Abstract
Cyclic nucleotide phosphodiesterases (PDEs) regulate intracellular levels of cAMP and cGMP by hydrolyzing them to their corresponding 5′ monophosphates. We report here the cloning and characterization of a novel cAMP-specific PDE from mouse testis. This unique phosphodiesterase contains a catalytic domain that overall shares <40% sequence identity to the catalytic domain of all other known PDEs. Based on this limited homology, this new PDE clearly represents a previously unknown PDE gene family designated as PDE8. The cDNA for PDE8 is 3,678 nucleotides in length and is predicted to encode an 823 amino acid enzyme. The cDNA includes a full ORF as it contains an in-frame stop codon before the start methionine. PDE8 is specific for the hydrolysis of cAMP and has a Km of 0.15 μM. Most common PDE inhibitors are ineffective antagonists of PDE8, including the nonspecific PDE inhibitor 3-isobutyl-1-methylxanthine. Dipyridamole, however, an inhibitor that is generally considered to be relatively specific for the cGMP selective PDEs, does inhibit PDE8 with an IC50 of 4.5 μM. Tissue distribution studies of 22 different mouse tissues indicates that PDE8 has highest expression in testis, followed by eye, liver, skeletal muscle, heart, 7-day embryo, kidney, ovary, and brain in decreasing order. In situ hybridizations in testis, the tissue of highest expression, shows that PDE8 is expressed in the seminiferous epithelium in a stage-specific manner. Highest levels of expression are seen in stages 7–12, with little or no expression in stages 1–6.
The second messengers cAMP and cGMP play important roles in mediating the biological effects of wide a variety of first messengers. The intracellular levels of cAMP and cGMP are controlled by their rates of synthesis by cyclases and their rates of degradation by phosphodiesterases (PDEs). Previously eight families of PDEs have been described in the literature (1–3). Each PDE family has characteristic kinetic and regulatory properties, sequence homology, and inhibitor profiles. Several lines of evidence have established an important role for PDEs in a wide variety of physiologic processes. Genetic studies have indicated that different PDEs regulate such processes as learning and memory (4), development (5), and visual signal transduction (6). Molecular and pharmacological studies have suggested that PDEs regulate such disparate functions as platelet aggregation (7), aldosterone production (8), insulin secretion (9), and olfactory signal transduction (10, 11).
We report here the cloning and description of a previously unrecognized cyclic nucleotide phosphodiesterase family, PDE8, from mouse. A similar isoform has been independently identified recently in humans as well (12). MMPDE8 has both a PDE catalytic domain at the C-terminus and a region at the N terminus homologous to the PAS/PAC domain found in many signal transduction proteins. PDE8, when expressed in Sf9 cells exhibits PDE catalytic activity that is specific for cAMP and is not inhibited well by many common PDE inhibitors. Additionally, this PDE appears to be most highly expressed in mouse testis, and in situ hybridization studies indicate PDE8 expression is restricted in the seminiferous epithelium in a spatial and temporal manner.
MATERIALS AND METHODS
Database Searching for Expressed Sequence Tags (EST) PDE Sequences.
The amino acid sequence of several known PDEs were used as queries to search the database of expressed sequence tags (13, 14). Initial searches were carried out by using the Basic Local Alignment Search Tool (blast) (15) accessed from the database search and analysis Search Launcher (16). This search resulted in many EST sequences with homology to PDEs. Each of these EST sequences were then used as queries in a blastn search of GenBank to determine whether they represented different but known PDEs or whether the EST sequence represented a truly unknown PDE. EST clone ID no. 585220 was isolated in this manner as a sequence that appeared to represent part of a distinct PDE.
Other Databases or Programs.
The PAS/PAC motif of PDE8 was identified by Hidden Markov Modeling (17) search of the Simple Modular Architecture Research Tool (smart) database. Homology of PDE8 N terminus to other PAS/PAC containing proteins was detected by Position-Specific Iterated blast (psi-blast) searches of the nonredundant GenBank database and by use of the Multiple Alignment Construction and Analysis Workbench (macaw) (18).
Sources of EST Clones.
Clones 585220 and 303522 were ordered from Genome Systems.
DNA Sequencing and Sequence Assembly.
Plasmid DNA was prepared by using the SNAP kit (Invitrogen). Primers were designed by using the program Amplify (freeware by William Engels, Genetics Department, University of Wisconsin, Madison). Sequencing was done by using ABI PRISM dye terminator cycle sequencing kit (Perkin–Elmer) and sequencing reactions were purified by using Centri-sep columns (Princeton separations, Adelphia, NJ). Sequences were assembled by using the program Sequencher 3.0 (Gene Codes, Ann Arbor, MI).
Primers.
8strt2: GTGCCGCCGCCGCCAGTATGGGCTGCGCCCCG; race8.as3:CAGCTTTCTCACATGCCCTGTGGAATC; race8.as1: GAAACAGGTAAGGACAGTCT GCACCTCC; race8.as2: CGGGGATATCTGTGGTCTATGATGATGATG; PDE8.AS1: GAGTTGGTTCTCCCAGGGTGATCCACG; PDE8.AS2: GCAACCTCGTCAATGCGATCTAAAGTTTCC; 585seq.as2: CTTAAATGTCTGCCTCGTTTGCTAGTG; AP1: CCATCCTAATACGACTCACTATAGGGC; AP2: ACTCACTATAGGGCTCGAGCGGC.
DNA Probe Synthesis and Northern Blot Analysis.
DNA probes were generated from EST clone 585220 by using Prime-It RmT random primer labeling kit (Stratagene). [α-32P]dCTP at 6,000 Ci/mmol (1 Ci = 37 GBq) was used and the reaction product was purified by using Centri-sep columns. Multiple tissue mRNA and mRNA dot blots were purchased from CLONTECH. Prehybridization, hybridization, and washing were done according manufacturer’s guidelines.
5′ Rapid Amplification of cDNA Ends (RACE).
Marathon-adapted cDNA and Advantage polymerase PCR mix were purchased from CLONTECH. Reactions were set up as follows: 0.5 ng of adapted cDNA, 0.2 μM AP1, or AP2 primer, 0.2 μM of gene-specific primer, 5 μl of 10× reaction buffer (supplied with Advantage polymerase), 0.2 mM dNTP, 1 μl of Advantage KlenTaq Polymerase mix, in a final volume of 50 μl. Reaction cycles were as follows: 94° for 1 min; 5 cycles of 94° for 30 sec, 72 degrees for 4 min; 5 cycles of 94° for 30 sec, 70° for 4 min; 25 cycles of 94° for 30 sec, 68° for 4 min.
Generation of Full Length MMPDE8.
The 5′ end of PDE8 was PCR amplified from testis cDNA. This reaction was set up as follows: 0.5 ng of adapted cDNA, 0.5 μM of strt.1 primer, 0.5 μM of PDE8.AS1 primer, 5 μl of 10× reaction buffer (supplied with Pfu Turbo polymerase), 0.8 mM of dNTP, 0.5 μl of Pfu Turbo polymerase (Stratagene), in a final volume of 50 μl. Reaction cycles were as follows: 94 degrees for 1 min; 25 cycles of 94° for 30 sec, 65 ° for 30 sec, 72° for 2 min. A single band of the correct size was observed by ethidium bromide staining after agarose gel electrophoresis. This band was subcloned into the TA Topo PCRII vector and sequenced. Comparison of this sequence to the sequence of both EST clone 303522 and the 5′ RACE clones demonstrated that no PCR errors were introduced into this PCR clone. This clone was then subcloned into EST clone 585220 by using the EcoRI site of the PCRII vector and an internal unique StuI site found in both the PCR clone and EST clone 585220. The resultant full-length PDE8 clone contained both the start methionine and the stop codon of PDE8. This clone was then subcloned into pFastBac HT (GIBCO/BRL) such that the resultant clone would be in the correct orientation and in-frame with the N-terminal histidine tag sequence. The cDNA as reported here has been confirmed to exist as a single mRNA species by PCR of testis cDNA using primers 8strt2 and 585seq.A2.
Expression of PDE8.
1 μg of PDE8 cDNA, subcloned into the pFastBac HT baculovirus vector was electroporated into 106 SF9 cells in a 0.4-cm electroporation cuvette at 71 μF and infinite resistance using 300 volts, 50 watts, and 50 mAmps and plated out into 60-mm dishes. Virus was amplified and titers were determined using standard procedures (19). Three days postinfection, cells were pelleted and harvested in homogenization buffer as described (2). Glycerol was added to a final concentration of 20–30% to all homogenates that were then stored at −20°C in aliquots without appreciable loss of activity over three weeks.
Kinetics and Inhibitor Studies.
All PDE assays were done according to the method of Hansen and Beavo (20) in a buffer containing 40 mM of Mops (pH 7.5), 0.8 mM of EGTA, 15.0 mM/Mg of acetate, 0.2 mg/ml BSA; and 50,000 cpm per reaction of [3H] cAMP in a final volume of 250 μl. All assays were done in triplicate and reaction times and enzyme amounts were kept such that the lowest substrate concentration gave no more than 30% hydrolysis. PDE8 activity is defined as the total specific activity in PDE8 infected SF9 cells minus the background-specific activity of control SF9 cells. cAMP hydrolytic activity was ≈90-fold higher in PDE8 infected cells compared with control SF9 cells at 0.15 μM cAMP. Thus the basal cAMP hydrolytic activity in SF9 cells was not a significant contributor to the total activity in PDE8 infected cells. Inhibitor studies were done at 0.01 μM cAMP, well below the Km of PDE8, so that IC50 values would approximate the Ki. 3-Isobutyl-1-methylxanthine (IBMX), erythro-9-[3-(2-hydrixynoyl)]adenine (EHNA), and dipyridamole were obtained from Sigma. Zaprinast was a gift from May & Baker (Dagenham, U.K.). Rolipram was obtained from Biomol (Plymouth Meeting, PA). {(+) cis-5,6a,7,8,9 hyl[phenylmethyl]-5-methyl-cylopent[4,5]imidao[2,1-b]purin-49(3H)one} (SCH 51866) was a gift from Schering-Plough Research Institute, and sildenafil was a gift from Pfizer. Enoximone was a gift from the Merrell Dow Research Institute.
Tissue Preparation and In Situ Hybridizations.
Adult male mice were anesthetized by CO2 inhalation and decapitated. Testis were rapidly isolated, soaked in 10–30% sucrose, and then fixed overnight in 4% paraformaldehyde. Tissues were embedded in paraffin and 20 μM sections were cut and mounted onto poly(l-lysine)-coated slides. Probes used were synthesized from clones 9N-term (nucleotides 126-1979) or full PDE8 clone (nucleotides 161–3023). The procedure for in situ hybridization was performed as described (21), except that slides were first dipped in xylene twice for 5 min, then rehydrated in 100, 95, 70, 50, and 30% ethanol for 5 min each before proteinase K treatment.
RESULTS
Cloning and Tissue Distribution of PDE8.
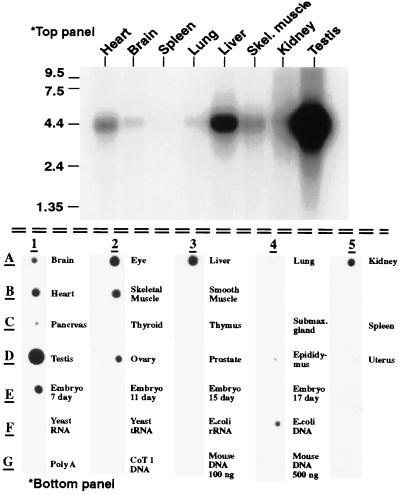
Using a bioinformatics approach, a cDNA was isolated from the database of ESTs (dBEST) that had a low but significant homology to PDEs. This EST, clone ID 585220, contained a sequence consistent with the PDEase signature sequence HDX2HX4N that is found within the catalytic domain of all class one PDEs, and therefore seemed likely to represent part of a cyclic nucleotide phosphodiesterase. Clone 585220 (nucleotides 1628–3678) was fully sequenced and found to contain a polyadenine [poly(A)] tail and part of the catalytic domain of what appeared to be a new PDE. This clone, however, seemed to be truncated at the 5′ end as it did not contain a full PDE catalytic domain. Northern blot analysis of multiple adult mouse tissues indicates the mRNA is 4.4 Kb in size and is most highly expressed in testis, followed by liver, heart, skeletal muscle, and kidney (Fig. 1 Upper). A poly(A) RNA dot blot of 22 mouse tissues also was screened to determine a wider tissue distribution of expression (Fig. 1 Lower). This blot not only confirmed the tissue distribution and levels of expression as determined by Northern blot analysis, but also indicated expression of PDE8 in eye, 7-day embryo, and ovary. Very little to no signal was observed in lung, smooth muscle, pancreas, thyroid, thymus, submaxillary gland, spleen, prostate, epididymus, uterus, or in embryos at days 11, 15, or 17.
Figure 1.
Tissue distribution of PDE8 by (Upper) Northern blot analysis and (Lower) Poly(A) RNA dot blot. Top panel: Northern blot of poly(A) RNA from multiple mouse tissues. A single band corresponding to a message size of 4.4 kb is seen. Bottom panel: Poly(A) RNA dot blot from mouse tissues. PDE8 expression is detected in a variety of tissues. Relative intensity of signal as determined by using the National Institutes of Health image program was testis>eye>skeletal muscle>heart>embryo 7d>kidney>ovary>brain. Note the signal found in the negative control “E. coli DNA” is most likely due to the fact that the probe template was prepared from E. coli hosts.
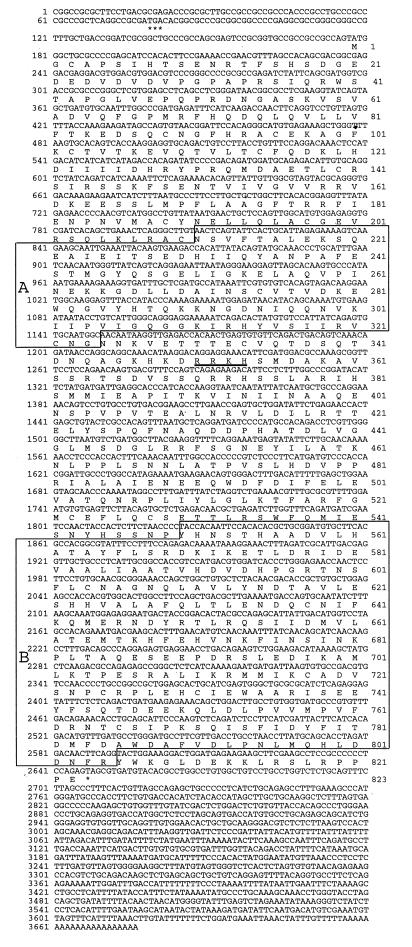
As both the Northern blot and poly(A) RNA dot blot indicated the highest level of expression of this new PDE in testis, 5′ RACE was performed by using specific primers for clone 585220 to PCR amplify the missing N-terminal end of this clone. This RACE reaction generated a PCR band 1.5 kb long that was subcloned into a TA vector and sequenced. This clone, clone 9 (1) (nucleotides 375–1926), not only contained the overlapping sequence of EST clone 585220, but also contained new sequence, which completed the catalytic domain for this PDE, and additional 5′ sequence that was different from all other PDEs, indicating that it belonged to a new PDE family. No in-frame stop codon was present, however, implying that it was still not full length. A second round of 5′ RACE was performed by using primers specific to the sequence of RACE clone 9 (1). This yielded two clones, clones C1 and B7, nucleotides 127–476, which extended the sequence of both clone 585220 and 9 (1), but also did not contain an in-frame stop codon. “Walking” the EST database was done by performing sequence homology database searches by using the new sequence obtained from clones C1 and B7 to obtain clones that might extend the sequence of PDE8 at the 5′ end. This search generated a second EST clone, clone 303522 (nucleotides 1–1036), which not only confirmed the 5′ sequence we had obtained from clones C1 and B7, but also extended in the 5′ direction the cDNA sequence by 127 nucleotides. Clone 303522 does contain an in-frame stop codon before a methionine that closely follows the rules of Kozak (22), producing the full ORF for PDE8. When combined with clones C1, B7, 9 (1), and clone 585220 the full-length cDNA has a combined length of 3,678 bp. The complete ORF predicts an 823 amino acid protein with a predicted molecular mass of 93,171 Da (see Fig. 2). A full-length clone was constructed by directly PCR amplifying from testis cDNA the full 5′ end of PDE8 and subcloning of this fragment (clone 9N-term) into an StuI site found in both 9N-term and clone 585220. Clone 9N-term was sequenced in its entirety and shown to be free of PCR errors by comparison to the 5′ RACE clones and clone 303522. The full-length ORF as reported here was then confirmed to exist as a single cDNA in testis by direct amplification of the full ORF from testis cDNA, subcloning the PCR product (full PDE8 clone), and sequencing of the ends.
Figure 2.
Nucleotide and amino acid sequence of PDE8. Note the in-frame stop codon above the predicted start methionine is marked by ∗∗∗. Underlined amino acid residues correspond to a nuclear localization consensus sequence. (A) The N-terminal PAS domain is homologous to many signaling proteins (see Fig. 5). (B) The catalytic domain of PDE8. ∗ denotes the stop codon at the end of the ORF.
Baculovirus Expression of PDE8 and Characterization of PDE8 Activity.
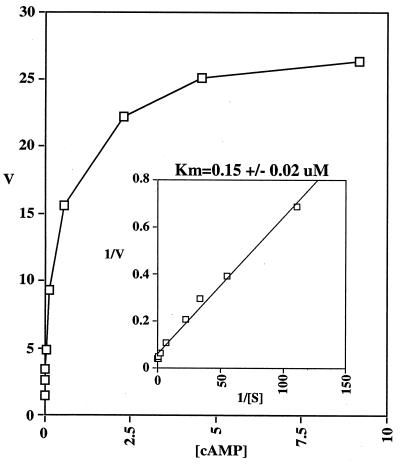
Full length PDE8 was subcloned into an N-terminal histidine tag baculovirus expression vector (pFastBac HT) and transfected into Sf9 cells to produce recombinant virus. Sf9 cells infected with PDE8 recombinant virus were assayed for PDE activity compared with that found in control uninfected Sf9 cells homogenates three days post infection. Assays for both cAMP and cGMP PDE activity were performed with a range of substrate concentrations from 0.01–9.2 μM. Sf9 cell homogenates infected with recombinant PDE8 virus had ≈90-fold more cAMP activity than the control Sf9 cells, but no cGMP PDE activity above background. cGMP did not effect cAMP hydrolysis at up to 100 μM of cGMP (data not shown). Two separate Sf9 cell infections were performed to prepare two independent batches of PDE8 expressing Sf9 cell homogenates and kinetic curve assays were done by using cAMP as substrate. The Km of PDE8 is 0.15 μM for cAMP (Fig. 3) (average of three separate experiments on two independent enzyme preparations). The effects of several PDE inhibitors also were determined for PDE8. Most PDE inhibitors tested were ineffective inhibitors of PDE8, including the nonselective PDE inhibitor IBMX and the PDE4 inhibitors rolipram and Ro 20–1724 (see Table 1). Papaverine, also a nonselective PDE inhibitor, was more potent than IBMX and had an IC50 of 174 μM. Interestingly however, dipyridamole, usually considered a PDE5 and PDE6 inhibitor, did inhibit PDE8 with an IC50 of 4.5 μM.
Figure 3.
PDE8 Kinetics. Concentration range of cAMP was 0.01–9.2 μM. Inset is a Lineweaver-Burke plot.
Table 1.
Inhibitor assays of PDE8
| Inhibitor | Selective for PDE Type (IC50) | IC50 for PDE8, μM |
|---|---|---|
| IBMX | non-selective (2–50 μM) | >200 |
| Zaprinast | PDE5/6 (0.76/0.15 μM) | >100 |
| EHNA | PDEZ (1 μM) | >100 |
| Enoximone | PDE3 (1 μM) | >100 |
| Dipyridamole | PDE5/6 (0.9/0.38 μM) | 4.5 |
| Sildenafil | PDE5 (3.9 μM) | >30 |
| SCH 51806 | PDE1/5 (0.1 μM) | >100 |
| Ro 20-1724 | PDE4 (2.0 μM) | >200 |
| Rolipram | PDE4 (2.0 μM) | >200 |
| Papaverine | nonselective (0.4–20 μM) | 174 |
Only dipyridamole and papaverine have any effect on PDE8 within the dose ranges. Rolipram, Ro 20-1724 and IBMX do not inhibit PDE8 within dose ranges considered specific.
In Situ Hybridization of PDE8 in Mouse Testis.
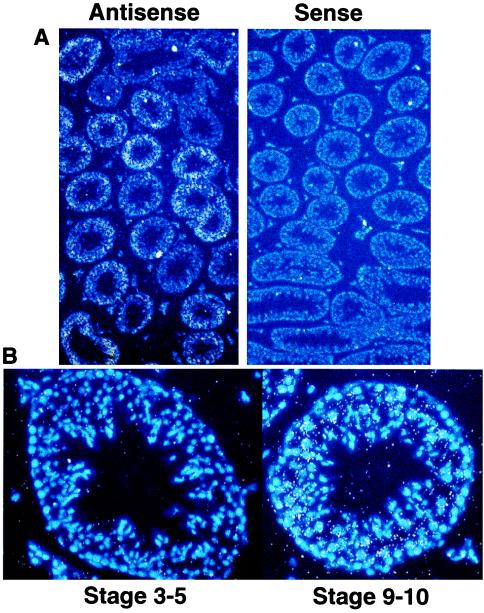
As PDE8 is most highly expressed in testis amongst the tissues examined thus far, in situ hybridizations were performed in testis by using two different probes (9N-term and full PDE clone). Both probes in the antisense orientation gave specific labeling of the seminiferous tubules, whereas the sense probes yielded little or no signal (see Fig. 4A). No significant signal was observed in either Leydig or Sertoli cells for either probe. Additionally, the signal patterns for both probes in the seminiferous tubules were uneven, indicating stage-specific expression for PDE8 (Fig. 4A). Staging of PDE8 expression by examination of Hoechst 33258 stained nuclei revealed that PDE8 expression is highest at stages 8–11, medium in stages 7–12, and nearly absent in stages 1–6. Fig. 5B compares a tubule representing stages 3–5, which has very little or no PDE8 expression, to a tubule at stage 9–10 that has high levels of expression. Additionally, expression of PDE8 was not uniform throughout the lumen of the tubules, but was restricted to the postmitotic pachytene spermatocytes.
Figure 4.
In situ hybridization of mouse testis with PDE8. Approximate stage was determined by using Hoechst 33258 to stain nuclei. (A) Antisense and sense view under fluorescent light to observe the nuclear stain (blue) and bright field to observe probe signal (white dots). Note the low probe signal with the sense probe and the uneven probe signal within the semifierous tubules in the antisense field, indicating a spatial and temporal regulation of PDE8 expression. (B) Representative high magnification fluorescent and bright field of a early stage tubule and late stage tubule. Note the lack of probe signal (white dots) above background at stage 3–5 vs. the intense signal at stage 9–10.
Figure 5.
Multiple sequence alignment of PDE PAS/PAC domain with homologous sequences. Abbreviations: Bat; putative bacterio-opsin activator from Halobacterium halobium (accession no. M23247). Nph1–2; nonphototropic hypocotyl 1 from Avena sativa (accession no. AF033097). Wc1; white collar 1 from Neurospora crassa (accession no. X94300). NifL; NifL from Enterobacter agglomerans (accession no. X89104). Signal kinase; putative signal-transducing histidine kinase—from Archaeoglobus fulgidus (accession no. AE000990).
DISCUSSION
The cDNA presented here demonstrates the existence of a formerly unrecognized PDE family, PDE8. Comparison of the catalytic domain of PDE8 to the catalytic domains of all other previously described PDE gene families reveals a sequence identity below 40 percent, demonstrating that this PDE represents a new gene family. What appears to be an additional member of the PDE8 family also has been independently cloned and characterized recently from human sources (12). The human and this mouse sequence of PDE8 share only 85% sequence identity within the catalytic domain. In contrast, sequence comparisons of all known PDE genes show that the same genes from different species all share >93% amino acid sequence identity within the catalytic domain region (not shown). Therefore, based on this analysis it is possible, but not yet certain, that the mouse PDE8 and human PDE8 isoforms are derived from different genes. If, however, the mouse and human clones are derived from the same gene, then the human clone is likely truncated at the 5′ end as the mouse clone contains an additional 112 amino acids above that reported for the human clone. Additional cloning from both species will be necessary to determine whether they represent two closely related genes in the same PDE family or whether they represent the same gene that has diverged between species more than the other PDE genes.
PDE8 also contains 531 amino acids N-terminal to the catalytic domain that is distinct from all other PDE families. Often these N-terminal, family specific regions contain regulatory domains that integrate other second messenger signals with the PDE catalytic function. Searches using the Simple Modular Architecture Research Tool database of signaling motifs reveals a single PAS/PAC domain [for Per, ARNT, and Sim proteins from which this domain was originally identified (23)] spanning amino acids 211 to 325 within the N terminus of PDE8. This motif may be present in single (putative bacterio-opsin activator) or multiple copies (nifL or per) (24, 25). psi-blast and multiple sequence alignments by using macaw reveals that this region of PDE8 is most homologous to several proteins from archaeal, eubacterial, and lower eukaryotic organisms that have been previously noted to contain the PAS/PAC domain, rather than the classic Period, ARNT, or Sim PAS/PAC repeats (see Fig. 5) (24–28). Although these domains have been found in a wide variety of different signaling molecules, a functional role for this domain for most of these proteins remains uncertain. Several functions have been proposed for this domain including binding of flavin adenine dinucleotide (29, 30), and involvement in protein/protein interactions (23, 30, 31). PDE8 also appears to contain a consensus sequence for nuclear localization of the type typified by simian virus 40 large T-antigen (32) (see Fig. 2) opening the possibility that the N-terminal region of PDE8 also could function to regulate its subcellular distribution. Further experiments will be necessary to determine the function, if any, of these domains in PDE8.
Tissue distribution studies of PDE8 expression at the mRNA level by Northern blot and poly(A) RNA dot blot analysis indicate that out of 22 mouse tissues surveyed, expression is highest in testis, followed by eye, liver, skeletal muscle, heart, 7-day embryo, kidney, ovary, and brain. In mouse embryo it is possible that this PDE may play some specialized role in very early embryogenesis, as the expression levels appear to drop significantly sometime between day 7 and day 11.
PDE8 is a low Km, cAMP-specific PDE. The Km of PDE8 for cAMP was determined to be 0.15 μM in Sf9 cell homogenates. This is lower than the Km reported for members of the PDE4 family, but similar to members of the PDE7 family, both of which also are referred to as low Km cAMP-specific PDEs. Although PDE8 and PDE7 have a similar substrate specificity and kinetics they do not appear to share a similar tissue distribution. PDE7 is expressed most highly in skeletal muscle and tissues and cells associated with immune function (33) whereas PDE8 does not appear to be expressed in either the thymus or spleen and is therefore likely to regulate distinct cAMP responses from those regulated by PDE7.
The pharmacology of PDE8 is different from most other PDEs. Although IBMX was an ineffective inhibitor of PDE8, another nonselective PDE inhibitor, papaverine had a weak potency with an IC50 of 174 μM. Most selective inhibitors were ineffective at dose ranges considered specific, however dipyridamole did inhibit PDE8 with an IC50 5–10-fold over the IC50 of this drug for PDE5 and PDE6 (Table1). It is surprising that an inhibitor that has generally been thought of as specific for the inhibition of cGMP-specific PDEs also has potency for a cAMP-specific PDE. Apparently, the catalytic sites of PDE5 and PDE6 share some similar topology to the catalytic site of PDE8 that is not related to substrate specificity. It is also important to note that PDE8 differs from the other cAMP-specific PDE4 PDEs in that neither the selective inhibitors rolipram or Ro 20–1724, nor the nonselective inhibitor IBMX inhibit PDE8. The insensitivity of PDE8 to rolipram and Ro 20–1724 is similar to the cAMP-specific PDE7.
To date PDE8 appears to be most highly expressed in testis in mouse, and in situ hybridizations show that its expression is regulated temporally and spatially in the seminiferous tubules. Additionally, PDE8 is most highly expressed in middle to late pachytene spermatocytes during stages 7–12. Consistent with our data, previous work has shown the majority of rolipram insensitive cAMP-PDE activity in the seminiferous tubules of rats to be present in stages 9–12 (34). The stage and cell type-specific expression of PDE8 suggests that PDE8 plays a role in cAMP regulation of germ cell development. Other cAMP PDEs also have been shown to have stage-specific gene expression (34–36). Several lines of evidence have suggested cAMP does play an important role in spermatogenesis. For instance, in addition to the stage-specific expression of PDE8 and PDE4, other cAMP signaling molecules also are known to be expressed in germ cells, including PKA (37), the PKA anchoring protein AKAP84 (38), and the cAMP response element modulator CREMtau (39). CREMtau gene expression also is found at high levels beginning in pachytene spermatocytes, and mice that are null for CREMtau by gene knockout produce no mature sperm and are sterile (39).
In conclusion, the identification and characterization of a full-length member of a new PDE family is described here. MMPDE8 is cAMP-specific and inhibited by dipyridamole and higher concentrations of papaverine. This PDE contains an N terminus that is homologous to many other signaling proteins and is similar to the PAS domain identified initially in Period, ARNT, and Sim. Additionally, the regulation of gene expression for PDE8 is restricted to middle and late pachytene spermatocytes in testis, and regulated during embryogenesis. The biologic significance of PDE8 and the functional role it may play in regulating cAMP responsive pathways will await the development of more selective inhibitors of PDE8 and or genetic approaches.
Acknowledgments
We thank Todd Berard and Rejean L. Iderda and the University of Washington Molecular Pharmacology DNA Core Facility for their DNA sequencing support services. We also would like to thank Douglas Fisher for sending us an advance copy of his manuscript; Pfizer Central Research (Sandwich, U.K.) for the gift of sildenafil; and the Schering-Plough Research Institute for the gift of SCH51866. This work was supported by National Institutes of Health Grants DK21723 and GMO7750.
ABBREVIATIONS
- PDE
phosphodiesterase
- IBMX
3-isobutyl-1-methylxanthine
- RACE
rapid amplification of cDNA ends
- EST
expressed sequence tag
- EHNA
erythro-9-[3-(2-hydroxynonyl)]adenine
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF067806).
References
- 1.Beavo J A. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 2.Soderling S H, Bayuga S J, Beavo J A. J Biol Chem. 1998;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- 3.Fisher D A, Smith J F, Pillar J S, St. Denis S H, Cheng J B. J Biol Chem. 1998;273:15559–15564. doi: 10.1074/jbc.273.25.15559. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar L M. J Neurosci. 1982;2:1347–1358. doi: 10.1523/JNEUROSCI.02-10-01347.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaulsky G, Escalante R, Loomis W F. Proc Natl Acad Sci USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin M E, Sandberg M A, Berson E L, Dryja T P. Nat Genet. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson N T, Jang E K, Haslam R J. Biochem J. 1997;323:371–377. doi: 10.1042/bj3230371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFarland R T, Zelus B D, Beavo J A. J Biol Chem. 1991;266:136–142. [PubMed] [Google Scholar]
- 9.Zhao A Z, Zhao H, Teague J, Fujimoto W, Beavo J A. Proc Natl Acad Sci USA. 1997;94:3223–3228. doi: 10.1073/pnas.94.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan C, Zhao A Z, Bentley J K, Loughney K, Ferguson K, Beavo J A. Proc Natl Acad Sci USA. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juilfs D M, Fulle H J, Zhao A Z, Houslay M D, Garbers D L, Beavo J A. Proc Natl Acad Sci USA. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher D A, Smith J F, Pillar J S, St. Denis S H, Cheng J B. Biochem Biophys Res Commun. 1998;246:570–577. doi: 10.1006/bbrc.1998.8684. [DOI] [PubMed] [Google Scholar]
- 13.Boguski M S, Lowe T M, Tolstoshev C M. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- 14.Hillier L D, Lennon G, Becker M, Bonaldo M F, Chiapelli B, Chissoe S, Dietrich N, DuBuque T, Favello A, Gish W, et al. Genome Res. 1996;6:807–828. doi: 10.1101/gr.6.9.807. [DOI] [PubMed] [Google Scholar]
- 15.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 16.Smith R F, Wiese B A, Wojzynski M K, Davison D B, Worley K C. Genome Res. 1996;6:454–462. doi: 10.1101/gr.6.5.454. [DOI] [PubMed] [Google Scholar]
- 17.Eddy S R, Mitchison G, Durbin R. J Comput Biol. 1995;2:9–23. doi: 10.1089/cmb.1995.2.9. [DOI] [PubMed] [Google Scholar]
- 18.Schuler G D, Altschul S F, Lipman D J. Proteins. 1991;9:180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly D R, Miller L K, Luckow V A. Baculovirus Expression Vectors: A Labortatory Manual. New York: Freeman; 1992. [Google Scholar]
- 20.Hansen R S, Beavo J A. Proc Natl Acad Sci USA. 1982;79:2788–2792. doi: 10.1073/pnas.79.9.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C, Bentley J K, Sonnenburg W K, Beavo J A. J Neurosci. 1994;14:973–984. doi: 10.1523/JNEUROSCI.14-03-00973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z J, Edery I, Rosbash M. Nature (London) 1993;364:259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhulin I B, Taylor B L, Dixon R. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]
- 25.Ponting C P, Aravind L. Curr Biol. 1997;7:R674–R677. doi: 10.1016/s0960-9822(06)00352-6. [DOI] [PubMed] [Google Scholar]
- 26.Huala E, Oeller P W, Liscum E, Han I S, Larsen E, Briggs W R. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 27.Linden H, Macino G. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crosthwaite S K, Dunlap J C, Loros J J. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 29.Rebbapragada A, Johnson M S, Harding G P, Zuccarelli A J, Fletcher H M, Zhulin I B, Taylor B L. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soderback E, Reyes-Ramirez F, Eydmann T, Austin S, Hill S, Dixon R. Mol Microbiol. 1998;1:179–192. doi: 10.1046/j.1365-2958.1998.00788.x. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Xu J, Li M. J Biol Chem. 1997;272:705–708. doi: 10.1074/jbc.272.2.705. [DOI] [PubMed] [Google Scholar]
- 32.Nigg E A. Nature (London) 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 33.Bloom T J, Beavo J A. Proc Natl Acad Sci USA. 1996;93:14188–14192. doi: 10.1073/pnas.93.24.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morena A R, Boitani C, de, G S, Stefanini M, Conti M. Endocrinology. 1995;136:687–695. doi: 10.1210/endo.136.2.7835302. [DOI] [PubMed] [Google Scholar]
- 35.Welch J E, Swinnen J V, O’Brien D A, Eddy E M, Conti M. Biol Reprod. 1992;46:1027–1033. doi: 10.1095/biolreprod46.6.1027. [DOI] [PubMed] [Google Scholar]
- 36.Naro F, Zhang R, Conti M. Endocrinology. 1996;137:2464–2472. doi: 10.1210/endo.137.6.8641200. [DOI] [PubMed] [Google Scholar]
- 37.Lonnerberg P, Parvinen M, Jahnsen T, Hansson V, Persson H. Biol Reprod. 1992;46:1057–1068. doi: 10.1095/biolreprod46.6.1057. [DOI] [PubMed] [Google Scholar]
- 38.Lin R Y, Moss S B, Rubin C S. J Biol Chem. 1995;270:27804–27811. doi: 10.1074/jbc.270.46.27804. [DOI] [PubMed] [Google Scholar]
- 39.Nantel F, Monaco L, Foulkes N S, Masquilier D, LeMeur M, Henriks’en K, Dierich A, Parvinen M, Sassone C P. Nature (London) 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]