Abstract
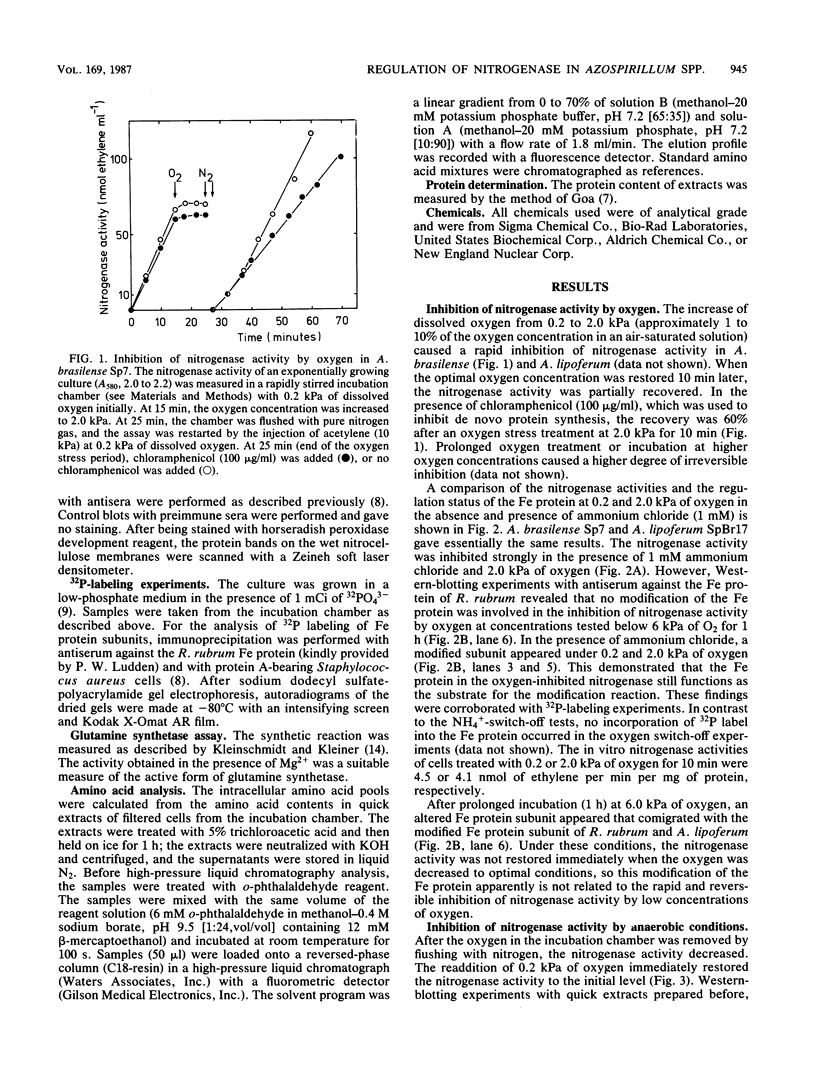
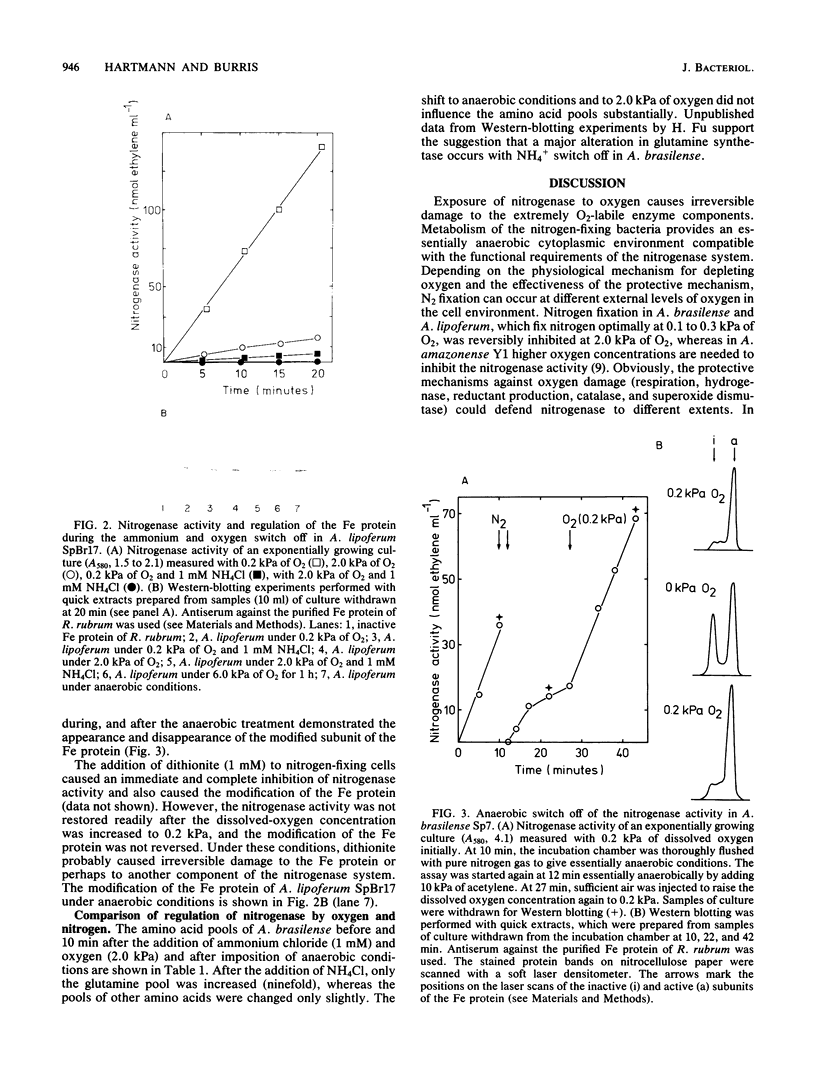
The nitrogenase activity of the microaerophilic bacteria Azospirillum brasilense and A. lipoferum was completely inhibited by 2.0 kPa of oxygen (approximately 0.02 atm of O2) in equilibrium with the solution. The activity could be partially recovered at optimal oxygen concentrations of 0.2 kPa. In contrast to the NH4+ switch off, no covalent modification of the nitrogenase reductase (Fe protein) was involved, as demonstrated by Western-blotting and 32P-labeling experiments. However, the inhibition of the nitrogenase activity under anaerobic conditions was correlated with covalent modification of the Fe protein. In contrast to the NH4+ switch off, no increase in the cellular glutamine pool and no modification of the glutamine synthetase occurred under anaerobic switch-off conditions. Therefore, a redox signal, independent of the nitrogen control of the cell, may trigger the covalent modification of the nitrogenase reductase of A. brasilense and A. lipoferum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burris R. H. Nitrogen fixation--assay methods and techniques. Methods Enzymol. 1972;24:415–431. doi: 10.1016/0076-6879(72)24088-5. [DOI] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Dingler C., Oelze J. Reversible and irreversible inactivation of cellular nitrogenase upon oxygen stress in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1985 Feb;141(1):80–84. doi: 10.1007/BF00446744. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Fu H., Burris R. H. Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol. 1986 Mar;165(3):864–870. doi: 10.1128/jb.165.3.864-870.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S., Turner G. L., Bergersen F. J. Synthesis and activity of nitrogenase in Klebsiella pneumoniae exposed to low concentrations of oxygen. J Gen Microbiol. 1984 May;130(5):1061–1067. doi: 10.1099/00221287-130-5-1061. [DOI] [PubMed] [Google Scholar]
- Hochman A., Burris R. H. Effect of oxygen on acetylene reduction by photosynthetic bacteria. J Bacteriol. 1981 Aug;147(2):492–499. doi: 10.1128/jb.147.2.492-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery R. G., Saari L. L., Ludden P. W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986 May;166(2):513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Okon Y., Burris R. H. The nitrogenase system of Spirillum lipoferum. Biochem J. 1978 Sep 1;173(3):1001–1003. doi: 10.1042/bj1731001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund S., Kanemoto R. H., Murrell S. A., Ludden P. W. Properties and regulation of glutamine synthetase from Rhodospirillum rubrum. J Bacteriol. 1985 Jan;161(1):13–17. doi: 10.1128/jb.161.1.13-17.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER C. A., SCUTT P. B. The effect of oxygen on nitrogen fixation by Azotobacter. Biochim Biophys Acta. 1960 Feb 26;38:230–238. doi: 10.1016/0006-3002(60)91236-1. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Bodmer S., Tabita F. R. Oxygen inactivation and recovery of nitrogenase activity in cyanobacteria. J Bacteriol. 1983 Jan;153(1):182–190. doi: 10.1128/jb.153.1.182-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L. Characterization of an oxygen-stable nitrogenase complex isolated from Azotobacter chroococcum. Biochem J. 1979 Sep 1;181(3):569–575. doi: 10.1042/bj1810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Song S. D., Hartmann A., Burris R. H. Purification and properties of the nitrogenase of Azospirillum amazonense. J Bacteriol. 1985 Dec;164(3):1271–1277. doi: 10.1128/jb.164.3.1271-1277.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Burris R. H. Nature of oxygen inhibition of nitrogenase from Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1972 Mar;69(3):672–675. doi: 10.1073/pnas.69.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Cantu M. Changes in the regulatory form of Rhodospirillum rubrum nitrogenase as influenced by nutritional and environmental factors. J Bacteriol. 1980 Jun;142(3):899–907. doi: 10.1128/jb.142.3.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]