Abstract
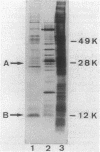
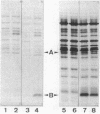
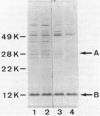
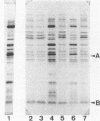
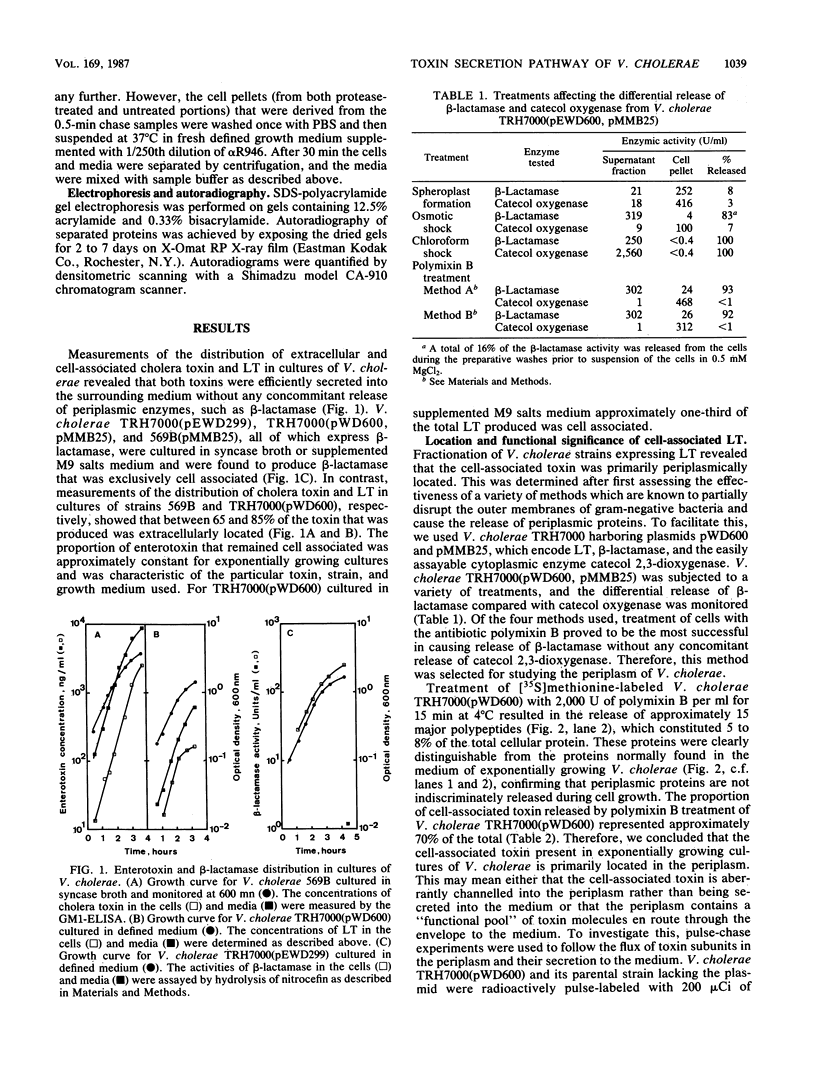
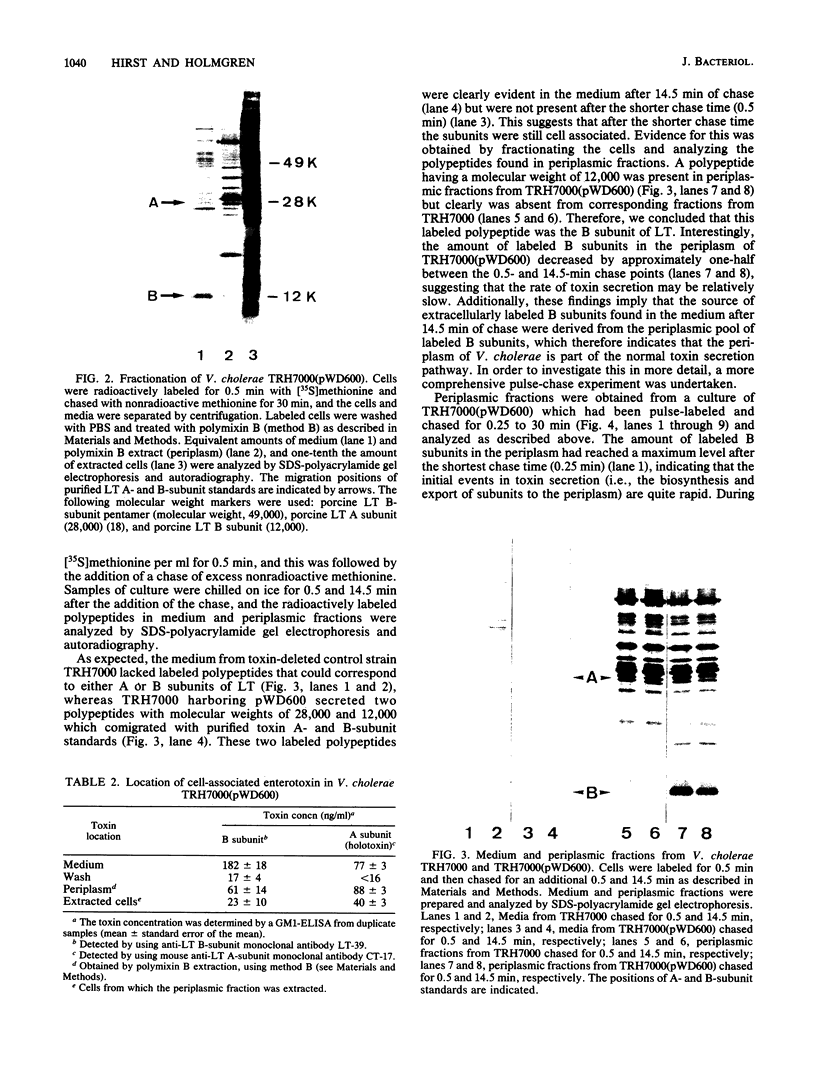
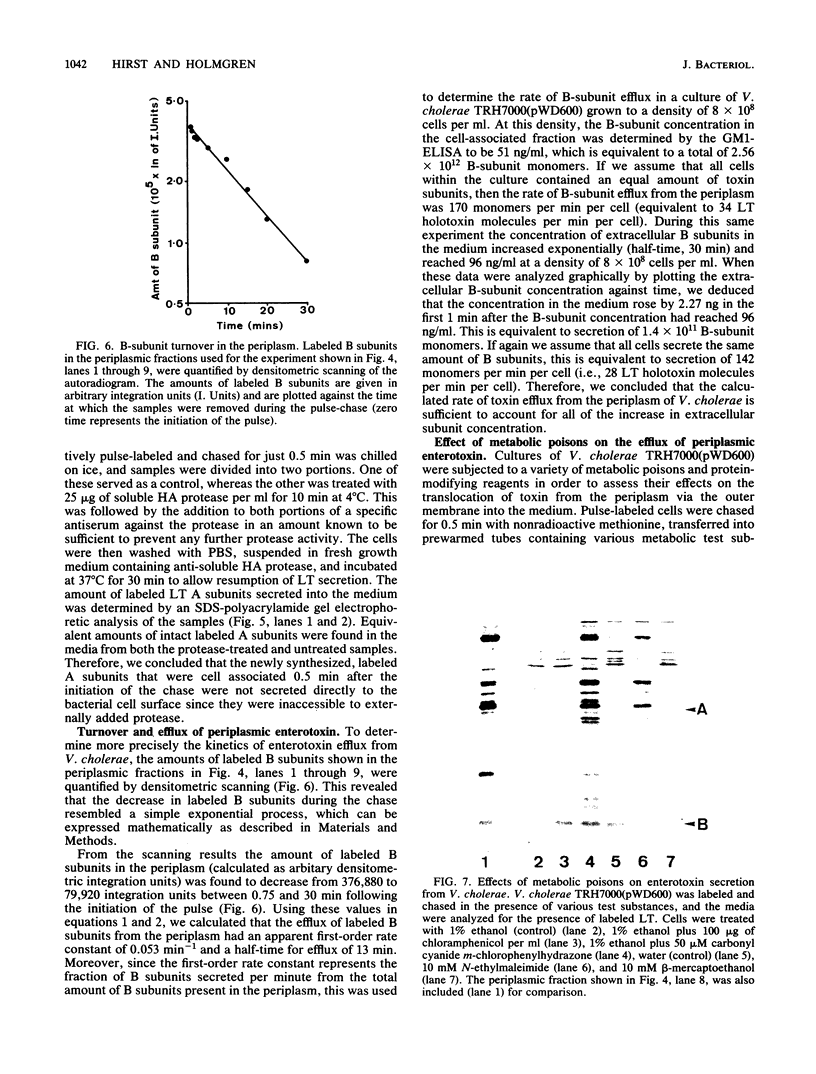
Cholera toxin and heat-labile enterotoxin (LT) are structurally similar oligomeric proteins which are capable of being efficiently secreted from Vibrio cholerae. Here we report that these proteins transiently enter the periplasm of V. cholerae as they traverse the cell envelope to reach the extracellular milieu. Pulse-chase experiments on V. cholerae TRH7000 harboring an LT-encoding plasmid revealed that radiolabeled LT A and B subunits entered the periplasm rapidly, followed by their slow efflux (half-time, 13 min) into the medium. LT B-subunit efflux from the periplasm was calculated to be at a rate of ca. 170 monomers per min per cell (which is equivalent to 34 assembled LT holotoxin molecules per min per cell). These values were estimated to be sufficient to account for the increase in extracellular enterotoxin concentration during exponential cell growth. Thus, all enterotoxin subunits which are secreted into the medium can be assumed to be channelled via the periplasm. These findings led to an improved model of the pathway of toxin secretion by V. cholerae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andro T., Chambost J. P., Kotoujansky A., Cattaneo J., Bertheau Y., Barras F., Van Gijsegem F., Coleno A. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J Bacteriol. 1984 Dec;160(3):1199–1203. doi: 10.1128/jb.160.3.1199-1203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J Bacteriol. 1985 Sep;163(3):877–881. doi: 10.1128/jb.163.3.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker J., Mulks M. H., Plaut A. G., Moxon E. R., Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 May;80(9):2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lowe K. L., Bonham L., el-Morshidy S. Intracellular distribution of heat-labile enterotoxin in a clinical isolate of Escherichia coli. Infect Immun. 1985 Oct;50(1):317–319. doi: 10.1128/iai.50.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S. Conformity between heat-labile toxin genes from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 May;40(2):647–652. doi: 10.1128/iai.40.2.647-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. The molecular nature of heat-labile enterotoxin (LT) of escherichia coli. Nature. 1979 Feb 1;277(5695):406–407. doi: 10.1038/277406a0. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Gill D. M., Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979 Sep;139(3):850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hirst T. R., Hardy S. J., Randall L. L. Assembly in vivo of enterotoxin from Escherichia coli: formation of the B subunit oligomer. J Bacteriol. 1983 Jan;153(1):21–26. doi: 10.1128/jb.153.1.21-26.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Randall L. L., Hardy S. J. Cellular location of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1984 Feb;157(2):637–642. doi: 10.1128/jb.157.2.637-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Sanchez J., Kaper J. B., Hardy S. J., Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Witholt B. Kinetics of synthesis, processing, and membrane transport of heat-labile enterotoxin, a periplasmic protein in Escherichia coli. J Biol Chem. 1984 Dec 25;259(24):15182–15187. [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol. 1985 Mar;161(3):1118–1124. doi: 10.1128/jb.161.3.1118-1124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Levner M. H., Urbano C., Rubin B. A. Lincomycin increases synthetic rate and periplasmic pool size for cholera toxin. J Bacteriol. 1980 Jul;143(1):441–447. doi: 10.1128/jb.143.1.441-447.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J. Subunit structure of cholera toxin. J Gen Microbiol. 1973 Jun;76(2):417–427. doi: 10.1099/00221287-76-2-417. [DOI] [PubMed] [Google Scholar]
- Mackman N., Holland I. B. Secretion of a 107 K dalton polypeptide into the medium from a haemolytic E. coli K12 strain. Mol Gen Genet. 1984;193(2):312–315. doi: 10.1007/BF00330686. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Richardson S. H. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest. 1978 Aug;62(2):281–285. doi: 10.1172/JCI109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Duggleby C. J., Sala-Trepat J. M., Williams P. A. The metabolism of benzoate and methylbenzoates via the meta-cleavage pathway by Pseudomonas arvilla mt-2. Eur J Biochem. 1972 Jul 24;28(3):301–310. doi: 10.1111/j.1432-1033.1972.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Neill R. J., Ivins B. E., Holmes R. K. Synthesis and secretion of the plasmid-coded heat-labile enterotoxin of Escherichia coli in Vibrio cholerae. Science. 1983 Jul 15;221(4607):289–291. doi: 10.1126/science.6857285. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Hirst T. R., Hardy S. J., Holmgren J., Randall L. Synthesis of a precursor to the B subunit of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1981 Apr;146(1):325–330. doi: 10.1128/jb.146.1.325-330.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]