Abstract
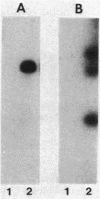
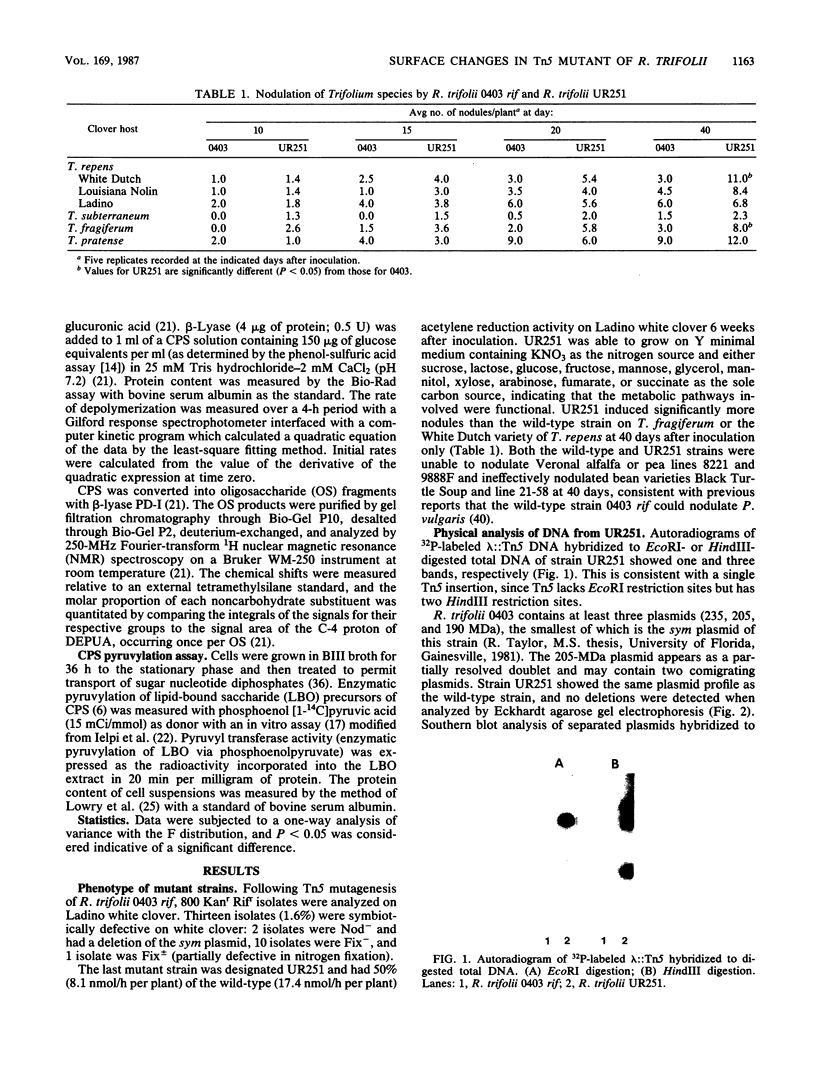
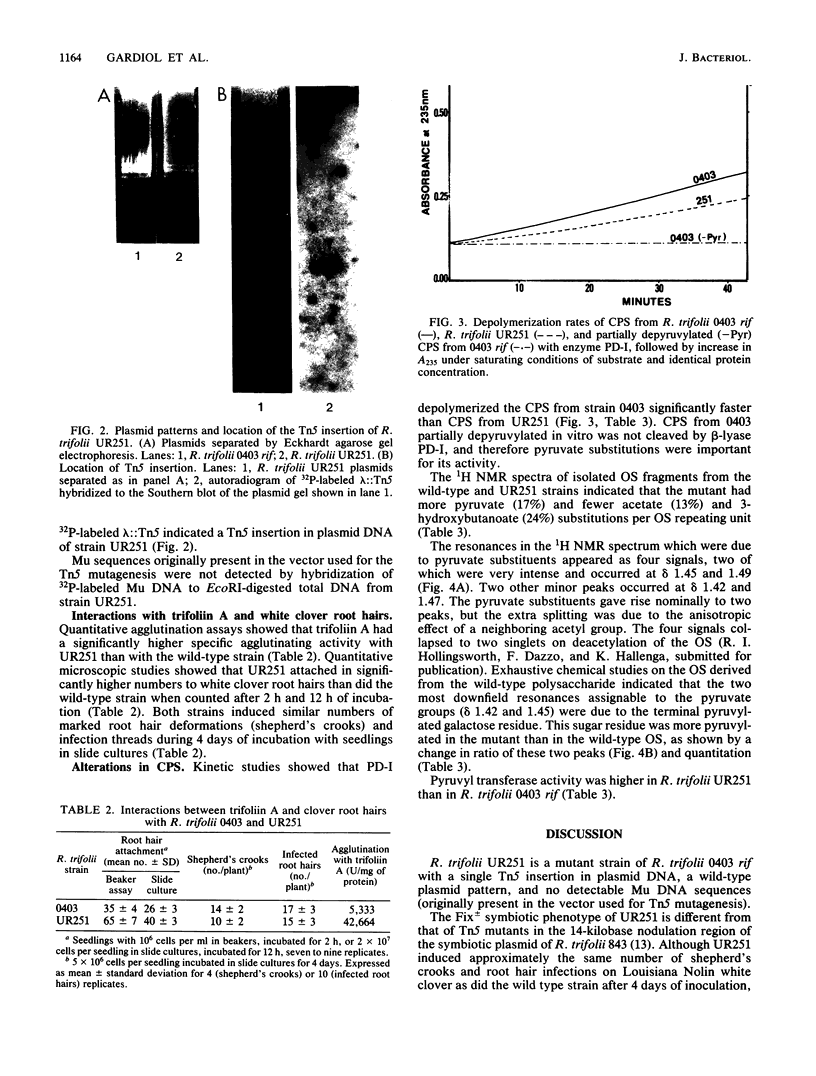
A symbiotically defective mutant strain of Rhizobium trifolii, UR251, was obtained by transposon Tn5 mutagenesis of R. trifolii 0403 rif and recognized by its partially ineffective (Fix +/-) phenotype on white clover plants. UR251 had a single Tn5 insertion in plasmid DNA, a wild-type plasmid pattern, and no detectable Mu DNA sequences originally present in the vector used for Tn5 mutagenesis. Agglutination by the clover lectin trifoliin A and attachment to clover root hairs was higher with UR251 than with the wild-type strain. The capsular polysaccharide (CPS) of UR251 was altered, as shown by a slower rate of CPS depolymerization with a CPS beta-lyase, PD-I; more pyruvate and less acetate and 3-hydroxybutanoate noncarbohydrate substitutions as quantitated by 1H nuclear magnetic resonance; and a higher pyruvyl transferase activity (enzymatic pyruvylation of lipid-bound saccharides). The site of increased pyruvylation in the CPS of UR251 was on the terminal galactose of the branch of the repeating oligosaccharide unit. These results show that the level of noncarbohydrate substitutions of the CPS as well as pyruvyl transferase activity are altered in R. trifolii UR251 and that trifoliin A-binding ability and clover root hair attachment are improved in this mutant strain of R. trifolii 0403 rif.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Sherwood J. E., Hollingsworth R. I., Dazzo F. B. Stimulation of clover root hair infection by lectin-binding oligosaccharides from the capsular and extracellular polysaccharides of Rhizobium trifolii. J Bacteriol. 1984 Nov;160(2):517–520. doi: 10.1128/jb.160.2.517-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bossio J. C., de Iannino N. I., Dankert M. A. In vitro synthesis of a lipid-linked acetylated and pyruvilated oligosaccharide in Rhizobium trifolii. Biochem Biophys Res Commun. 1986 Jan 14;134(1):205–211. doi: 10.1016/0006-291x(86)90548-6. [DOI] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Christensen A. H., Schubert K. R. Identification of a Rhizobium trifolii plasmid coding for nitrogen fixation and nodulation genes and its interaction with pJB5JI, a Rhizobium leguminosarum plasmid. J Bacteriol. 1983 Nov;156(2):592–599. doi: 10.1128/jb.156.2.592-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Histone genes are clustered but not tandemly repeated in the chicken genome. Proc Natl Acad Sci U S A. 1981 May;78(5):2856–2860. doi: 10.1073/pnas.78.5.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol A. E., Dazzo F. B. Biosynthesis of Rhizobium trifolii capsular polysaccharide: enzymatic transfer of pyruvate substitutions into lipid-bound saccharide intermediates. J Bacteriol. 1986 Dec;168(3):1459–1462. doi: 10.1128/jb.168.3.1459-1462.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F. J., Howe M. M. Involvement of the invertible G segment in bacteriophage mu tail fiber biosynthesis. Virology. 1984 Apr 30;134(2):296–317. doi: 10.1016/0042-6822(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth R. I., Abe M., Sherwood J. E., Dazzo F. B. Bacteriophage-induced acidic heteropolysaccharide lyases that convert the acidic heteropolysaccharides of Rhizobium trifolii into oligosaccharide units. J Bacteriol. 1984 Nov;160(2):510–516. doi: 10.1128/jb.160.2.510-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ielpi L., Couso R. O., Dankert M. A. Pyruvic acid acetal residues are transferred from phosphoenolpyruvate to the pentasaccharide-P-P-lipid. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1400–1408. doi: 10.1016/s0006-291x(81)80167-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Paau A. S., Leps W. T., Brill W. J. Agglutinin from Alfalfa Necessary for Binding and Nodulation by Rhizobium meliloti. Science. 1981 Sep 25;213(4515):1513–1515. doi: 10.1126/science.213.4515.1513. [DOI] [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Sherwood J. E., Vasse J. M., Dazzo F. B., Truchet G. L. Development and trifoliin A-binding ability of the capsule of Rhizobium trifolii. J Bacteriol. 1984 Jul;159(1):145–152. doi: 10.1128/jb.159.1.145-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Industrially useful microbial polysaccharides. Microbiol Sci. 1986 Jan;3(1):5–9. [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Tolmasky M. E., Staneloni R. J., Ugalde R. A., Leloir L. F. Lipid-bound sugars in Rhizobium meliloti. Arch Biochem Biophys. 1980 Aug;203(1):358–364. doi: 10.1016/0003-9861(80)90187-3. [DOI] [PubMed] [Google Scholar]
- Ugalde R. A., Handelsman J., Brill W. J. Role of galactosyltransferase activity in phage sensitivity and nodulation competitiveness of Rhizobium meliloti. J Bacteriol. 1986 Apr;166(1):148–154. doi: 10.1128/jb.166.1.148-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R., Jaskunas S. R., Nomura M. Insertion of DNA carrying ribosomal protein genes of Escherichia coli into Charon vector phages. J Biol Chem. 1977 Oct 25;252(20):7344–7354. [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]