Abstract
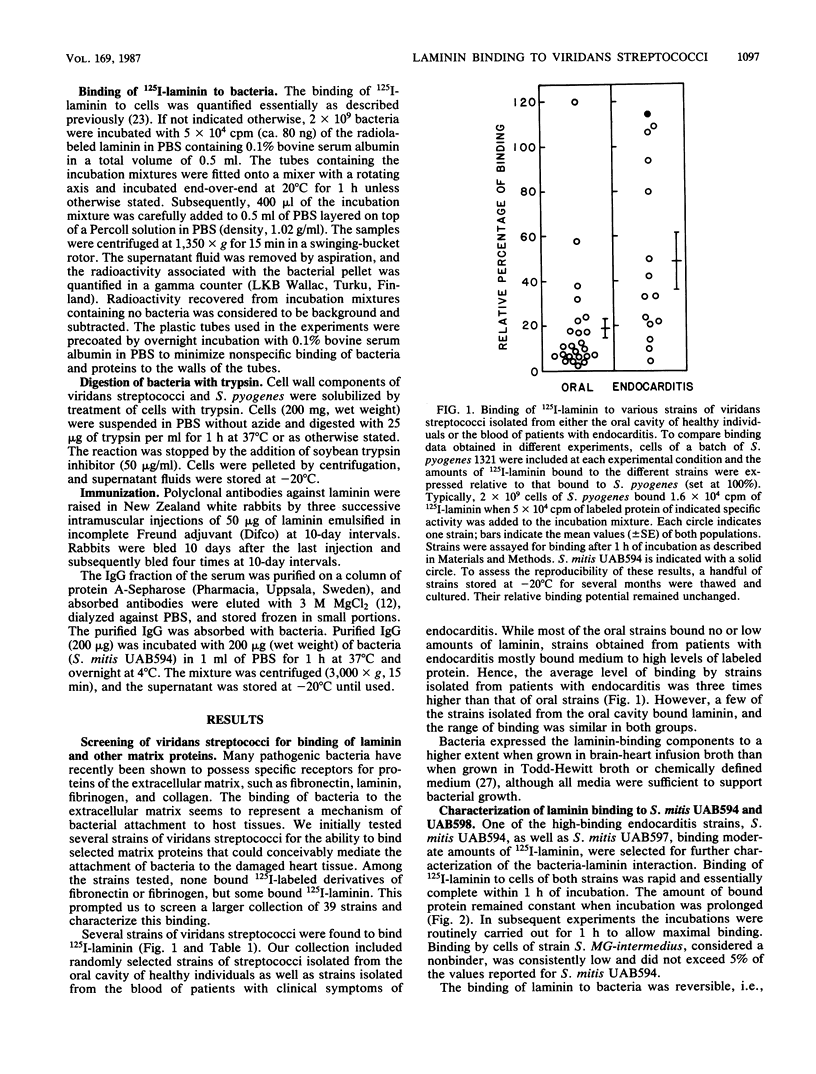
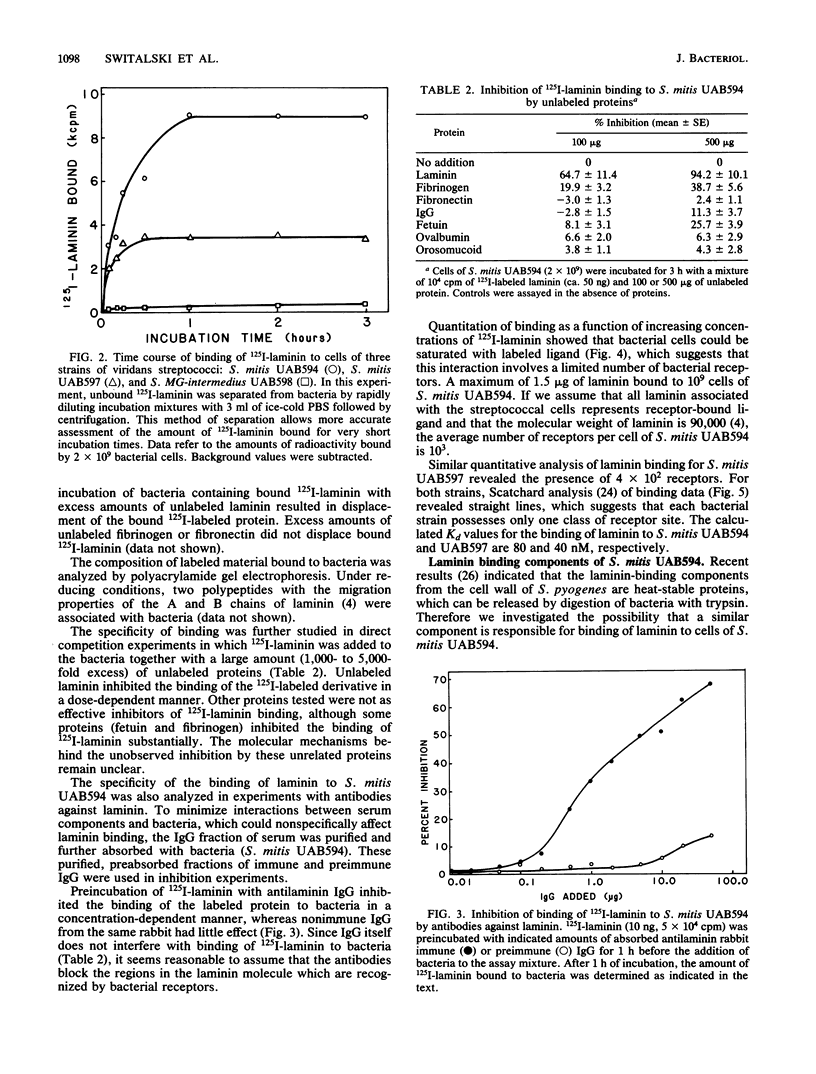
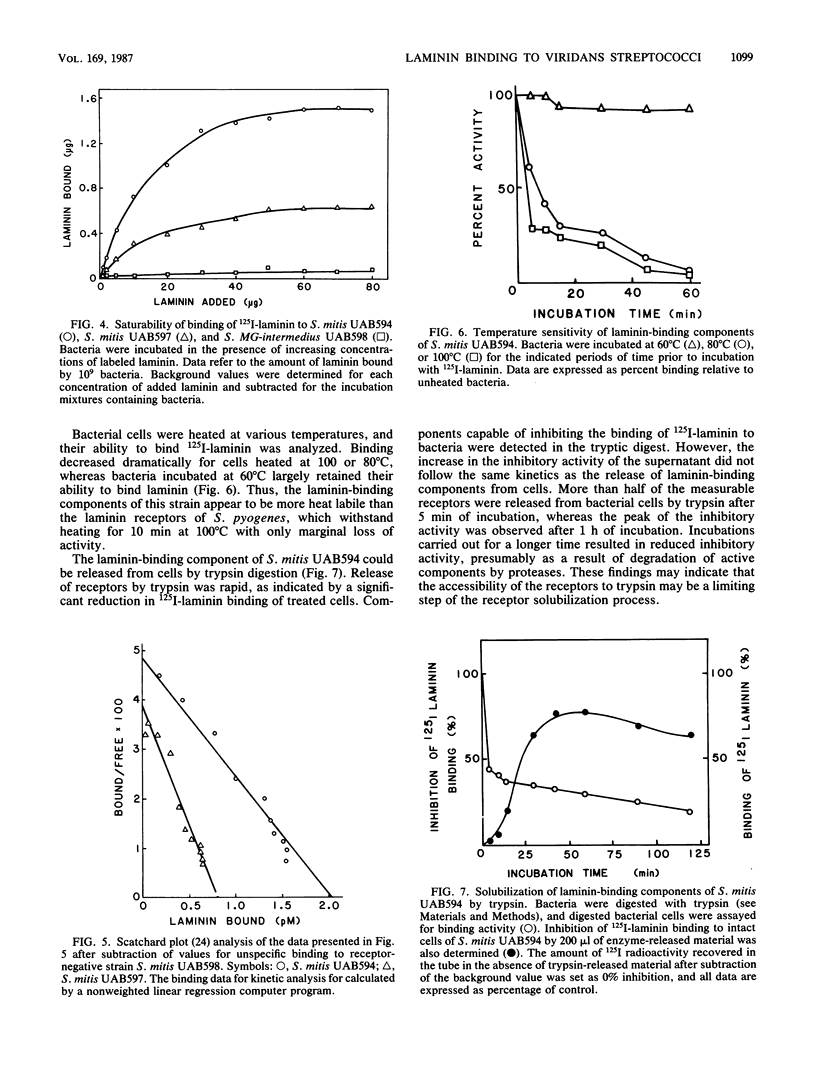
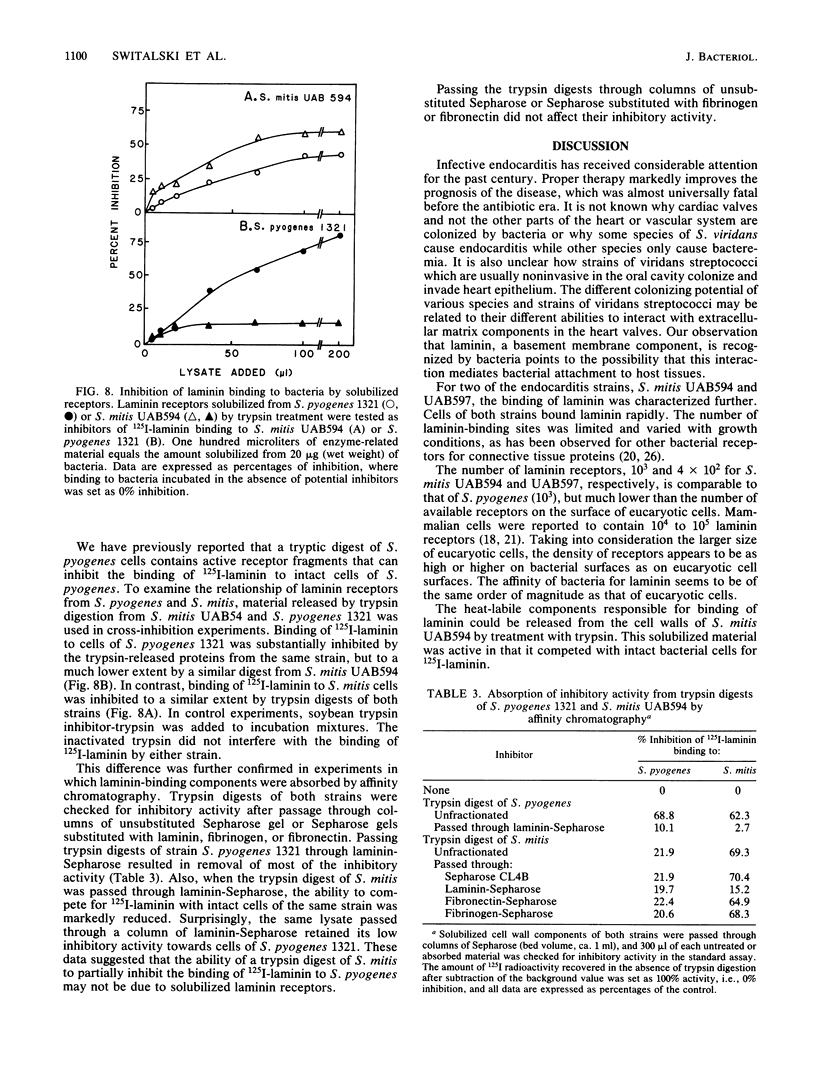
Attachment of bacteria to the host tissue is regarded as a crucial step in the development of many types of infections. Recent studies by us and others have shown that matrix proteins which serve as adhesion proteins for eucaryotic cells may also be recognized by some bacteria. In the present communication, we report that several strains of viridans streptococci are able to bind to laminin. Most strains isolated from blood and heart valves of patients with endocarditis expressed laminin receptors, whereas only a few of the strains isolated from the oral cavity recognized this protein. This observation indicates that laminin binding might be an important factor in the pathogenesis of viridans endocarditis. Laminin binding to two strains (Streptococcus mitis UAB594 and UAB597) isolated from patients with endocarditis was characterized further. The bacterial cells expressed a limited number of laminin receptors (4 X 10(2) to 1 X 10(3) per cell) which bound the protein in a high-affinity interaction (Kd, 40 to 80 nM). This receptor of S. mitis UAB594 was heat labile and could be solubilized from bacteria by brief digestion with trypsin. Solubilized receptors which competed with cell-bound receptors for 125I-laminin could be adsorbed on laminin-Sepharose but not on Sepharose substituted with fibrinogen or fibronectin. Comparison of laminin receptors from S. mitis with those previously described for Streptococcus pyogenes suggest that different sites in the laminin molecule are recognized by the two bacteria and hence that the corresponding receptor molecules are not identical.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Bayliss R., Clarke C., Oakley C. M., Somerville W., Whitfield A. G., Young S. E. The microbiology and pathogenesis of infective endocarditis. Br Heart J. 1983 Dec;50(6):513–519. doi: 10.1136/hrt.50.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg R. O., Giuliani E. R., Wilson W. R., Geraci J. E. Infective endocarditis--a 25 year overview of diagnosis and therapy. J Am Coll Cardiol. 1983 Jan;1(1):280–291. doi: 10.1016/s0735-1097(83)80029-1. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Etienne J., Reverdy M. E., Mouren C., Fleurette J. Etude bactériologique de cent vingt-cinq endocardites infectieuses à streptocoque. Pathol Biol (Paris) 1982 Oct;30(8):707–710. [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- KRASSE B. The proportional distribution of Streptococcus salivarius and other streptococci in various parts of the mouth. Odontol Revy. 1954;5(3):203–211. [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]


