Abstract
In normal vision, shifts of attention are usually followed by saccadic eye movements. Neurons in extrastriate area V4 are modulated by focal attention when eye movements are withheld, but they also respond in advance of visually guided saccadic eye movements. We have examined the visual selectivity of saccade-related responses of area V4 neurons in monkeys making delayed eye movements to receptive field stimuli of varying orientation. This task did not require the monkey to attend to orientation per se but merely to foveate the receptive field stimulus. We present evidence that the presaccadic enhancement exhibited by V4 neurons, quite separate from the response at stimulus onset, is a resurgent visual representation that seems as selective as the response is when the stimulus first appears. The presaccadic enhancement appears to provide a strengthening of a decaying featural representation immediately before an eye movement is directed to visual targets. We suggest that this reactivation provides a mechanism by which a clear perception of the saccade goal can be maintained during the execution of the saccade, perhaps for the purpose of establishing continuity across eye movements.
Keywords: extrastriate cortex/area V4/attention/saccades
Although we can direct visual attention away from the center of gaze, shifts of attention seldom take place without accompanying eye movements. The vast majority of neurophysiological studies of spatial attention in macaque area V4 have involved monkeys trained to attend to particular locations while withholding eye movements to the relevant stimuli (1–5). In general, this method has proven critical in dissociating brain mechanisms of attention from oculomotor ones (6–7). Yet, such a dissociation is somewhat artificial. Visually guided saccadic eye movements most often closely follow shifts of attention (8–11). This fact suggests that under normal circumstances, the neural activity reflecting attentional shifts and oculomotor planning are temporally contiguous. One might argue, therefore, that an understanding of the neural mechanisms involved in the sort of attention related to saccadic eye movements is particularly relevant to normal vision.
The visual responses of neurons in extrastriate area V4 are enhanced when visual stimuli within their receptive fields (RFs) are used as targets for saccadic eye movements (12–13). The saccade-related activation can be separated from the visual onset response by delaying the saccadic eye movement to the RF stimulus. The visual selective properties of the saccade-related activity, however, have not yet been examined. We have studied the visual selectivity of presaccadic responses of V4 neurons in monkeys making delayed saccadic eye movements to stimuli of varying orientation. Our results show that the saccade-related activity in area V4 is a resurgent visually selective response that provides a representation of the saccade goal immediately before an eye movement. We suggest that the presaccadic response ensures that a featural representation of the saccade goal is present during the eye movement, perhaps for the purpose of facilitating continuity across displacements of the eye.
METHODS
We recorded from single neurons in extrastriate area V4 of two macaque monkeys (Maccaca mulatta) by using standard electrophysiological techniques described previously (14). Single cell responses were isolated and studied while monkeys performed a delayed saccade task (15). In this task, a visual stimulus was presented to the RF of a neuron while a monkey fixated within a <1° window and waited for the appearance of a saccade target (0.25°) at one of two locations distant from the RF (>5.0°). On two-thirds of the trials, the saccade target appeared when the fixation spot was extinguished and the monkey was required to make a saccadic eye movement to the target. On the remaining trials, the saccade target did not appear; when the fixation spot was extinguished, the monkey was required to make a saccadic eye movement to the RF stimulus. Both conditions were identical up until the cue to saccade (disappearance of the fixation spot) and were pseudorandomly interleaved. The delay between stimulus onset and the cue to saccade (0.5–1.0 sec) allowed us to examine separately for each cell, the activity related to the onset of the stimulus and the activity related to the eye movement. By varying the orientation of the stimulus, we also were able to assess the degree to which the saccade-related activity carried information about the stimulus. At no time was the monkey required to discriminate the orientation of the stimulus, nor had they been previously trained to do so. RF stimuli were oriented light and dark bars presented on a 34 × 27 cm video monitor (Sony) driven by a Number Nine graphics board (640 × 480 resolution). Eye position was monitored continuously at 200 Hz via a schleral search coil (16). Neural activity and eye position data were both stored for off-line clustering (Datawaves Corporation) and analysis.
The presaccadic response was measured during a 100-ms period of activity beginning before saccade onset and extended 30 ms into the saccade itself. Visual latencies of V4 neurons in our sample were >60 ms and typically 70 ms, a measurement that matches previous observations (17). Therefore, responses extending 30 ms into the saccade should include no activity corresponding to visual stimulation caused by movement of the eye. In addition, it is crucial to determine whether or not stability of the eye before saccade onset can account for any differences in presaccadic activity between saccade conditions. We therefore examined the presaccadic eye velocities in both conditions, and we compared them in the same manner as we compared the corresponding neural activity. We computed the instantaneous velocity for each successive pair of 200-Hz eye position samples during a 150-ms period immediately before onset of all saccadic eye movements from which corresponding neural activity was recorded. This generated large populations of velocities for both saccade conditions. The components of eye velocity in the direction of the RF were statistically indistinguishable between the two populations (Wilcoxon sign rank test, P > 0.3).
RESULTS
We first contrasted the amount of activity preceding saccadic eye movements to the RF stimulus with the activity preceding saccades to a contralateral saccade target for a sample of 51 visually responsive neurons. The population of neurons as a whole showed significantly greater presaccadic activity when eye movements were directed to the RF stimulus (Wilcoxon signed rank test, P < 0.001). Individually, 19 of the 51 cells (37%) had presaccadic activity that differed between the two saccade conditions (χ2, P < 0.05). Of these cells, 18 responded greater before saccadic eye movements to the RF stimulus and 1 responded greater before eye movements directed away from the RF. Cells that responded significantly greater when eye movements were directed to RF stimuli were enhanced by a median factor of 1.7:1. However, the likelihood of a cell showing enhancement depended on which stimulus was in the RF. Our focus, therefore, was on the degree to which the presaccadic activation depended on the stimulus itself and whether or not this selectivity matched the selectivity seen at the time of stimulus onset.
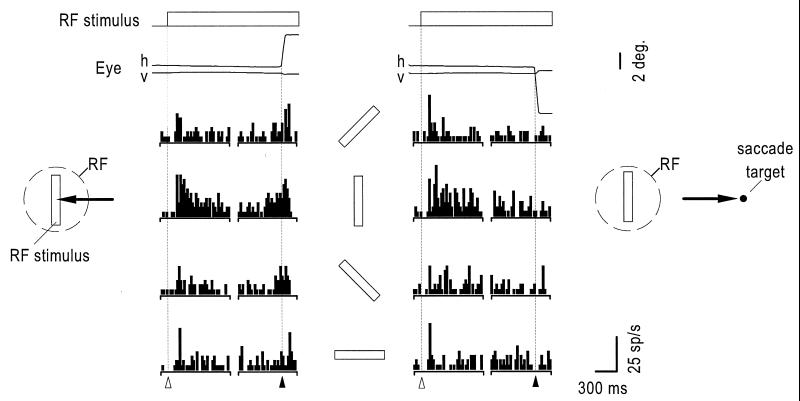
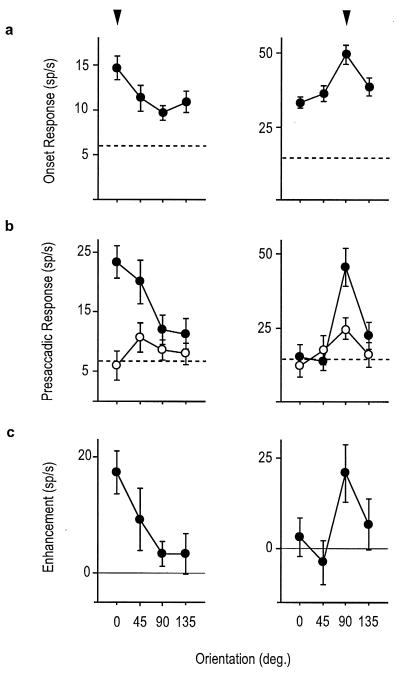
Fig. 1 shows an example of a V4 neuron that responded before saccadic eye movements to the RF stimulus. During the period immediately following stimulus onset, the cell was clearly selective for orientation but responsiveness declined for all orientations within a few hundred milliseconds. The cell was reactivated however just before saccadic eye movements were directed to the preferred RF stimulus. In comparison, the presaccadic activity was much less pronounced when eye movements were made to nonpreferred stimulus orientations. Eye movements directed to a target in the opposite hemifield elicited little or no presaccadic activity, regardless of stimulus orientation. Fig. 2 shows the presaccadic orientation-tuning curves of two other cells and their correspondence with the selectivity evident immediately after stimulus onset. In both cases, the magnitude of the enhancement above the control condition was greatest at the preferred stimulus orientation.
Figure 1.
Histograms showing the response of a V4 neuron after the appearance of an oriented bar in the RF and immediately before a saccadic eye movement either to the RF stimulus (Left) or a saccade target appearing 7° in the opposite hemifield (Right). In each column, spikes from the first half of the trial are aligned to the onset of the RF stimulus (▵) and then to the onset of the eye movement (▴) in 20 ms bins. Above each set of histograms are the mean horizontal (h) and vertical (v) eye traces. Bars between columns show the orientation of the RF stimulus. The RF of this neuron was located 4.2° from the fovea and centered on the horizontal meridian.
Figure 2.
Visual onset and presaccadic orientation tuning curves for two neurons. Arrows above the right and left set of curves highlight the tuning peaks for each of the two cells. (a) The orientation selectivity evident within the first 200 ms after stimulus onset, at which time the trial was identical for both saccade conditions. (b) The orientation selectivity within the 100 ms presaccadic period when eye movements were directed to the RF stimulus (•) or a saccade target in the opposite hemifield (○). (c) Enhancement tuning curves computed from the average trial by trial difference in the presaccadic activity between the two saccade conditions. Dotted lines in a and c represent the baseline firing rates. (Error bars: means ± SE.)
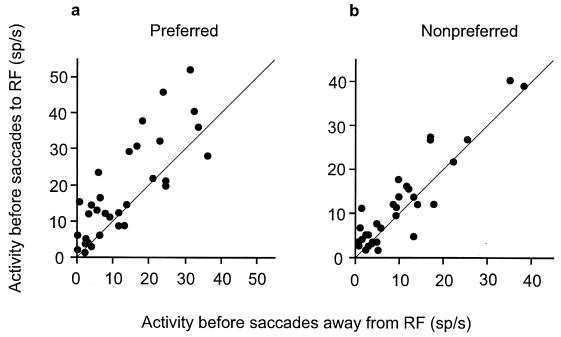
We examined the presaccadic enhancement of a population of 35 neurons that were clearly tuned at the time the RF stimulus first appeared. We contrasted the presaccadic enhancement for eye movements directed to the preferred orientation with that of saccades directed to the nonpreferred orientation for the entire population. For both preferred and nonpreferred stimuli, the population showed significant presaccadic enhancement (preferred, P < 0.005; nonpreferred, P < 0.05, Wilcoxon signed rank test). However, the presaccadic enhancement was greatest when the monkey made eye movements to the preferred orientation (Fig. 3). The difference between the activity before saccades to the RF and saccades away from it was significantly greater when the preferred stimulus was in the RF (Wilcoxon signed rank test, P < 0.05). Thus, the effect of saccade direction depended on which stimulus was in the RF, the preferred stimulus producing greater modulation. Interestingly, at the time the monkey made an eye movement away from the RF stimulus, the population activity could no longer distinguish between the preferred and nonpreferred orientations, which were still in the RF (Wilcoxon signed rank test, P > 0.1). In contrast, the presaccadic activation was clearly tuned at the time the monkey made an eye movement to the RF stimulus (Wilcoxon signed rank test, P < 0.0003).
Figure 3.
Presaccadic activity of 35 orientation selective neurons when saccadic eye movements were made to RF stimuli plotted against the presaccadic activity for saccades directed away from the RF when the preferred (a) or nonpreferred stimulus (b) was in the RF. Nonpreferred stimuli were those orientations that activated the cell the least.
DISCUSSION
Our results show that the presaccadic activity in V4 is a visually selective representation of the targets of saccadic eye movements. We interpret the general phenomenon of presaccadic reactivation as a consequence of the fact that attentional shifts typically precede shifts in the direction of gaze (8–11). Several studies have established that neurons in V4 are modulated by focal attention in the absence of accompanying eye movements (1–5). However, the fact that the enhancement is visually selective does not necessarily follow from the notion that it reflects a presaccadic attentional shift. Previous studies have shown that the degree to which monkeys must scrutinize visual discriminanda affects the tuning of V4 neurons (4) and that attention to particular features (e.g., color or orientation) can determine the degree to which those features are represented by neurons (5). Our behavioral task did not require the monkey to attend to orientation per se and saccades to any set of oriented bars requires a movement to only a single “center of gravity.” One might have thus predicted that the saccade-related enhancement would be independent of orientation. Our results therefore suggest that the resurgence of visual selectivity in the presaccadic period occurs by default, as it does when a visual stimulus is initially presented.
We suggest that this resurgent representation may be useful in maintaining a clear visual perception of the saccade goal during the execution of an eye movement and that this may facilitate continuity of perception across displacements of the eye. A persisting question in vision research is how our perceptions of the world appear continuous despite the interruptions brought about by saccadic eye movements, which rapidly sweep visual stimuli across the retina (18). Eye movements at saccade velocities should result in a period of smeared vision, and this period should disrupt perceptual continuity across saccadic eye movements. Yet our perception is seamless, and we do not experience smeared images despite the fact that our eyes are in flight almost 10% of the time. One major hypothesis offered as the solution to the problem of retinal smear is that it is eliminated by the forward and backward visual “masking” resulting from a clear image before and after the saccade (19–21). Masking essentially results from the relative weakness of intrasaccadic visual stimulation as compared with the input preceding and following a saccadic eye movement. In addition to the masking of retinal image blur, perceptual continuity requires the integration of pre- and postsaccadic perceptions across changes in eye position. Psychophysical evidence suggests that presaccadic visual information at the saccade goal aids in postsaccadic visual detection once a saccadic eye movement brings an object of interest onto the fovea (22–26). This effect depends on the similarity of pre- and postsaccadic stimuli and implies a mechanism by which the two are compared.
Both masking and transaccadic integration phenomena suggest a mechanism by which strong visual representations are provided immediately before and immediately after an eye movement. Although it is simple to imagine how readily strong, postsaccadic representations are provided by the visual system, the fact that visual responses in extrastriate cortex often decay shortly after stimulus presentation suggests a lack of strong, presaccadic visual representations when eye movements are made to stable visual targets. The presaccadic reactivation described here appears to provide a strengthening of the decaying featural representation immediately before an eye movement is made to such targets. This representation could serve in the forward masking of retinal image blur as well as in facilitating featural integration across saccadic eye movements. This idea suggests that the normal relationship between eye movements and attention may provide a convenient, visual outcome. Presumably, if the function of visual attention is to selectively process only relevant stimuli, this should be of particular use during saccadic eye movements, when visual clarity is jeopardized. It is during eye movements that the representation of the saccade goal competes not only with that of nontarget stimuli but also with the visual disturbance caused by the movement itself. Dodge (27) seems to have had a similar view nearly a century ago.
It should also be noted, however, that by considering the visual consequences of presaccadic reactivation, this does not rule out an oculomotor role of presaccadic signals. It is known that many extrastriate visual areas project inputs to the superior colliculus, (28) and it is therefore possible, if not likely, that these inputs affect oculomotor commands. For some stimulus properties, such as motion, it is reasonable to suppose that extrastriate visual activity aids directly in moving the fovea appropriately to targets of interest. Microstimulation of the middle temporal visual area (MT), for example, has been shown to affect saccade programming and in a manner predicted by the direction selective properties of neurons in the stimulated cortical columns (29). It should thus follow that presaccadic enhancement in this area, if present, might be used to synchronize target velocity information with the saccade command for the purpose of foveating moving visual targets. Indeed, the fact that attention and eye movement control mechanisms seem to overlap in the brain (30–31) invites consideration of joint perceptual and motor influences of presaccadic activation in extrastriate visual cortex.
Acknowledgments
We thank M. Graziano, K. L. Nace, and E. J. Tehovnik for making valuable comments on the manuscript and W. M. Slocum for technical assistance. This work was supported by National Insitutes of Health Grant EY0067 and the McDonnell-Pew Foundation.
ABBREVIATION
- RF
receptive field
References
- 1.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 2.Heanny P E, Schiller P H. Exp Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- 3.Motter B C. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer H, Desimone R, Moran J. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- 5.Motter B C. J Neurosci. 1994;14:2178–2189. doi: 10.1523/JNEUROSCI.14-04-02178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushnell M C, Goldberg M E, Robinson D L. J Neurophysiol. 1981;46:755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg M E, Bushnell M C. J Neurophysiol. 1981;46:773–787. doi: 10.1152/jn.1981.46.4.773. [DOI] [PubMed] [Google Scholar]
- 8.Remington R W. J Exp Psychol Hum Percept Perform. 1980;6:726–744. doi: 10.1037//0096-1523.6.4.726. [DOI] [PubMed] [Google Scholar]
- 9.Posner M I, Cohen Y. In: Attention and Performance X. Bouma H, Bouwhuis G G, editors. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- 10.Shepherd M, Findlay J M, Hockey R J. Quart J Exp Psychol. 1986;38:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman J E, Subramaniam B. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- 12.Fischer B, Boch R. Exp Brain Res. 1981;41:431–433. doi: 10.1007/BF00238903. [DOI] [PubMed] [Google Scholar]
- 13.Fischer B, Boch R. Exp Brain Res. 1981;44:129–137. doi: 10.1007/BF00237333. [DOI] [PubMed] [Google Scholar]
- 14.Zipser K, Lamme V A F, Schiller P H. J Neurosci. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg M E, Wurtz R H. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- 16.Judge S J, Richmond B J, Chu F C. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 17.Nowak L G, Bullier J. In: Cerebral Cortex. Rockland K S, Kaas J H, Peters A, editors. Vol. 12. New York: Plenum; 1997. pp. 205–237. [Google Scholar]
- 18.Dodge R. Psychol Rev. 1900;7:454–465. [Google Scholar]
- 19.Matin E, Clymer A B, Matin L. Science. 1972;178:170–181. doi: 10.1126/science.178.4057.179. [DOI] [PubMed] [Google Scholar]
- 20.Campbell F W, Wurtz R H. Vision Res. 1978;18:1297–1303. doi: 10.1016/0042-6989(78)90219-5. [DOI] [PubMed] [Google Scholar]
- 21.Judge S J, Wurtz R H, Richmond B J. J Neurophysiol. 1980;43:1133–1155. doi: 10.1152/jn.1980.43.4.1133. [DOI] [PubMed] [Google Scholar]
- 22.Dodge, R. (1907) Psychol. Rev. Monogr. Suppl. 8, No. 37.
- 23.Wolf W, Hauske G, Lupp U. Vision Res. 1980;20:117–125. doi: 10.1016/0042-6989(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 24.Pollatsek A, Rayner K, Collins W E. J Exp Psychol Gen. 1984;113:426–442. doi: 10.1037//0096-3445.113.3.426. [DOI] [PubMed] [Google Scholar]
- 25.Irwin D E. Cognit Psychol. 1991;23:420–456. doi: 10.1016/0010-0285(91)90015-g. [DOI] [PubMed] [Google Scholar]
- 26.Juttner M, Rohler R. Percept Psychophys. 1993;53:210–220. doi: 10.3758/bf03211731. [DOI] [PubMed] [Google Scholar]
- 27.Dodge R. Psychol Rev. 1905;3:193–199. [Google Scholar]
- 28.Fries W. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- 29.Groh J M, Born R T, Newsome W T. J Neurosci. 1997;17:4312–4330. doi: 10.1523/JNEUROSCI.17-11-04312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheliga B M, Riggio L, Rizzolatti G. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- 31.Kustov A A, Robinson D L. Nature (London) 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]