Abstract
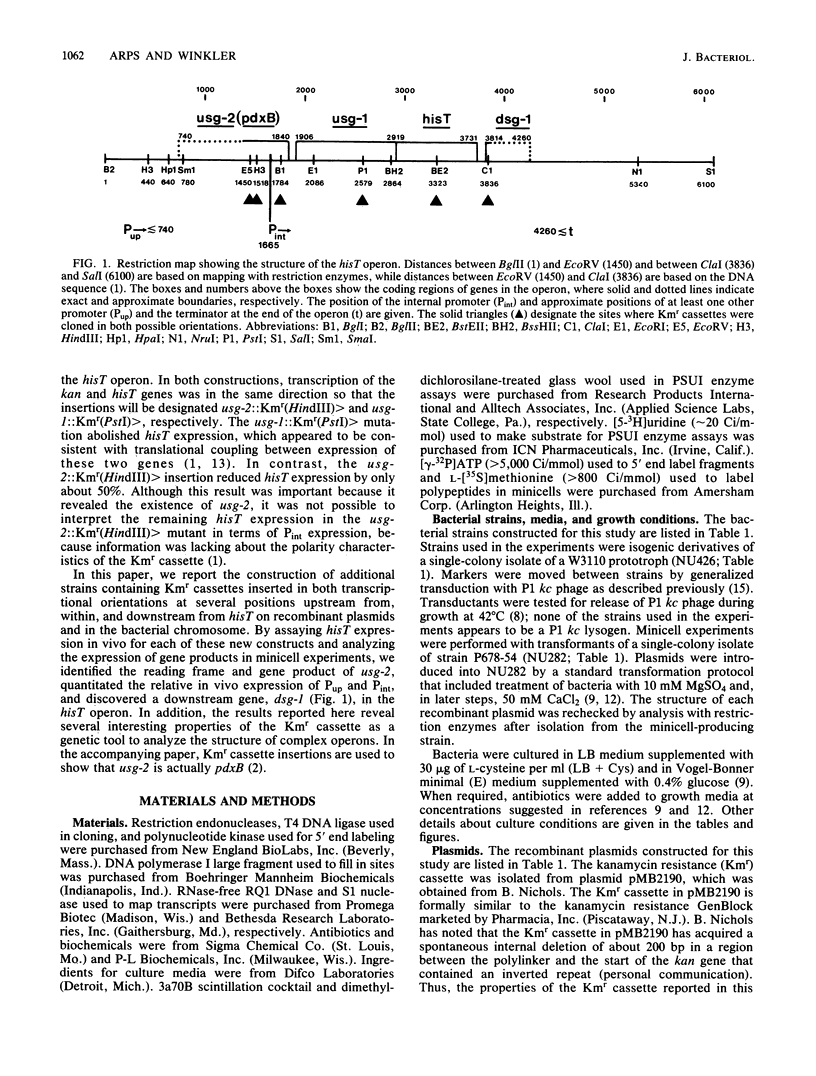
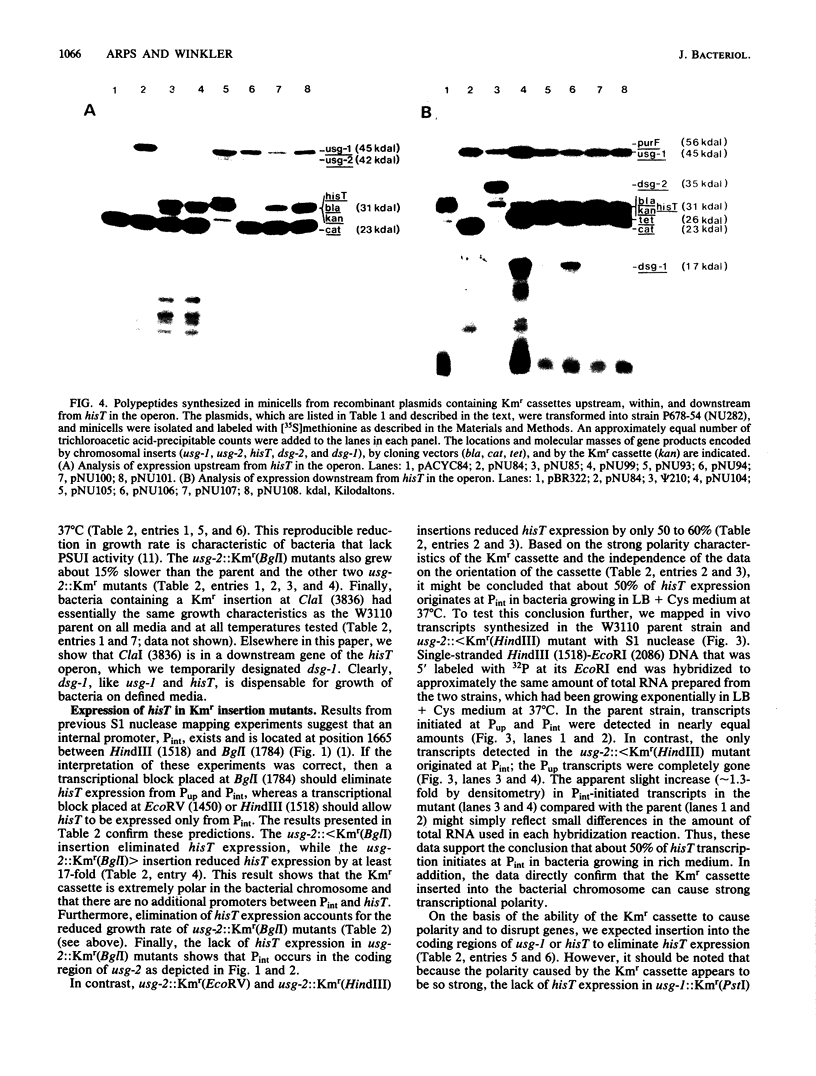
We constructed a series of recombinant plasmids containing a kanamycin resistance (Kmr) cassette upstream from, within, and downstream from hisT, which encodes the tRNA modification enzyme pseudouridine synthase I. These Kmr insertions were then crossed directly into the bacterial chromosome. We determined growth characteristics, assayed in vivo hisT expression, and mapped in vivo hisT operon transcripts for the Kmr insertion mutants. We also analyzed polypeptides synthesized in minicells from plasmids containing Kmr cassettes. The combined results from these experiments demonstrate new features concerning the structure and expression of the complex operon that contains hisT. We show that the minimum size of the operon is approximately 3,500 base pairs and that it contains at least four genes, which are arranged in the order usg-2 (pdxB), usg-1, hisT, and dsg-1 and encode polypeptides with apparent molecular masses of 42,000, 45,000, 31,000, and 17,000 daltons, respectively. Of these genes, only the functions of usg-2 (pdxB) and hisT are known, and genetic evidence suggests that these two genes do not require usg-1 or dsg-1 for function, usg-2 (pdxB) is required for growth of bacteria on minimal medium at 37 degrees C. In contrast, the three genes at the end of the hisT operon are dispensable and form a transcription unit that is expressed from a relatively strong internal promoter. The phenotypes of the Kmr insertion mutants and results from gene expression experiments further confirm the position of the internal promoter and locate additional genetic signals in the DNA sequence around hisT. The experiments reported here also indicate several interesting properties of the Kmr cassette as a tool for probing complex operons.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arps P. J., Marvel C. C., Rubin B. C., Tolan D. A., Penhoet E. E., Winkler M. E. Structural features of the hisT operon of Escherichia coli K-12. Nucleic Acids Res. 1985 Jul 25;13(14):5297–5315. doi: 10.1093/nar/13.14.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Winkler M. E. An unusual genetic link between vitamin B6 biosynthesis and tRNA pseudouridine modification in Escherichia coli K-12. J Bacteriol. 1987 Mar;169(3):1071–1079. doi: 10.1128/jb.169.3.1071-1079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D. H., Matsumura P. Identification of Escherichia coli region III flagellar gene products and description of two new flagellar genes. J Bacteriol. 1984 Nov;160(2):577–585. doi: 10.1128/jb.160.2.577-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi M. S., Schmid M. B., Roth J. R. Transposon Tn10 provides a promoter for transcription of adjacent sequences. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5016–5020. doi: 10.1073/pnas.79.16.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri R. K., Gots J. S. A DNA-binding protein with specificity for pur genes in Escherichia coli. J Biol Chem. 1980 Oct 25;255(20):9594–9598. [PubMed] [Google Scholar]
- Marvel C. C., Arps P. J., Rubin B. C., Kammen H. O., Penhoet E. E., Winkler M. E. hisT is part of a multigene operon in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):60–71. doi: 10.1128/jb.161.1.60-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Palmer D. T., Blum P. H., Artz S. W. Effects of the hisT mutation of Salmonella typhimurium on translation elongation rate. J Bacteriol. 1983 Jan;153(1):357–363. doi: 10.1128/jb.153.1.357-363.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro A., Spena A., Santonastaso V., Conini P. Stringency without ppGpp accumulation. Nature. 1981 May 21;291(5812):256–258. doi: 10.1038/291256a0. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




