shaw et al. (51), who observed that immediately after challenge infection, IFN-γ–producing CD4+ T cells from T. gondii–vaccinated mice transiently acquire the capacity to simultaneously secrete IL-10.
As already noted, the major down-regulatory cytokine IL-10 can be produced by multiple CD4+ T lymphocyte populations. The immunobiological significance of this redundancy is a topic of great interest. IL-10 production by Th2 cells appears to arm them with the capacity to suppress their own function as well as to cross-regulate Th1 responses (52, 53, 2, 15). In the case of naturally occurring CD25+CD4+ T reg cells, IL-10 synthesis appears to be constitutive and primarily functions to control autoreactivity or, when hijacked by certain pathogens, to promote latent infection (21). Interestingly, because previous studies have demonstrated an inhibitory effect of TLR signaling on CD25+CD4+ T reg cell activity (54), their functional capacity may be limited during infection with agents such as T. gondii that express potent TLR agonist activity (41). The IL-10–producing Th1 lymphocytes studied here have an overlapping but distinct profile of immunoregulatory properties that allows their expansion in the context of strong Ag-specific Th1 cell priming and serve the primary purpose of limiting collateral host damage while maintaining a potent effector response. Their IL-10 synthesis is deliberately unstable to avoid sustained suppression of effector function. We speculate that these regulatory cells are present during a wide variety of different infections but will be particularly prominent in those triggering extensive and highly polarized Th1 responses.
MATERIAL AND METHODS
Experimental animals.
C57BL/6 and BALB/c mice were obtained from Taconic Farms, and C57BL/10 IL-10 KO and C57BL/6 RAG2 × IL-10 KO mice were obtained from the NIAID contract facility maintained by the same supplier. IL-10 GFP knock-in tiger mice were generated in the laboratory of R.A. Flavell, as previously described (31), and bred as homozygotes for the transgene. In the characterization of these animals, GFP and IL-10 expression were found to closely correlate in all cell populations tested (31). All mice were maintained at an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee. Age (8–12 wk)- and sex-matched mice were used in all experiments.
Infection procedure and Ag preparation.
T. gondii cysts from the avirulent strain ME-49 were prepared from the brains of infected C57BL/6 mice. For experimental infections, mice were inoculated i.p. with an average of 20 cysts/animal. When indicated in the figures, cyst preparations were pepsin treated to eliminate potential contamination with host cells (55). Tachyzoites of the virulent RH strain of T. gondii were harvested from infected monolayers of human foreskin fibroblasts. STAg was prepared as previously described (56).
In vivo mAb treatment.
WT mice were injected i.p. with 1 mg anti–IL-10R (1B1.3a) (57), anti-CD25 (PC61) (58), or control mAb (GL113) (57) on days −2 and +2, or on days +28, 30, and 33. In one set of experiments, 1 mg anti-CD4 mAb (GK1.5) (59) was administered i.p. alone or together with 1 mg anti–IL-10R on days −2 and +2. FACS analysis on splenocytes from anti-CD25 mAb– or anti-CD4 mAb–treated mice performed with an mAb with a fine specificity distinct from the one used for the depletion (PE-labeled anti-CD25 mAb [clone 3C7] and anti-CD4 mAb [clone RM4-5]; BD Biosciences) confirmed a >90 and >99% depletion of CD25+CD4+ and CD4+ T cells, respectively.
Body weight, serum cytokine, and hepatic enzyme measurement.
Individual mice were weighed before and every 3–4 d after infection and mAb treatment, and the percent change in body weight was calculated for each animal. On days 0 and +8, 28 and 35 infected mice were bled, and levels of AST were determined using a commercial kit (Boehringer) in an automatic analyzer (model 917; Hitachi). Levels of IL-12, IFN-γ, and IL-10 were determined by ELISA in the same samples on days 0 and +8 after infection.
Cell purification for adoptive transfer.
Whole CD4+ T cells were purified from spleens and lymph nodes of naive WT and IL-10 KO mice by negative selection (Miltenyi Biotec). For adoptive transfer, recipients received 15 × 106 CD4+ cells i.v.
Cell preparation, purification, and culture conditions.
Single-cell suspensions (4 × 106 cells/ml) were prepared from pooled peritonea or spleens from naive and day 7 T. gondii–infected animals (n = 2–5). At 2, 4, and 10 wk after infection, splenocytes from individual infected mice were cultured in RPMI 1640 complete medium (60) for 72 h with 5 μg/ml STAg, and supernatants were removed for cytokine assays. For purification of CD4+, CD4+CD25−, CD4+CD25+, and CD4+CD25+CD45RBhigh and CD4+CD25+CD45RBlow populations, pooled splenocytes from 6 wk–infected animals (n = 3) were stained with CyChrome-labeled anti-CD4 (RM4-5), FITC–anti-CD45Rb (16A), and biotin–anti-CD25 (7D4) mAb and streptavidin-PE (all obtained from BD Biosciences) and sorted on a FACStarPlus/SE or a FACSAria (BD Biosciences). FACS-purified CD4+ subpopulations (>98%) were cultured (3 × 105 cells/200 μl) in flat-bottom 96-well plates with 106 irradiated splenocytes/200 μl from naive mice, plus 5 μg/ml STAg, and their proliferative and cytokine responses were analyzed.
In a separate set of experiments, peritoneum exudate cells (PECs) and splenocytes from naive and day 7–infected IL-10gfp mice were stained with Cy-labeled anti-CD4 (RM4-5) and APC-labeled anti-CD44 (M7; BD Biosciences), and CD4+CD44+ GFP− and CD4+CD44+ GFP+ cells were purified (>96%) by sorting. The two populations were tested in an in vitro microbicidal assay or cultured for 12, 24, and 48 h in wells coated with anti-CD3 mAb, followed by reexamination of their GFP phenotype.
Proliferation assay.
At 6 wk after infection, splenic CD4+, CD4+CD25+, CD4+CD25−, CD4+CD25−CD45RBhigh and CD4+CD25−CD45RBlow FACS-purified populations were cultured with irradiated splenocytes from naive mice as APCs and 5 μg/ml STAg, as described in the previous section. After 48 h, cultures were pulsed with 0.5 μCi [3H]TdR (New England Nuclear) per well for 18 h, and the incorporated isotype was measured.
NO and cytokine measurements.
NO2 − levels were used as an indicator of reactive nitrogen intermediates released in culture supernatants and were measured by the Griess assay (61). IFN-γ, IL-12, and IL-10 were measured by ELISA (62, 29, 63). The limits of detection were as follows: IFN-γ = 125 pg/ml; IL-12 = 200 pg/ml; and IL-10 = 600 pg/ml.
Intracellular staining.
Analyses of intracellular cytokine expression were performed either on freshly isolated PECs and splenocytes stimulated with anti-CD3 mAb in the presence of 10 μg/ml Brefeldin A (Sigma-Aldrich) for 5 h, or on the same in vitro spleen cell cultures used for cytokine secretion assays. In the latter case, the cells received an additional 18-h incubation in fresh medium and were subsequently stimulated with 1 ng/ml PMA (Sigma-Aldrich) and 1 μg/ml inonomycin (Sigma-Aldrich) for 4 h with the addition of Brefeldin A during the last 2 h, as previously described (60). In additional experiments, freshly isolated PECs and splenocytes were stained with either PE–anti-Foxp3 (FJK-16s; eBioscience) or FITC– or PE–anti–T-bet mAb (4B10; Santa Cruz Biotechnology, Inc.) using a similar protocol. Cell fluorescence was measured with a FACScan or a FACSCalibur (BD Biosciences), and data was analyzed using CELLQuest (BD Biosciences) or FlowJo (TreeStar, Inc.) software.
Measurement of CD4+ T cell–dependent macrophage microbicidal activity.
Inflammatory macrophages were harvested from IL-10 KO mice, inoculated i.p. 3 d earlier with 1.5 ml 3% thioglycolate (Sigma-Aldrich). 2 × 105 cells/100 μl were distributed in flat-bottom 96-well plates incubated in complete medium for 2 h, at which time an equal number of irradiated RH tachyzoites were added to each well. After an additional 2–4-h incubation, the plates were centrifuged (8 min at 800 RPM), and 100 μl of medium was replaced with the same volume containing different numbers of FACS-purified CD4+ T lymphocytes. The plates were incubated for a further 24 h, and aliquots of culture supernatants were removed for quantitation of both NO and cytokines. The remaining cultures were next pulsed with 0.5 μCi [5,6-3H]Uracil (MP Biomedicals) per well for an additional 18 h, and the incorporated radioactivity was determined (64). The percentage of microbicidal activity was calculated at each cell concentration tested by determining the ratio of incorporated counts per minute between wells receiving CD4+ lymphocytes and the wells containing macrophages alone. Where indicated in the figures, 10 μg/ml control (GL113) or anti–IL-10R (1B1.3a) mAb was added to the cultures before the CD4+ lymphocytes.
Generation and maintenance of T. gondii–specific CD4+ T cell clones.
FACS-purified CD4+ T cells isolated from spleens of C57BL/6 mice immunized repeatedly with STAg (34) were cloned by limiting dilution (0.3 cells/well) in 0.1 ml cells/well in round-bottom 96-well plates with 3 × 105 irradiated syngeneic spleen cells and 5 μg/ml STAg (60). On the next day, 0.1 ml rIL-2 (20 U/ml) was added to each well. At biweekly intervals, the cultures were given fresh medium containing APCs and Ag, followed by rIL-2 48 h later. Wells containing proliferating cells were identified visually and further expanded by Ag/APC restimulation and culture in rIL-2–containing medium at 4-wk intervals. The resulting clones were characterized for their cytokine secretion profile by ELISA and by intracellular staining and were found to display a stable phenotype over a period of 6 mo in culture.
Statistical analysis.
The statistical significance of differences between means between data groups was determined using an unpaired, two-tailed Student's t test.
Online supplemental material.
Fig. S1 shows the coexpression of IFN-γ and IL-10 by CD4+, but not CD8+, T lymphocytes in mice acutely infected with T. cruzi (provided by A. Rothfuchs and A. Bafica, NIH, Bethesda, MD). Fig. S2 shows a model for the cellular dynamics and function of IL-10–producing Th1 lymphocytes in T. gondii infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20062175/DC1.
Supplemental Material
Acknowledgments
We thank Calvin Eigsti for performing FACS sorting and Pat Caspar and Sara Hieny for excellent technical assistance throughout this study. We also thank Drs. Antony Rothfuchs and Andre Bafica for providing T. cruzi-infected mice, Dr. Stephanie James for advice regarding the in vitro microbicidal assay, and Drs. Yasmine Belkaid, Ethan Shevach, and George Yap for critical reading of the manuscript.
This research was supported by the Intramural Research Program of the NIAID, NIH.
The authors have no conflicting financial interests.
Abbreviations used: Ag, antigen; AST, aspartate aminotransferase; ICS, intracellular cytokine staining; NO, nitrite; PEC, peritoneum exudate cell; STAg, soluble tachyzoite Ag.
M.C. Kullberg's present address is Immunology and Infection Unit, Dept. of Biology, University of York and Hull York Medical School, York YO10 5YW, England, UK.
C.M. Collazo's present address is Division of Vaccines and Related Products Applications, Center for Biologics Evaluation and Research, Food and Drug Administration, Rockville, MD 20852.
References
- 1.Gajewski, T.F., and F.W. Fitch. 1988. Anti-proliferative effect of IFN-γ in immune regulation. I. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 140:4245–4252. [PubMed] [Google Scholar]
- 2.Fiorentino, D.F., M.W. Bond, and T.R. Mosmann. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 5.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. [DOI] [PubMed] [Google Scholar]
- 6.Shevach, E.M. 2002. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 7.De Smedt, T., M. Van Mechelen, G. De Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229–1235. [DOI] [PubMed] [Google Scholar]
- 8.Enk, A.H., V.L. Angeloni, M.C. Udey, and S.I. Katz. 1993. Inhibition of Langerhans cell antigen-presenting function by IL-10. J. Immunol. 151:2390–2398. [PubMed] [Google Scholar]
- 9.Fiorentino, D.F., A. Zlotnik, T.R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815–3822. [PubMed] [Google Scholar]
- 10.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X., D.N. Koldzic, L. Izikson, J. Reddy, R.F. Nazareno, S. Sakaguchi, V.K. Kuchroo, and H.L. Weiner. 2004. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int. Immunol. 16:249–256. [DOI] [PubMed] [Google Scholar]
- 12.Suffia, I.J., S.K. Reckling, C.A. Piccirillo, R.S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrat, F.J., D.J. Cua, A. Boonstra, D.F. Richards, C. Crain, H.F. Savelkoul, R. de Waal-Malefyt, R.L. Coffman, C.M. Hawrylowicz, and A. O'Garra. 2002. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)– and Th2-inducing cytokines. J. Exp. Med. 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira, P.L., J.R. Christensen, S. Minaee, E.J. O'Neill, F.J. Barrat, A. Boonstra, T. Barthlott, B. Stockinger, D.C. Wraith, and A. O'Garra. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172:5986–5993. [DOI] [PubMed] [Google Scholar]
- 15.Del Prete, G., M. De Carli, F. Almerigogna, M.G. Giudizi, R. Biagiotti, and S. Romagnani. 1993. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 150:353–360. [PubMed] [Google Scholar]
- 16.O'Garra, A., R. Chang, N. Go, R. Hastings, G. Haughton, and M. Howard. 1992. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 22:711–717. [DOI] [PubMed] [Google Scholar]
- 17.Corinti, S., C. Albanesi, A. la Sala, S. Pastore, and G. Girolomoni. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166:4312–4318. [DOI] [PubMed] [Google Scholar]
- 18.Katakura, T., M. Miyazaki, M. Kobayashi, D.N. Herndon, and F. Suzuki. 2004. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J. Immunol. 172:1407–1413. [DOI] [PubMed] [Google Scholar]
- 19.Murray, P.J., and R.A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs, M., N. Brown, N. Allie, R. Gulert, and B. Ryffel. 2000. Increased resistance to mycobacterial infection in the absence of interleukin-10. Immunology. 100:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belkaid, Y., K.F. Hoffmann, S. Mendez, S. Kamhawi, M.C. Udey, T.A. Wynn, and D.L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti–IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazzinelli, R.T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798–805. [PubMed] [Google Scholar]
- 23.Abrahamsohn, I.A., and R.L. Coffman. 1996. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 84:231–244. [DOI] [PubMed] [Google Scholar]
- 24.Hunter, C.A., L.A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F.G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311–3316. [PubMed] [Google Scholar]
- 25.Chmiel, J.F., M.W. Konstan, A. Saadane, J.E. Krenicky, H. Lester Kirchner, and M. Berger. 2002. Prolonged inflammatory response to acute Pseudomonas challenge in interleukin-10 knockout mice. Am. J. Respir. Crit. Care Med. 165:1176–1181. [DOI] [PubMed] [Google Scholar]
- 26.Villegas, E.N., U. Wille, L. Craig, P.S. Linsley, D.M. Rennick, R. Peach, and C.A. Hunter. 2000. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect. Immun. 68:2837–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wille, U., E.N. Villegas, L. Craig, R. Peach, and C.A. Hunter. 2002. Contribution of interleukin-12 (IL-12) and the CD28/B7 and CD40/CD40 ligand pathways to the development of a pathological T-cell response in IL-10-deficient mice. Infect. Immun. 70:6940–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roers, A., L. Siewe, E. Strittmatter, M. Deckert, D. Schluter, W. Stenzel, A.D. Gruber, T. Krieg, K. Rajewsky, and W. Muller. 2004. T cell–specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 200:1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazzinelli, R.T., M. Wysocka, S. Hayashi, E.Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533–2543. [PubMed] [Google Scholar]
- 30.Suzuki, Y., A. Sher, G. Yap, D. Park, L.E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375–5382. [DOI] [PubMed] [Google Scholar]
- 31.Kamanaka, M., S.T. Kim, Y.Y. Wan, F.S. Sutterwala, M. Lara-Tejero, J.E. Galan, E. Harhaj, and R.A. Flavell. Expression of IL-10 in intestinal lymphocytes detected by an IL-10 reporter knockin tiger mouse. Immunity. 25:941–952. [DOI] [PubMed] [Google Scholar]
- 32.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10−/− setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 33.Aliberti, J., C. Serhan, and A. Sher. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J. Exp. Med. 196:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R.A. Kastelein, C. Saris, and C.A. Hunter. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 19:645–655. [DOI] [PubMed] [Google Scholar]
- 35.Stumhofer, J.S., A. Laurence, E.H. Wilson, E. Huang, C.M. Tato, L.M. Johnson, A.V. Villarino, Q. Huang, A. Yoshimura, D. Sehy, et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7:937–945. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy, M.K., K.S. Picha, W.C. Fanslow, K.H. Grabstein, M.R. Alderson, K.N. Clifford, W.A. Chin, and K.M. Mohler. 1996. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur. J. Immunol. 26:370–378. [DOI] [PubMed] [Google Scholar]
- 37.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 38.Sawitzki, B., C.I. Kingsley, V. Oliveira, M. Karim, M. Herber, and K.J. Wood. 2005. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J. Exp. Med. 201:1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levings, M.K., S. Gregori, E. Tresoldi, S. Cazzaniga, C. Bonini, and M.G. Roncarolo. 2005. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 105:1162–1169. [DOI] [PubMed] [Google Scholar]
- 40.Wakkach, A., N. Fournier, V. Brun, J.P. Breittmayer, F. Cottrez, and H. Groux. 2003. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 18:605–617. [DOI] [PubMed] [Google Scholar]
- 41.Sher, A., F. Yarovinsky, R. Goldszmid, J. Aliberti, and D. Jankovic. 2006. Sentinel and regulatory functions of dendritic cells in the immune response to Toxoplasma gondii. In Handbook of Dendritic Cells. Vol. 2. M.B. Lutz, N. Romani, and A. Steinkasserer, editors. Wiley-VCH, Weinheim, Germany. 693–705.
- 42.Stock, P., O. Akbari, G. Berry, G.J. Freeman, R.H. Dekruyff, and D.T. Umetsu. 2004. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat. Immunol. 5:1149–1156. [DOI] [PubMed] [Google Scholar]
- 43.Anderson, C.F., M. Oukka, V.J. Kuchroo, and D. Sacks. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stager, S., A. Maroof, S. Zubairi, S.L. Sanos, M. Kopf, and P.M. Kaye. 2006. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. 36:1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svetic, A., Y.C. Jian, P. Lu, F.D. Finkelman, and W.C. Gause. 1993. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-γ in CD4+ T cells. Int. Immunol. 5:877–883. [DOI] [PubMed] [Google Scholar]
- 46.Gerosa, F., C. Nisii, S. Righetti, R. Micciolo, M. Marchesini, A. Cazzadori, and G. Trinchieri. 1999. CD4+ T cell clones producing both interferon-γ and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 92:224–234. [DOI] [PubMed] [Google Scholar]
- 47.Herrero, C., X. Hu, W.P. Li, S. Samuels, M.N. Sharif, S. Kotenko, and L.B. Ivashkiv. 2003. Reprogramming of IL-10 activity and signaling by IFN-γ. J. Immunol. 171:5034–5041. [DOI] [PubMed] [Google Scholar]
- 48.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628–631. [DOI] [PubMed] [Google Scholar]
- 49.Park, A.Y., B. Hondowicz, M. Kopf, and P. Scott. 2002. The role of IL-12 in maintaining resistance to Leishmania major. J. Immunol. 168:5771–5777. [DOI] [PubMed] [Google Scholar]
- 50.Feng, C.G., D. Jankovic, M. Kullberg, A. Cheever, C.A. Scanga, S. Hieny, P. Caspar, G.S. Yap, and A. Sher. 2005. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J. Immunol. 174:4185–4192. [DOI] [PubMed] [Google Scholar]
- 51.Shaw, M.H., G.J. Freeman, M.F. Scott, B.A. Fox, D.J. Bzik, Y. Belkaid, and G.S. Yap. 2006. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-γ-dependent IL-10 reactivation. J. Immunol. 176:7263–7271. [DOI] [PubMed] [Google Scholar]
- 52.Wynn, T.A., R. Morawetz, T. Scharton-Kersten, S. Hieny, H.C. Morse III, R. Kuhn, W. Muller, A.W. Cheever, and A. Sher. 1997. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J. Immunol. 159:5014–5023. [PubMed] [Google Scholar]
- 53.Grunig, G., D.B. Corry, M.W. Leach, B.W. Seymour, V.P. Kurup, and D.M. Rennick. 1997. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 185:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 55.Yap, G.S., T. Scharton-Kersten, D.J. Ferguson, D. Howe, Y. Suzuki, and A. Sher. 1998. Partially protective vaccination permits the development of latency in a normally virulent strain of Toxoplasma gondii. Infect. Immun. 66:4382–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grunvald, E., M. Chiaramonte, S. Hieny, M. Wysocka, G. Trinchieri, S.N. Vogel, R.T. Gazzinelli, and A. Sher. 1996. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect. Immun. 64:2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kullberg, M.C., D. Jankovic, P.L. Gorelick, P. Caspar, J.J. Letterio, A.W. Cheever, and A. Sher. 2002. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus–induced colitis. J. Exp. Med. 196:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowenthal, J.W., P. Corthesy, C. Tougne, R. Lees, H.R. MacDonald, and M. Nabholz. 1985. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J. Immunol. 135:3988–3994. [PubMed] [Google Scholar]
- 59.Dialynas, D.P., Z.S. Quan, K.A. Wall, A. Pierres, J. Quintans, M.R. Loken, M. Pierres, and F.W. Fitch. 1983. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J. Immunol. 131:2445–2451. [PubMed] [Google Scholar]
- 60.Jankovic, D., M.C. Kullberg, P. Caspar, N. Noben-Trauth, W.E. Paul, and A. Sher. 2000. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for in vivo and in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J. Immunol. 164:3047–3055. [DOI] [PubMed] [Google Scholar]
- 61.Green, L.C., D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, and S.R. Tannenbaum. 1982. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal. Biochem. 126:131–138. [DOI] [PubMed] [Google Scholar]
- 62.Scott, P., P. Natovitz, R.L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosmann, T.R., J.H. Schumacher, D.F. Fiorentino, J. Leverah, K.W. Moore, and M.W. Bond. 1990. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J. Immunol. 145:2938–2945. [PubMed] [Google Scholar]
- 64.Scharton-Kersten, T.M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.