Abstract
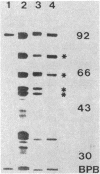
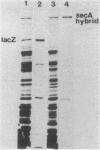
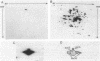
To define the anti-SecA-LacZ antiserum, immunoprecipitates produced with either whole anti-SecA-LacZ rabbit antiserum or affinity-purified antibodies were used to analyze nondenatured lysates of Escherichia coli. The antiserum contains antibodies that recognize different proteins. Antibody purified by preadsorption to the SecA-LacZ hybrid protein precipitated only the SecA protein from extracts. In contrast, antibody purified from the intact SecA protein precipitated several additional proteins with SecA protein. Ribosomal protein L7L12 is one of the polypeptides coprecipitated with SecA protein by antibody purified by immunoadsorption to the intact SecA protein as well as by unfractionated anti-SecA-LacZ antiserum. Two-dimensional gel electrophoresis of the SecA protein immunoprecipitated by either antiserum or purified antibody indicated that the SecA protein exists in at least two, and probably four, isoforms. Only one of the SecA isoforms is present in a ribosomal preparation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Honma M., Beckwith J. The product of gene secC is involved in the synthesis of exported proteins in E. coli. Cell. 1984 Aug;38(1):211–217. doi: 10.1016/0092-8674(84)90542-7. [DOI] [PubMed] [Google Scholar]
- Gross C. A., Grossman A. D., Liebke H., Walter W., Burgess R. R. Effects of the mutant sigma allele rpoD800 on the synthesis of specific macromolecular components of the Escherichia coli K12 cell. J Mol Biol. 1984 Jan 25;172(3):283–300. doi: 10.1016/s0022-2836(84)80027-3. [DOI] [PubMed] [Google Scholar]
- Langley D., Guest J. R. Biochemical genetics of the alpha-keto acid dehydrogenase complexes of Escherichia coli K12: genetic characterization and regulatory properties of deletion mutants. J Gen Microbiol. 1978 May;106(1):103–117. doi: 10.1099/00221287-106-1-103. [DOI] [PubMed] [Google Scholar]
- Meyer L. J., Milburn S. C., Hershey J. W. Immunochemical characterization of mammalian protein synthesis initiation factors. Biochemistry. 1982 Aug 31;21(18):4206–4212. doi: 10.1021/bi00261a003. [DOI] [PubMed] [Google Scholar]
- Moore P. B. The preparation of deuterated ribosomal materials for neutron scattering. Methods Enzymol. 1979;59:639–655. doi: 10.1016/0076-6879(79)59119-8. [DOI] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Blobel G. Protein export in Escherichia coli requires a soluble activity. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7737–7741. doi: 10.1073/pnas.81.24.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Vaughn V., Phillips T. A., Bloch P. L. Gene-protein index of Escherichia coli K-12. Microbiol Rev. 1983 Jun;47(2):231–284. doi: 10.1128/mr.47.2.231-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J Bacteriol. 1982 May;150(2):686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Plumbridge J. A., Deville F., Sacerdot C., Petersen H. U., Cenatiempo Y., Cozzone A., Grunberg-Manago M., Hershey J. W. Two translational initiation sites in the infB gene are used to express initiation factor IF2 alpha and IF2 beta in Escherichia coli. EMBO J. 1985 Jan;4(1):223–229. doi: 10.1002/j.1460-2075.1985.tb02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Bassford P. J., Jr Both linked and unlinked mutations can alter the intracellular site of synthesis of exported proteins of Escherichia coli. J Bacteriol. 1985 Jan;161(1):258–264. doi: 10.1128/jb.161.1.258-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeh S., Pedersen S. Post-translational modification of Escherichia coli ribosomal protein S6. Mol Gen Genet. 1979 Jun 7;173(2):183–187. doi: 10.1007/BF00330309. [DOI] [PubMed] [Google Scholar]
- Rombauts W., Peeters B., Wittmann H. G. Comparison of peptide patterns from isolated 30 S and 50 S ribosomal proteins of Escherichia coli by column chromatography. FEBS Lett. 1971 Oct 15;18(1):164–168. doi: 10.1016/0014-5793(71)80435-0. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. 2-Oxoacid dehydrogenase complexes of Escherichia coli: cellular amounts and patterns of synthesis. J Bacteriol. 1983 Oct;156(1):81–88. doi: 10.1128/jb.156.1.81-88.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers E., Jr, Cuatrecasas P., Pollard H. B. The purification of beta-galactosidase from Escherichia coli by affinity chromatography. J Biol Chem. 1971 Jan 10;246(1):196–200. [PubMed] [Google Scholar]
- Steitz J. A., Wolin S. L., Rinke J., Pettersson I., Mount S. M., Lerner E. A., Hinterberger M., Gottlieb E. Small ribonucleoproteins from eukaryotes: structures and roles in RNA biogenesis. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):893–900. doi: 10.1101/sqb.1983.047.01.103. [DOI] [PubMed] [Google Scholar]