Abstract
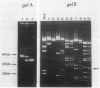
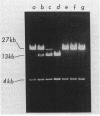
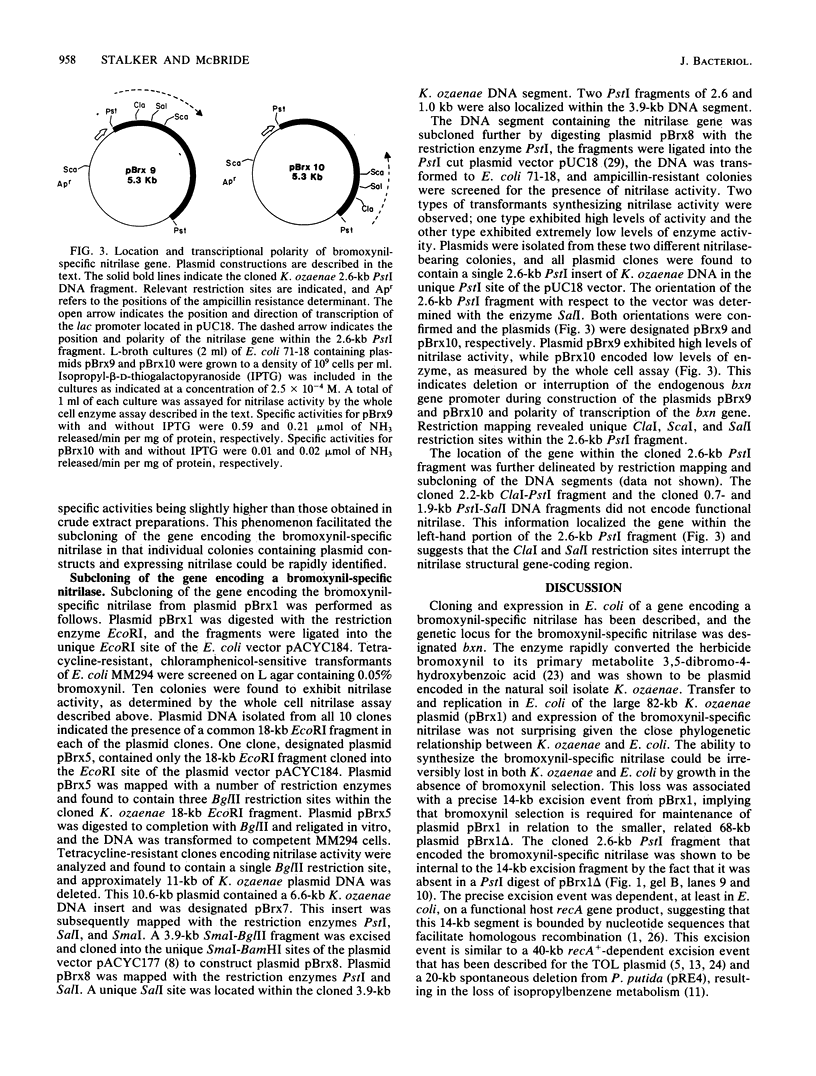
An enzyme (nitrilase) that converts the herbicide bromoxynil (3,5-dibromo-4-hydroxybenzonitrile) to its metabolite 3,5-dibromo-4-hydroxybenzoic acid was shown to be plasmid encoded in the natural soil isolate Klebsiella ozaenae. The bromoxynil-specific nitrilase was expressed in Escherichia coli by direct transfer and stable maintenance in E. coli of a naturally occurring 82-kilobase K. ozaenae plasmid. Irreversible loss of the ability to metabolize bromoxynil both in E. coli and K. ozaenae was associated with the conversion of the 82-kilobase plasmid to a 68-kilobase species. In E. coli this conversion was the result of a host recA+-dependent recombinational event. A gene, designated bxn, encoding the bromoxynil-specific nitrilase was constitutively expressed in K. ozaenae and E. coli and subcloned on a 2.6-kilobase PstI DNA segment. The polarity and the location of the gene were determined by assaying hybrid constructs of the bromoxynil-specific nitrilase gene fused with the heterologous lac promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Amy P. S., Schulke J. W., Frazier L. M., Seidler R. J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985 May;49(5):1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Nagasawa T., Asano Y., Fujishiro K., Tani Y., Yamada H. Purification and Characterization of Benzonitrilases from Arthrobacter sp. Strain J-1. Appl Environ Microbiol. 1986 Feb;51(2):302–306. doi: 10.1128/aem.51.2.302-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley S. A., Duggleby C. J., Worsey M. J., Williams P. A., Hardy K. G., Broda P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol Gen Genet. 1977 Jul 20;154(2):203–204. doi: 10.1007/BF00330838. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandsch R., Faller W., Schneider K. Plasmid pAO1 of Arthrobacter oxidans encodes 6-hydroxy-D-nicotine oxidase: cloning and expression of the gene in Escherichia coli. Mol Gen Genet. 1986 Jan;202(1):96–101. doi: 10.1007/BF00330523. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol. 1982 Jan;149(1):40–46. doi: 10.1128/jb.149.1.40-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Timmis K. N. Spontaneous deletion of a 20-kilobase DNA segment carrying genes specifying isopropylbenzene metabolism in Pseudomonas putida RE204. J Bacteriol. 1986 Oct;168(1):428–430. doi: 10.1128/jb.168.1.428-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. R., Appleton J., Pemberton J. M. Isolation and characterization of the pesticide-degrading plasmid pJP1 from Alcaligenes paradoxus. J Bacteriol. 1978 Sep;135(3):798–804. doi: 10.1128/jb.135.3.798-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Genes specifying degradation of 3-chlorobenzoic acid in plasmids pAC27 and pJP4. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1638–1642. doi: 10.1073/pnas.82.6.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Harper D. B. Characterization of a nitrilase from Nocardia sp. (Rhodochrous group) N.C.I.B. 11215, using p-hydroxybenzonitrile as sole carbon source. Int J Biochem. 1985;17(6):677–683. doi: 10.1016/0020-711x(85)90364-7. [DOI] [PubMed] [Google Scholar]
- Harper D. B. Fungal degradation of aromatic nitriles. Enzymology of C-N cleavage by Fusarium solani. Biochem J. 1977 Dec 1;167(3):685–692. doi: 10.1042/bj1670685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns J. S., Duttagupta S., Chakrabarty A. M. Regulation of 2,4,5-trichlorophenoxyacetic acid and chlorophenol metabolism in Pseudomonas cepacia AC1100. Appl Environ Microbiol. 1983 Nov;46(5):1182–1186. doi: 10.1128/aem.46.5.1182-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil H., Keil S., Pickup R. W., Williams P. A. Evolutionary conservation of genes coding for meta pathway enzymes within TOL plasmids pWW0 and pWW53. J Bacteriol. 1985 Nov;164(2):887–895. doi: 10.1128/jb.164.2.887-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride K. E., Kenny J. W., Stalker D. M. Metabolism of the herbicide bromoxynil by Klebsiella pneumoniae subsp. ozaenae. Appl Environ Microbiol. 1986 Aug;52(2):325–330. doi: 10.1128/aem.52.2.325-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulien P., Downing R. G., Broda P. Excision of the 40kb segment of the TOL plasmid from Pseudomonas putida mt-2 involves direct repeats. Mol Gen Genet. 1981;184(1):97–101. doi: 10.1007/BF00271202. [DOI] [PubMed] [Google Scholar]
- Monticello D. J., Bakker D., Finnerty W. R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985 Apr;49(4):756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Recombination activities of E. coli recA protein. Cell. 1981 Jul;25(1):3–4. doi: 10.1016/0092-8674(81)90224-5. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Lehrbach P. R., Timmis K. N. Use of cloned genes of Pseudomonas TOL plasmid to effect biotransformation of benzoates to cis-dihydrodiols and catechols by Escherichia coli cells. Appl Environ Microbiol. 1985 Dec;50(6):1409–1413. doi: 10.1128/aem.50.6.1409-1413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]