Abstract
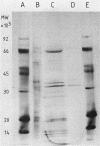
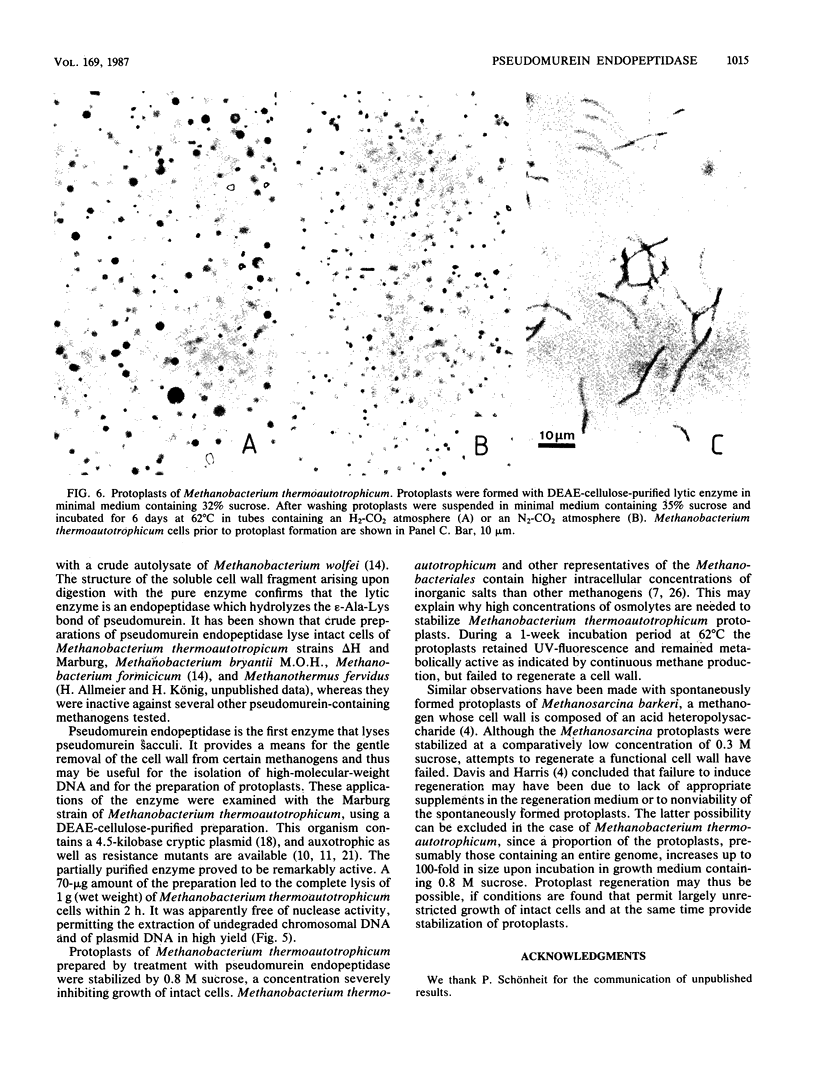
The pseudomurein-degrading enzyme from autolysates of Methanobacterium wolfei was purified approximately 500-fold to electrophoretic homogeneity by ion-exchange chromatography under anaerobic conditions. Analysis of the soluble cell wall fragments produced by the pure enzyme from a cell wall preparation of M. thermoautotrophicum indicated that it is a peptidase hydrolyzing the epsilon-Ala-Lys bond of pseudomurein. A partially purified preparation of pseudomurein endopeptidase was free of nuclease activity and thus proved useful for the preparation in high yields of undegraded chromosomal and plasmid DNA from M. thermoautotrophicum. The partially purified enzyme was also used for the preparation of protoplasts, which were stabilized by 0.8 M sucrose. Under growth conditions the protoplasts produced methane and increased up to 100-fold in size, but failed to regenerate a cell wall.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush J. W. Enzymatic lysis of the pseudomurein-containing methanogen Methanobacterium formicicum. J Bacteriol. 1985 Jul;163(1):27–36. doi: 10.1128/jb.163.1.27-36.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Bowers B., Stadtman T. C. Methanococcus vannielii: ultrastructure and sensitivity to detergents and antibiotics. J Bacteriol. 1977 Jun;130(3):1357–1363. doi: 10.1128/jb.130.3.1357-1363.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Kandler O., Jensen M., Rietschel E. T. The primary structure of the glycan moiety of pseudomurein from Methanobacterium thermoautotrophicum. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):627–636. doi: 10.1515/bchm2.1983.364.1.627. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meile L., Kiener A., Leisinger T. A plasmid in the archaebacterium Methanobacterium thermoautotrophicum. Mol Gen Genet. 1983;191(3):480–484. doi: 10.1007/BF00425766. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Silver staining methods for polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:230–239. doi: 10.1016/s0076-6879(83)96021-4. [DOI] [PubMed] [Google Scholar]
- Mountfort D. O., Mörschel E., Beimborn D. B., Schönheit P. Methanogenesis and ATP synthesis in a protoplast system of Methanobacterium thermoautotrophicum. J Bacteriol. 1986 Nov;168(2):892–900. doi: 10.1128/jb.168.2.892-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Mahadevan S., Erfle J. D. Methane synthesis by membrane vesicles and a cytoplasmic cofactor isolated from Methanobacterium thermoautotrophicum. Biochem J. 1984 Jul 1;221(1):61–69. doi: 10.1042/bj2210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Jarrell K. F. K+, Na+, and Mg2+ content and permeability of Methanospirillum hungatei and Methanobacterium thermoautotrophicum. Can J Microbiol. 1981 Apr;27(4):444–451. doi: 10.1139/m81-067. [DOI] [PubMed] [Google Scholar]