Abstract
Growth factor-derived mitogenic signals from the cell surface are transmitted to the nucleus via receptor tyrosine kinases (RTKs), the adaptor proteins Shc and Grb2, and a Ras-dependent protein kinase cascade that activates the extracellular signal regulated kinase (ERK) subfamily of mitogen-activated protein kinases. ERKs also are activated by hormones that stimulate G protein-coupled receptors (GPCRs). We report here that, in agreement with previous data, the epidermal growth factor receptor (EGFR) is a signaling intermediate in ERK activation by GPCRs. Of import, we show that cross-talk between two classes of surface receptors, RTKs and GPCRs, is a general feature. Lysophosphatidic acid not only induces ligand-independent tyrosine autophosphorylation of EGFR but also of platelet-derived growth factor β receptor (PDGF-β-R) as shown by detection of tyrosine phosphorylation and by the use of specific inhibitors of RTKs. The cross-talk appears to be cell type-specific: In L cells that lack EGFR, lysophosphatidic acid-induced Shc and ERK activation is prevented completely by specific inhibition of PDGFR, whereas in COS-7 cells expressing only EGFR, the pathway via EGFR is chosen. In Rat-1 cells, however, that express both EGFR and PDGFR, the EGFR pathway dominates.
Keywords: epidermal growth factor receptor/G protein-coupled receptors/MAP kinase/cross-talk
The proliferative response of mammalian cells is executed, at least in part, by transcription factors that are controlled by the extracellular signal regulated kinase (ERK) subfamily of mitogen-activated protein kinases. ERKs are at the end of one or several signaling cascades through which extracellular mitogenic stimuli are converted into proliferative responses (reviewed in ref. 1). The best studied cascade is triggered by growth factors that stimulate receptor tyrosine kinase (RTK) autophosphorylation and tyrosine phosphorylation of the adaptor proteins Shc and Grb2, followed by recruitment of the Ras guanine nucleotide exchange factor Sos. Assembly of this signaling complex leads to activation of the small GTP-binding protein Ras and subsequently of a protein kinase cascade reaching ERK (reviewed in refs. 2 and 3).
A diverse group of agonists (e.g., peptide hormones, neurotransmitters, and phospholipids) that do not directly interact with RTKs but rather act through G protein-coupled receptors (GPCRs) also induce proliferative responses and even cellular transformation in a variety of cell lines and tissues (reviewed in refs. 4–9). The major mitogenic activity in serum, in fact, is represented by a GPCR ligand, lysophosphatidic acid (LPA). LPA induces, e.g., proliferation of fibroblasts or of smooth muscle cells (reviewed in refs. 10 and 11) and tumor cell invasion (12). ERK activation or DNA synthesis induced by GPCRs involves receptor-specific activation of heterotrimeric G proteins, both pertussis toxin (PTX)-sensitive (Gi/o) and -insensitive (Gq) (reviewed in refs. 13 and 14). It is thought that both the α and the βγ subunits of heterotrimeric G proteins can lead to ERK activation. Giβγ subunits would account for the PTX-sensitive pathway (17–19). Gαq subunits appear to activate ERK through protein kinase C (PKC) (16, 19). Of interest, ERK activation by either Gi- or Gq-coupled receptors can be inhibited by genistein (15, 18, 20) and several GPCR agonists such as LPA cause tyrosine phosphorylation of a number of proteins including the adaptor protein Shc and induce Shc-Grb2 complex formation (15, 21–26). The nature of the tyrosine kinase involved has been subject to intense investigation. Recently, a novel pathway has been discovered: ligand-independent activation of RTKs (27–31). LPA, endothelin, or thrombin activate the epidermal growth factor receptor in Rat-1 cells in the absence of EGF. Inhibition of EGFR kinase activity leads to failure of these stimuli to activate ERK and cfos (31).
We report here the GPCR-mediated ligand-independent activation of PDGFR. This step becomes limiting in cells lacking EGFR. The data presented support the existence of crosstalk between GPCRs and several RTKs as a general mechanism.
MATERIALS AND METHODS
Reagents and Antibodies.
Culture media and Lipofectamine were from GIBCO, Protein A-Sepharose was from Pharmacia, platelet-derived growth factor receptor (PDGF)-BB was from BioMol (Plymouth Meeting, PA). All inhibitors were from Calbiochem. All other reagents were purchased from Sigma; radiochemicals were purchased from DuPont/NEN.
Antibodies were from the following suppliers: monoclonal 12CA5 anti-hemagglutinin (HA), Boehringer Mannheim; monoclonal anti-phosphotyrosine 4G10, Upstate Biotechnology; rabbit polyclonal anti-PDGF-β-R, Santa Cruz Biotechnology; rabbit polyclonal anti-EGFR, Santa Cruz Biotechnology; sheep polyclonal anti-EGFR, Upstate Biotechnology; rabbit polyclonal anti-ERK2, Santa Cruz Biotechnology; rabbit polyclonal anti-Shc, Transduction Laboratories (Lexington, KY); phosphospecific anti-ERK, New England Biolabs. Secondary antibodies were from Dianova (Hamburg, Germany). The enhanced chemiluminescence system was from Amersham.
Cell Culture and Transfections.
COS-7 (Genentech) and Rat-1 cells were cultured in DMEM containing 10% FCS, and L cells were cultured in α-MEM containing 10% FCS. All media were supplemented with penicillin (50 units/ml) and streptomycin (50 μg/ml).
COS-7 and L cells were transfected transiently at 70% confluency by using Lipofectamine as described by the manufacturer. For transfection of COS-7 cells in 12-well dishes, cells were incubated for 6 h in 0.4 ml of serum-free medium containing 4 μl of Lipofectamine and 0.8 μg of total plasmid DNA per well. With this protocol, transfection efficiency in COS-7 cells reached 80–90% measured by transfection of a β-galactosidase-containing construct. For transfection of L cells in 10-cm dishes, 4 ml of serum-free medium, 40 μl of Lipofectamine, and 10 μg of total plasmid DNA were used. At 48 h after transfection, cells were serum-starved for 16 h, then treated and lysed. Stable transfectants with the PDGF-β-R or the C terminus of the β-adrenergic receptor kinase (βARK-ct) were selected with 400 μg/ml Geneticin (G418; GIBCO) and tested by immunoblotting for expression. For 32P-labeling of cells, cells were incubated in phosphate-deficient medium for 16 h and then supplemented with 500 μCi/ml 32P-orthophosphate for 2–3 h.
Cell Lysis, Immunoprecipitation, SDS/PAGE, and Immunoblotting.
Cells grown to confluency were washed with PBS, serum-starved for 16 h, and then treated with inhibitors and agonists as indicated. Before lysis, cells were washed once with PBS and lysed for 10 min on ice in lysis buffer containing 50 mM Hepes (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10% glycerol, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml aprotinin. Lysates were precleared by centrifugation, supernatants diluted with an equal volume of dilution buffer containing 20 mM Hepes (pH 7.5), 150 mM NaCl, 0,1% Triton X-100, and 10% glycerol and subsequently were subjected to immunoprecipitation at 4°C overnight by using the antibodies indicated in the figures. Subsequently, 30 μl of Protein A-Sepharose beads (12.5%, wt/vol) was added, incubated for 3–4 h, and precipitated by centrifugation. Precipitates were washed three times with dilution buffer, resuspended in SDS sample buffer, boiled for 3 min, and subjected to SDS/PAGE. After SDS/PAGE, proteins were transferred to a nitrocellulose membrane and immunoblotted.
ERK Mobility Shift Assay.
Cells were grown to confluency in 6-well dishes, washed once with PBS, serum-starved for 16 h, washed again, and lysed on ice in SDS sample buffer. Lysates were then subjected to SDS/PAGE on 10% gels, and ERK isoforms were visualized by immunoblotting.
In Vitro ERK Assay.
Epitope-tagged HA-ERK2 or endogenous ERK was immunoprecipitated from lysates obtained from 12-well dishes by using 2.5 μg of 12CA5 antibody or 5 μl of anti-ERK2 antibody, respectively, and was washed three times with 0.25 ml of dilution buffer (see above) and once with 0.4 ml of kinase buffer containing 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM DTT, and 200 μM sodium-orthovanadate. Subsequently, kinase reactions were performed in 30 μl of kinase buffer supplemented with 0.5 mg/ml myelin basic protein, 50 μM ATP, and 1 μCi [γ-32P]ATP for 10 min at room temperature. Reactions were stopped by addition of 30 μl of 2× sample buffer and subjected to SDS/15%PAGE. The upper part of nitrocellulose transfers were immunoblotted with anti-ERK2. Labeled myelin basic protein on the lower part (below 20–25 kDa) was quantified by phosphorimaging (Fuji).
RESULTS
Cell Type-Specific Differences in LPA-Mediated Mitogenic Activity.
In a comparison of GPCR-mediated mitogenic pathways in COS-7, Rat-1, and L cells, widely studied model systems for GPCR-signaling, we came across a striking difference in LPA-induced ERK activation. In Rat-1 and COS-7 but not L cells, LPA treatment led to strong ligand-independent tyrosine phosphorylation of the endogenous EGFR, approximately equivalent to that seen after stimulation with 3 ng/ml EGF (Fig. 1 and ref. 32). The phosphorylated EGFR was functional in that it tyrosine-phosphorylated the adaptor protein Shc, a critical step in RTK-mediated stimulation of Ras-dependent ERK activity (reviewed in refs. 2 and 3), as detected in immunoprecipitated Shc isoforms from either COS-7 or Rat-1 cells (Fig. 1 and see also ref. 32). A tyrosine-phosphorylated 180-kDa protein coprecipitated with Shc from COS-7 cell lysates (Fig. 1, longer exposure) also stained with the anti-EGFR antibody (data not shown), suggesting that EGFR was responsible for LPA-mediated Shc tyrosine phosphorylation in COS-7 cells. In further support of functionality of GPCR-activated EGFR, the inhibition of EGFR by either the EGFR kinase-selective tyrphostin AG1478 (33) or by expression of a truncated kinase-deficient dominant negative EGFR mutant, HER-CD533 (34), eliminated LPA-induced activation of Shc and ERK (in vitro kinase assay) both in Rat-1 and COS-7 cells (Fig. 1, Rat-1 and COS-7). Other tyrosine kinase inhibitors, e.g., the PDGFR kinase-selective tyrphostin AG1296, were ineffective (Fig. 1, Rat-1).
Figure 1.
Effect of receptor tyrosine kinase inhibition on LPA-mediated signaling in Rat-1, COS-7, and L cells. (Left, Rat-1) Quiescent Rat-1 cells were pretreated with either AG1296 or AG1478 at the concentrations indicated and stimulated for 5 min with 1% BSA (control, CO), 10 μM LPA, 10 ng/ml EGF, or 30 ng/ml PDGF-BB as indicated. (Top) Lysates were immunoprecipitated (IP) with anti-EGFR (αEGFR) followed by SDS/PAGE and immunoblotting with anti-phosphotyrosine (αPY). The filter then was reprobed with αEGFR. (Middle) Lysates were immunoprecipitated (IP) with anti-Shc (αShc), and proteins were resolved by SDS/PAGE and immunoblotted with anti-phosphotyrosine (αPY). (Bottom) Lysates from Rat-1 cells were immunoprecipitated (IP) with anti-ERK (αERK). In vitro kinase activity of precipitated endogenous ERK with myelin basic protein (MBP) as substrate was assessed as described in Materials and Methods. Phosphorylated myelin basic protein was visualized by autoradiography. (Center, L cells) After pretreatment with solvent (DMSO) or with 1 μM AG1478, quiescent L cells were stimulated for 5 min with 1% BSA (control, CO), 25 μM LPA, 2 units/ml thrombin receptor peptide (TRP), UV light (100 J/m2 UV C), 10 ng/ml EGF, or 10 ng/ml PDGF-BB. An ERK mobility shift assay was used to detect ERK activation (see Materials and Methods). (Right, COS-7) Confluent serum-starved COS-7 cells were treated with agonists as above. (Top) Lysates from COS-7 cells transiently transfected with either vector alone or dominant negative EGFR (HER-CD533) were immunoprecipitated (IP) with anti-EGFR (αEGFR). After SDS/PAGE, immunoblotting with anti-phosphotyrosine (αPY) was performed. The filter was reprobed with anti-EGFR (αEGFR). (Middle) COS-7 cell lysates were processed as described under Rat-1, middle panel. Shc isoforms with different molecular weights are indicated on the right (p66, p52, p46). The filter was reprobed with anti-Shc (αShc). Two different exposures of the same blot are shown. (Lower) COS-7 cells were transfected with HA-ERK2, pretreated with AG1478, or control-treated. HA-ERK2 was immunoprecipitated with anti-HA (αHA), and kinase activity was assessed with an in vitro ERK assay (see Materials and Methods).
In L cells, LPA also caused ERK activation as measured by ERK mobility-shift assays (Fig. 1, L cells). In contrast to Rat-1 and COS-7 cells, however, the LPA action was not inhibited by either AG1478 (Fig. 1, L cells) or the dominant negative EGFR mutant (data not shown). Also, ERK activation by another inducer of a ligand-independent EGFR pathway, UV irradiation (27), was not inhibited by AG1478 (Fig. 1, L cells) or the EGFR mutant (data not shown). In contrast, AG1478 and the dominant negative EGFR mutant blocked the response to UV irradiation in other cells (27, 29). This result prompted us to investigate whether L cells expressed a functional EGFR at all and how LPA nevertheless could activate ERK in L cells.
LPA-Mediated Mitogenic Activity in EGFR-Deficient Cells.
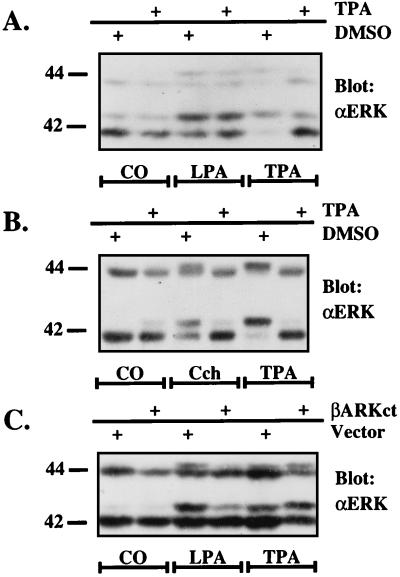
EGF concentrations of up to 100 ng/ml had no effect on ERK activity in L cells (Fig. 1, L cells), and by Western blot analysis no EGFR could be detected (data not shown). We conclude therefore that L cells do not express EGFR. In search of an alternative pathway that could be used by LPA to activate ERK in L cells, we considered two possibilities: (i) a pathway linking an LPA receptor (LPAR) to Gq/PKC or (ii) involvement of another receptor tyrosine kinase. We have shown previously that LPA-mediated inositol phosphate accumulation in L cells is 60–80% sensitive to PTX pretreatment, indicating predominant involvement of GI proteins (35). A minor contribution of Gq/PKC, however, cannot be excluded. To explore this possibility, we either depleted PKC by prolonged pretreatment of the cells with phorbol 12-tetradecanoate 13-acetate (TPA) (Fig. 2A) or blocked PKC activity by short term incubations with the specific PKC inhibitor GF109203X (36) (data not shown). Both treatments had no effect on LPA-stimulated ERK activity, whereas ERK activation after stimulation of stably expressed Gq-coupled m5-muscarinic receptor (m5R), supposedly acting through PKC (35, 37), was completely inhibited by TPA pretreatment (Fig. 2B) or by the PKC inhibitor (data not shown). These results suggested that LPA-mediated ERK activity in L cells depended on Gi proteins.
Figure 2.
Effect of PKC inhibition on ERK activation via the LPA receptor and the m5-muscarinic receptor (A + B). Effect of βARKct on LPA-mediated ERK activation in L cells (C). Quiescent L cells were pretreated with DMSO or with 100 ng/ml TPA for 16 h (A and B) or not pretreated at all (C). The cells were then stimulated as indicated with agonists [1% BSA (control, CO), 25 μM LPA, or 100 nM carbachol (Cch), 100 ng/ml TPA], lysed and subjected to an ERK mobility shift assay (see Materials and Methods). (A and B) After a 5-min incubation of cells with 1% BSA (control, CO), 25 μM LPA, or 100 ng/ml TPA, ERK activation was compared in L cells stably transfected with vector alone or with the βARKct plasmid (C).
ERK activation by Gi- in contrast to Gq-coupled receptors should be sensitive to the Gβγ sequestrants βARKct or transducin α (16, 17, 19). Indeed, in L cells stably expressing the Gβγ-scavenger βARKct (17, 35), LPA-induced ERK activation was strongly inhibited (Fig. 2C). Therefore, we conclude that LPA in L cells is linked to the ERK pathway in a Giβγ-dependent manner and does not involve PKC.
LPA-Mediated Mitogenic Activity in L Cells Requires the PDGFR as an Essential Signaling Intermediate.
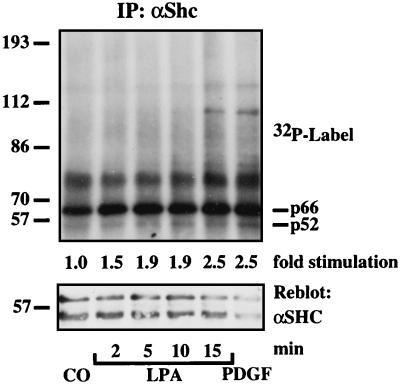
To explore the possible involvement of other RTKs in GPCR-mediated ERK activation in L cells, we first analyzed tyrosine phosphorylation of the adaptor protein Shc as an indicator of RTK activity. In Shc immunoprecipitates of 32P-labeled L cells, we detected comparable levels of LPA- and PDGF-stimulated Shc tyrosine phosphorylation within 15 min (Fig. 3). Despite 24 h of serum starvation of L cells, basal Shc tyrosine phosphorylation remained relatively high, indicating unavoidable spontaneus Shc activation. Candidate RTKs possibly responsible for the LPA-dependent increase in Shc tyrosine phosphorylation were detected by Western blotting: PDGF-β-receptor and Her2/neu, an EGFR-family member (data not shown). To sensitively assay for a ligand-independent autophosphorylation of RTKs in response to LPA, we first enriched all glycosylated RTKs by precipitation with agarose-coupled lens culinaris lectin. The precipitates were subjected to SDS/PAGE and probed with anti-phosphotyrosine. In these fractions, a 190-kDa protein was phosphorylated consistently in response to LPA. It comigrated with a protein phosphorylated in response to PDGF and corresponded in size to the PDGFR (not shown).
Figure 3.
Shc activation in response to LPA in L cells. Confluent L cells were serum-starved in phosphate-deficient medium for 16 h, pulsed with 500 μCi/ml 32P-orthophosphate for 3 h, and stimulated with 1% BSA (15 min, CO), 25 μM LPA (indicated time points), or 10 ng/ml PDGF-BB (15 min). Incorporation of 32P into Shc was measured by autoradiography and densitometric scanning. Shc isoforms of different molecular masses are indicated (p66, p52). The filter was reprobed with anti-Shc (αShc).
Most interesting to note, when L cells were pretreated with the PDGFR kinase-selective inhibitor AG1296 (38), LPA-induced ERK activation was attenuated strongly (as measured by ERK mobility-shift assays; Fig. 4). These results support the idea that, in L cells, PDGF-β-receptor autophosphorylation is an essential signaling step in ERK activation by LPA. Furthermore, LPA caused tyrosine phosphorylation of the immunoprecipitated PDGF-β-R in hPDGF-β-R (39) overexpressing L cells (Fig. 5A), which again was abrogated by AG1296 (Fig. 5B) as was Shc tyrosine phosphorylation and ERK activation (data not shown). Immunoprecipitated HER2/neu, in contrast, was not phosphorylated (data not shown). In a transient cotransfection assay, overexpression of a truncated kinase-deficient dominant negative PDGFR mutant (PDGF-β-R-CD504) (40) abolished LPA- and PDGF-stimulated activation of an epitope-tagged version of ERK2, HA-ERK2 (41) (Fig. 5C). These data imply that activation of the intrinsic kinase activity of the receptor is necessary for the LPA signal and demonstrate a role for PDGF-β-R in LPA/Gi-stimulated ERK activation in L cells.
Figure 4.
Effect of AG1296 and AG1478 on LPA-induced ERK activation in L cells. Confluent, serum-starved L cells were pretreated with DMSO, AG1296, or AG1478 at the indicated concentrations. Before lysis, the cells were incubated for 5 min with 1% BSA (control, CO), 25 μM LPA, or 10 ng/ml PDGF-BB. ERK activation was detected with an ERK mobility shift assay (see Materials and Methods).
Figure 5.
LPA signaling to ERK in L cells involves PDGFR. Confluent 15-cm dishes were serum-starved for 16 h and stimulated with 1% BSA (control, CO) (10 min), 3 ng/ml PDGF-BB (10 min), or 25 μM LPA (indicated time points). Lysates were immunoprecipitated with anti-PDGF-β-R (αPDGF-β-R) followed by SDS/PAGE and immunoblotting with anti-phosphotyrosine (αPY) (Left). In a similar experiment, the filter was reprobed with anti-PDGF-β-R (αPDGF-β-R) (Right) (A). Quiescent L cells were preincubated for 10 min with DMSO or 10 μM AG1296. Tyrosine phosphorylation of the precipitated PDGF-β-R was determined by immunoblotting with anti-phosphotyrosine (αPY) (B). L cells were transfected transiently with HA-ERK2 and either vector alone or a dominant negative PDGF-β-R. The cells were serum-starved for 16 h and stimulated (5 min) before lysis with 1% BSA (control, CO), 10 ng/ml PDGF-BB, or 25 μM LPA. Lysates were immunoprecipitated with anti-HA (αHA) and subjected to SDS/PAGE. ERK activation was detected by immunoblotting with a phopho-specific anti-ERK (αPY-ERK) that only detects tyrosine-phosphorylated activated ERKs (C).
LPA-Mediated Mitogenic Activity in Rat-1 Cells Expressing Both EGFR and PDGFR.
To investigate which RTK, PDGFR or EGFR or both, mediated mitogenic activity induced by LPA in a cell line functionally expressing both RTKs (31), we chose Rat-1 cells. In these cells, LPA-induced, ligand-independent EGFR tyrosine phosphorylation and ERK activation were unaffected by AG1296 (Fig. 1, Rat-1), whereas AG1296 inhibited the effect of PDGF. On the other hand, AG1478 (100 nM) completely blocked LPA- and EGF-induced ERK activity and EGFR tyrosine phosphorylation (31), leaving PDGF signaling unaltered (Fig. 1, Rat-1). In agreement with these observations, AG1296, even at the high concentration of 30 μM, was unable to attenuate LPA-mediated Shc tyrosine phosphorylation, whereas that stimulated by PDGF was already fully blocked with 10 μM AG1296 (Fig. 1, Rat-1). In contrast, AG1478 at the low concentration of 100 nM completely blocked LPA-induced but not PDGF-induced Shc tyrosine phosphorylation (Fig. 1, Rat-1). These results prove the specific action of the inhibitors and suggest that, in cells expressing EGFR plus PDGFR such as Rat-1 cells, EGFR and Her2/neu (31) are used preferentially to mediate ERK activation in response to GPCR ligands, whereas PDGFR does not seem to be involved to a detectable degree.
DISCUSSION
In the present paper, we demonstrate that ligand-independent receptor tyrosine kinase activity involving different RTKs is a necessary intermediate in the mitogenic response to LPA, a stimulus to which most cells are responsive (reviewed in ref. 10). Stimulation of an endogenous GPCR for LPA in COS-7 and Rat-1 cells resulted in ligand-independent tyrosine phosphorylation and activation of EGFR and of the adaptor protein Shc. Most important to note, we found that, in EGFR-deficient L cells, PDGF-β-R can substitute for EGFR in mediating ERK activation by LPA. Inhibition of RTK activity abolished LPA-mediated RTK and Shc tyrosine phosphorylation as well as ERK activation. Our results document a cross-talk at the receptor level between a GPCR for LPA and at least two RTKs, PDGFR and EGFR. Other stimuli, e.g., UV irradiation, calcium, and hyperosmotic shock, also cause ligand-independent tyrosine phosphorylation and activation of a variety of RTKs (27–30). Provided the mechanisms are similar (to be discussed below), one may speculate that GPCRs link to many RTKs, suggesting that the crosstalk between GPCRs and RTKs represents a general principle of GPCR action. The generalization is supported further by reports on ligand-independent phosphorylation of the PDGF-β-R (42), of the insulin-like growth factor-1 receptor, of insulin receptor substrate-1, and of phospholipase Cγ in response to angiotensin II and thrombin in primary rat vascular smooth muscle cells (43, 44).
Ligand-independent activation of RTKs by GPCRs occurs in a cell type-specific manner, possibly reflecting either relative abundance of RTKs or preferential coupling to particular RTKs. In COS-7 cells devoid of functional endogenous PDGFR and also in Rat-1 cells where PDGFR is coexpressed endogenously with high levels of EGFR, EGFR inhibition by AG1478 resulted in a complete loss of LPA-stimulated ERK activity. On the contrary, in L cells that lack EGFR, the PDGFR represented the dominant signaling intermediate in ERK activation by LPA. The fact that, in contrast to Rat-1 cells (31), Her2/neu was not activated in response to LPA in L cells possibly can be explained by the absence of EGFR; ligand-independent activation of Her2/neu by LPA or UV irradiation appears to require heterodimer formation of Her2/neu with EGFR (29, 31). However, we cannot exclude that the failure to detect Her2/neu activation by GPCRs reflects inefficient coupling of Her2/neu to the Gi-mediated signaling pathway in the absence of EGFR.
In many cells, LPA stimulates G proteins of the Gi-, Gq-, and G12/13 families (reviewed in ref. 11), and Gα as well as Gβγ subunits may be involved in ERK activation by GPCRs (17–19). In L cells, the effects of LPA on ERK activity are mediated by Giβγ subunits because the signal is PTX-sensitive and is inhibited by coexpression of the Gβγ scavenger βARKct. Although PTX-insensitive G proteins are expressed in L cells (35), Gq does not seem to participate in the mitogenic response to LPA because the mitogenic response is not blocked by PKC inhibition. Finally, the PTX-insensitive G12/13 proteins are linked functionally to Jun-N-terminal kinase rather than to ERK (41, 45, 46). G12/13 may contribute to the LPA- (and serum-) induced overall mitogenic response by activation of an as-yet-unidentified pathway via the small GTP-binding protein Rho (47).
RTKs act as dimers: one subunit phosphorylating the other and vice versa. According to current models (reviewed in refs. 2 and 3), the RTK ligands induce dimerization and thereby autophosphorylation. The discovery of ligand-independent RTK-activation (27–31) strongly suggests that dimerization, at least, cannot be the only mechanism of activation. Moreover, ligand-independent activation of RTKs by UV irradiation causes prolonged lifetime of their tyrosine phosphates and regulation can be directly attributed to protein tyrosine phosphatases (PTPs) (ref. 29 and S. Groβ, A.K., T. Tenev, A. Neininger, A. Deck, P.H., M. Gaestel, and F. Böhmer, unpublished work). Inhibition of a specific PTP also may account for GPCR-mediated RTK activation because antioxidants (which are thought to prevent inhibition of PTPases) were shown to inhibit LPA-induced activation of the ERK pathway in HeLa cells (48).
Remarkably, UV- or Ca2+-induced signaling through RTKs requires c-Src function (49, 50). Recently, it has been suggested that Src-family kinases mediate LPA/Giβγ-dependent phosphorylation of EGFR and subsequently ERK activation in COS-7 cells independently of RTK activity (51). Our results demonstrate, however, that, in the case of LPA-mediated ERK activation, ligand-independent autophosphorylation of RTKs is necessary because the kinase-selective tyrphostins AG1478 and AG1296 as well as dominant negative mutants of both PDGFR and EGFR abolished LPA-mediated RTK tyrosine phosphorylation and activation of ERK. However, RTKs and nonreceptor tyrosine kinases may cooperate in the mitogenic response to GPCRs; c-Src is necessary for mitogenic signaling by EGF and PDGF (52–54) and associates with (55, 56) and phosphorylates (57–59) both RTKs at nonautophosphorylation sites. In the case of EGFR, this confers binding of c-Src to EGFR (58). Mutation of such a c-Src-dependent phosphorylation site in the PDGFR reduces the mitogenic response to PDGF (59). One therefore could imagine that ligand-independent autophosphorylation of RTKs is enhanced by c-Src phosphorylation of the receptors, and both kinases may act as scaffolds for adaptor molecules like Shc. Shc then would be accessible for phosphorylation by RTKs and/or c-Src or a c-Src-like kinase (60). A recent report suggests that other cytoplasmic tyrosine kinases could possibly connect GPCRs to ERK activation independently of Src and Shc tyrosine phosphorylation via a novel, yet unidentified 100-kDa Grb2-binding protein (61).
In the past, signal transduction pathways were perceived as linear processes from the cell surface to the nucleus. We now begin to understand signaling as a complex network, so far established at the level of downstream signaling molecules (62). A new level of complexity is added with the realization that cross-talk also exists between different classes of surface receptors: GPCRs and RTKs. Our contribution here is the demonstration that such cross-talk occurs in a cell type-specific manner via ligand-independent activation of different RTKs by the same GPCR ligand.
Acknowledgments
We are grateful to S. Gutkind, C.-H. Heldin, C. Sachsenmaier. and J. Cooper for supplying us with different plasmids and reagents. We thank H. J. Rahmsdorf for stimulating discussions. A.H. was supported by the Boehringer Ingelheim Fonds. T.G. and G.S. were supported by grants from Deutsche Forschungsgemeinschaft, and H.D. and A.U. were supported by grants from Sugen, Inc.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: βARKct, C terminus of the β-adrenergic receptor kinase; EGFR, epidermal growth factor receptor; ERK, extracellular signal regulated kinase; GPCR, G protein-coupled receptor; HA, influenza virus hemagglutinin; LPA, lysophosphatidic acid; m5R, m5 muscarinic acetylcholine receptor; PDGFR, platelet-derived growth factor receptor; PKC, protein kinase C; PTX, pertussis toxin; RTK, receptor tyrosine kinase; TPA, phorbol 12-tetradecanoate 13-acetate.
References
- 1.Treisman R. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 2.Weiss F U, Daub H, Ullrich A. Curr Opin Genet Dev. 1997;7:80–86. doi: 10.1016/s0959-437x(97)80113-x. [DOI] [PubMed] [Google Scholar]
- 3.Heldin C H. Cancer Surv. 1996;27:7–24. [PubMed] [Google Scholar]
- 4.Dhanasekaran N, Heasley L E, Johnson G L. Endocr Rev. 1995;16:259–270. doi: 10.1210/edrv-16-3-259. [DOI] [PubMed] [Google Scholar]
- 5.Shenker A, Laue L, Kosugi S, Merendino J J, Jr, Minegishi T, Cutler G B., Jr Nature (London) 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- 6.Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, Dumont J, Vassart G. Nature (London) 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- 7.Landis C A, Masters S B, Spada A, Pace A M, Bourne H R, Vallar L. Nature (London) 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 8.Lyons J, Landis C A, Harsh G, Vallar L, Grunewald K, Feichtinger H, Duh Q Y, Clark O H, Kawasaki E, Bourne H R, et al. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- 9.Hermouet S, Merendino J J, Jr, Gutkind J S, Spiegel A M. Proc Natl Acad Sci USA. 1991;88:10455–10459. doi: 10.1073/pnas.88.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalink K, Hordijk P L, Moolenaar W H. Biochim Biophys Acta. 1994;1198:185–196. doi: 10.1016/0304-419x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 11.Moolenaar W H. Curr Opin Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 12.Imamura F, Shinkai K, Mukai M, Yoshioka K, Komagome R, Iwasaki T, Akedo H. Int J Cancer. 1996;65:627–632. doi: 10.1002/(SICI)1097-0215(19960301)65:5<627::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Endocr Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 14.van Corven E J, Groenink A, Jalink K, Eichholtz T, Moolenaar W H. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 15.van Corven E J, Hordijk P L, Medema R H, Bos J L, Moolenaar W H. Proc Natl Acad Sci USA. 1993;90:1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawes B E, van Biesen T, Koch W J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 17.Koch W J, Hawes B E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 19.Faure M, Voyno-Yasenetskaya T A, Bourne H R. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 20.Sadoshima J, Izumo S. EMBO J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]
- 21.Hordijk P L, Verlaan I, van Corven E J, Moolenaar W H. J Biol Chem. 1994;269:645–651. [PubMed] [Google Scholar]
- 22.Cazaubon S M, Ramos-Morales F, Fischer S, Schweighoffer F, Strosberg A D, Couraud P O. J Biol Chem. 1994;269:24805–24809. [PubMed] [Google Scholar]
- 23.Ohmichi M, Matuoka K, Takenawa T, Saltiel A R. J Biol Chem. 1994;269:1143–1148. [PubMed] [Google Scholar]
- 24.van Biesen T, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Nature (London) 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 25.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Grall D, Salcini A E, Pelicci P G, Pouyssegur J, Van Obberghen-Schilling E. EMBO J. 1996;15:1037–1044. [PMC free article] [PubMed] [Google Scholar]
- 27.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf H J. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 28.Coffer P J, Burgering B M, Peppelenbosch M P, Bos J L, Kruijer W. Oncogene. 1995;11:561–569. [PubMed] [Google Scholar]
- 29.Knebel A, Rahmsdorf H J, Ullrich A, Herrlich P. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen L B, Greenberg M E. Proc Natl Acad Sci USA. 1996;93:1113–1118. doi: 10.1073/pnas.93.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daub H, Weiss F U, Wallasch C, Ullrich A. Nature (London) 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 32.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 34.Kashles O, Yarden Y, Fischer R, Ullrich A, Schlessinger J. Mol Cell Biol. 1991;11:1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrlich A, Kühn B, Grosse R, Schmid A, Schultz G, Gudermann T. J Biol Chem. 1996;271:16764–16772. doi: 10.1074/jbc.271.28.16764. [DOI] [PubMed] [Google Scholar]
- 36.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 37.Liao C F, Schilling W P, Birnbaumer M, Birnbaumer L. J Biol Chem. 1990;265:11273–11284. [PubMed] [Google Scholar]
- 38.Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin C H, Waltenberger J, Bohmer F D, Levitzki A. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 39.Kashishion A, Kazlauskas A, Copper J. EMBO J. 1992;11:1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strawn L M, Mann E, Elliger S S, Chu L M, Germain L L, Niederfellner G, Ullrich A, Shawver L K. J Biol Chem. 1994;269:21215–21222. [PubMed] [Google Scholar]
- 41.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 42.Linseman D A, Benjamin C W, Jones D A. J Biol Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 43.Rao G N, Delafontaine P, Runge M S. J Biol Chem. 1995;270:27871–27875. doi: 10.1074/jbc.270.46.27871. [DOI] [PubMed] [Google Scholar]
- 44.Du J, Sperling L S, Marrero M B, Phillips L, Delafontaine P. Biochem Biophys Res Commun. 1996;218:934–939. doi: 10.1006/bbrc.1996.0165. [DOI] [PubMed] [Google Scholar]
- 45.Dhanasekaran N, Dermott J M. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 46.Collins L R, Minden A, Karin M, Brown J H. J Biol Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- 47.Hill S, Wynne J, Treisman R. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 48.Chen Q, Olashaw N, Wu J. J Biol Chem. 1995;270:28499–28502. doi: 10.1074/jbc.270.48.28499. [DOI] [PubMed] [Google Scholar]
- 49.Devary Y, Gottlieb R A, Smeal T, Karin M. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 50.Rusanescu G, Qi H, Thomas S M, Brugge J S, Halegoua S. Neuron. 1995;15:1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 51.Luttrell L M, Della Rocca G J, van Biesen T, Luttrell D K, Lefkowitz R J. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 52.Twamley-Stein G M, Pepperkok R, Ansorge W, Courtneidge S A. Proc Natl Acad Sci USA. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erpel T, Alonso G, Roche S, Courtneidge S A. J Biol Chem. 1996;271:16807–16812. doi: 10.1074/jbc.271.28.16807. [DOI] [PubMed] [Google Scholar]
- 54.Broome M A, Hunter T. J Biol Chem. 1996;271:16798–16806. doi: 10.1074/jbc.271.28.16798. [DOI] [PubMed] [Google Scholar]
- 55.Kypta R M, Goldberg Y, Ulug E T, Courtneidge S A. Cell. 1990;62:481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- 56.Luttrell D K, Lee A, Lansing T J, Crosby R M, Jung K D, Willard D, Luther M, Rodriguez M, Berman J, Gilmer T M. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wasilenko W J, Payne D M, Fitzgerald D L, Weber M J. Mol Cell Biol. 1991;11:309–321. doi: 10.1128/mcb.11.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stover D R, Becker M, Liebetanz J, Lydon N B. J Biol Chem. 1995;270:15591–15597. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 59.Hansen K, Johnell M, Siegbahn A, Rorsman C, Engstrom U, Wernstedt C, Heldin C H, Ronnstrand L. EMBO J. 1996;15:5299–5313. [PMC free article] [PubMed] [Google Scholar]
- 60.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 61.Kranenburg O, Verlaan I, Hordijk P L, Moolenaar W H. EMBO J. 1997;16:3097–3105. doi: 10.1093/emboj/16.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gudermann T, Kalkbrenner F, Schultz G. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]