Abstract
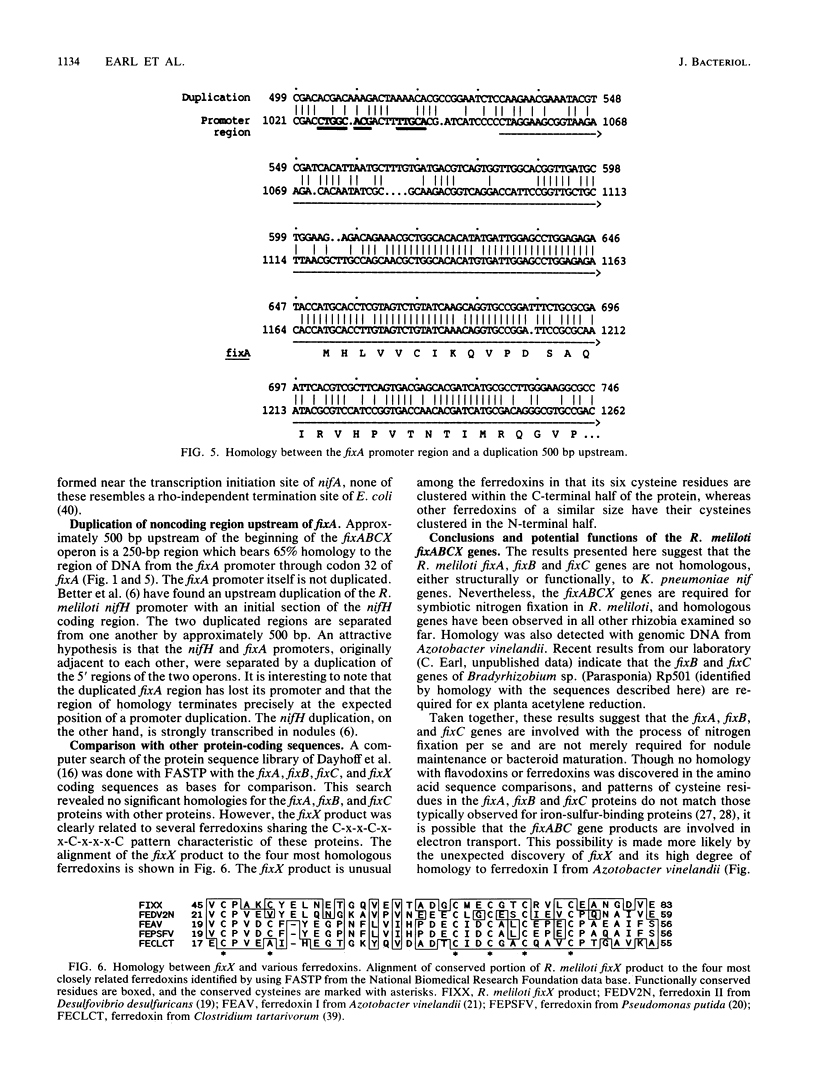
The fixA, fixB, fixC, and fixX genes of Rhizobium meliloti 1021 constitute an operon and are required for nitrogen fixation in alfalfa nodules. DNA homologous to the R. meliloti fixABC genes is present in all other Rhizobium and Bradyrhizobium species examined, but fixABC-homologous sequences were found in only one free-living diazotroph, Azotobacter vinelandii. To determine whether the fixABCX genes share sequence homology with any of the 17 Klebsiella pneumoniae nif genes, we determined the entire nucleotide sequence of the fixA, fixB, fixC, and fixX genes and defined four open reading frames that code for polypeptides of molecular weights 31,146, 37,786, 47,288, and 10,937, respectively. Neither DNA nor amino acid sequence homology to the R. meliloti fixA, -B, -C, and -X genes was found in the K. pneumoniae nif operon. The fixX gene contains a cluster of cysteine residues characteristic of ferredoxins and is highly homologous to an Azotobacter ferredoxin which has been shown to donate electrons to nitrogenase. The fixABC operon contains a promoter region that is highly homologous to other nifA-activated promoters. We also found a duplication of the 5' end of the fixABCX operon; a 250-bp region located 520 bp upstream of the fixABCX promoter bears more than 65% homology to the 5' end of the transcribed region, including the first 32 codons of fixA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Kapp D., Pühler A. Characterization of a Rhizobium meliloti fixation gene (fixF) located near the common nodulation region. J Bacteriol. 1985 Oct;164(1):245–254. doi: 10.1128/jb.164.1.245-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Better M., Ditta G., Helinski D. R. Deletion analysis of Rhizobium meliloti symbiotic promoters. EMBO J. 1985 Oct;4(10):2419–2424. doi: 10.1002/j.1460-2075.1985.tb03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brown S. E., Ausubel F. M. Mutations affecting regulation of the Klebsiella pneumoniae nifH (nitrogenase reductase) promotor. J Bacteriol. 1984 Jan;157(1):143–147. doi: 10.1128/jb.157.1.143-147.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Klingensmith J. A., Gibbons S. L., Ausubel F. M. Conservation of structure and location of Rhizobium meliloti and Klebsiella pneumoniae nifB genes. J Bacteriol. 1987 Mar;169(3):1120–1126. doi: 10.1128/jb.169.3.1120-1126.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Buikema W. J., Szeto W. W., Lemley P. V., Orme-Johnson W. H., Ausubel F. M. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 1985 Jun 25;13(12):4539–4555. doi: 10.1093/nar/13.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon F. C., Dixon R. A., Postgate J. R. Derivation and properties of F-prime factors in Escherichia coli carrying nitrogen fixation genes from Klebsiella pneumoniae. J Gen Microbiol. 1976 Mar;93(1):111–125. doi: 10.1099/00221287-93-1-111. [DOI] [PubMed] [Google Scholar]
- Carter K. R., Rawlings J., Orme-Johnson W. H., Becker R. R., Evans H. J. Purification and characterization of a ferredoxin from Rhizobium japonicum bacteroids. J Biol Chem. 1980 May 10;255(9):4213–4223. [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Guerlesquin F., Bruschi M., Bovier-Lapierre G., Bonicel J., Couchoud P. Primary structure of the two (4 Fe-4 S) clusters ferredoxin from Desulfovibrio desulfuricans (strain Norway 4). Biochimie. 1983 Jan;65(1):43–47. doi: 10.1016/s0300-9084(83)80027-3. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Matsubara H. Pseudomonas ovalis ferredoxin: similarity to Azotobacter and Chromatium ferredoxins. FEBS Lett. 1978 Jul 15;91(2):315–319. doi: 10.1016/0014-5793(78)81200-9. [DOI] [PubMed] [Google Scholar]
- Howard J. B., Lorsbach T. W., Ghosh D., Melis K., Stout C. D. Structure of Azotobacter vinelandii 7Fe ferredoxin. Amino acid sequence and electron density maps of residues. J Biol Chem. 1983 Jan 10;258(1):508–522. [PubMed] [Google Scholar]
- Merrick M., Filser M., Kennedy C., Dixon R. Polarity of mutations induced by insertion of transposons Tn5, Tn7 and Tn10 into the nif gene cluster of Klebsiella pneumoniae. Mol Gen Genet. 1978 Sep 20;165(1):103–111. doi: 10.1007/BF00270382. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Riedel G. E., Brown S. E., Ausubel F. M. Nitrogen fixation by Klebsiella pneumoniae is inhibited by certain multicopy hybrid nif plasmids. J Bacteriol. 1983 Jan;153(1):45–56. doi: 10.1128/jb.153.1.45-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Matsueda G., Yasunobu K. T., Himes R. H., Akagi J. M., Barnes E. M., Devanathan T. The primary structure of the Clostridium tartarivorum ferredoxin, a heat-stable ferredoxin. J Biol Chem. 1971 Jun 25;246(12):3953–3960. [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Reiländer H., Pühler A. Mapping and expression of a regulatory nitrogen fixation gene (fixD) of Rhizobium meliloti. EMBO J. 1985 Nov;4(11):2751–2756. doi: 10.1002/j.1460-2075.1985.tb03999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]



