Abstract
An overactive renin-angiotensin-aldosterone system (RAAS) has a central role in the pathogenesis of hypertension and cardiac hypertrophy, precursors of cardiac failure. Natriuretic peptides and NO acting through their second messenger, cGMP, increase natriuresis and diuresis, and inhibit renin release; however the mechanism by which this inhibition of the RAAS system functions is obscure. We recently reported cloning of the cDNA for type II cGMP-dependent protein kinase (cGK II), elucidated its first known function of inhibiting the cystic fibrosis transmembrane conductance regulator in rat intestine, and initially described its location in rat kidney juxtaglomerular (JG) cells, the ascending thin limb, and the brush border of proximal tubules. Here, we demonstrate inhibition of isoproterenol- or forskolin-stimulated renin release by 8-para-chlorophenylthio-cGMP (8-pCPT-cGMP), a selective activator of cGK, and prevention of this inhibition by a selective inhibitor of cGK, Rp-8-pCPT-cGMPS. In systems of differing complexity, inhibition by 8-pCPT-cGMP was nearly complete in isolated perfused kidney and microdissected afferent arterioles but only ≈25% in isolated JG cells. Expression of either cGK II or cGK I in JG cells by using adenoviral vectors enhanced the inhibition of forskolin-stimulated renin release by 8-pCPT-cGMP to 50%. Our results indicate that cGK II, and possibly cGK I, can mediate cGMP inhibitory effects on renin release and are physiological components of the cGMP signal transduction system which opposes the RAAS.
Inhibitors of the renin-angiotensin-aldosterone system (RAAS) have longstanding clinical use in the control of hypertension and its frequent sequels of cardiac hypertrophy and heart failure (1). Recently, the potential of the RAAS system to generate pathology was demonstrated directly by the development of severe hypertension and cardiac hypertrophy in double transgenic rats and mice expressing human angiotensinogen and renin DNA (2). Treatment with either an angiotensin I-converting enzyme inhibitor or with a renin inhibitor normalized blood pressure. In contrast, NO has been shown to have profound restraining effects on the RAAS because long-term blockade of NO synthase (NOS) by chronic administration of Nω-nitro-l-arginine methyl ester (l-NAME) to rats increased plasma renin activity, cardiac tissue angiotensin converting enzyme, vascular medial thickening, arterial systolic hypertension, and cardiac hypertrophy (3). eNOS knockout mice display increased blood pressure and plasma renin activity twice the normal (4). Some, but not all, effects of NO are mediated via cGMP, as are also effects of atrial natriuretic peptide (ANP), another inhibitor of the vasoconstrictor and volume-retention properties of angiotensin (5, 6). ANP receptor A knockout mice develop hypertension, cardiac hypertrophy, and sudden death (7). However, knowledge of cGMP-activated signal transduction pathways controlling the RAAS is still rudimentary.
Major mediators of the effects of cGMP include cGMP-gated channels, cGMP-regulated phosphodiesterases (PDEs), and cGMP-dependent protein kinases (cGK) (5). Mammalian cGK exists as two major forms, cGK I, a soluble enzyme consisting of α and β isoforms derived from alternative splicing from one gene, and cGK II, a myristoylated, membrane-associated enzyme derived from a second gene (8, 12, and reviewed in ref. 9). Considerable experimental evidence supports the concept that cGK I lowers intracellular Ca2+ and inhibits vascular smooth muscle contraction, platelet activation, and endothelial cell permeability, thereby opposing events leading to hypertension, thrombosis, and atherosclerosis (9). In kidney, cGK I is present in vascular smooth muscle cells, mesangial cells, and contractile interstitial cells (10) and is a candidate mediator of cGMP effects on renal hemodynamics and glomerular filtration rate.
Although less widely studied in comparison to cGK I, cGK II often has a quite different localization and certain distinct and specific functions. For example, cGK II, but not cGK I, phosphorylates and activates the cystic fibrosis transmembrane conductance regulator (CFTR) in intestinal mucosa in response to cGMP-elevating agents such as the Escherichia coli heat stable toxin and a structurally similar endogenous intestinal peptide, guanylin (reviewed in ref. 9). A cGK II knockout mouse indeed shows resistance to heat stable toxin-induced intestinal secretion and furthermore displays a defect in bone endochondral ossification resulting in dwarfism (11). Obviously neither defect is rescued by cGK I and also not by cAMP-kinase. No altered phenotype with respect to renal function has yet been reported.
We recently cloned cGK II (12) and identified primary sites of extra-intestinal cGK II in kidney (juxtaglomerular (JG), ascending thin limb, and proximal tubule cells) (13), adrenal, and brain (S.G., unpublished results). The sites of cGK II localization in kidney suggested that cGK II may mediate described effects of ANP (14) via cGMP on inhibition of renin release from JG cells and inhibition of angiotensin II-stimulated sodium and water reabsorption in the proximal tubule. Furthermore, chronic treatment of rats with the type I angiotensin II receptor (AT1R) inhibitor, losartan, stimulated the level of both renin and cGK II mRNA and protein in JG cells, suggesting a potential involvement of cGK II in renin regulation (13). In the present study, we focus on the role of cGK in renin release. Analyses from three systems of differing complexity demonstrated that a cGK-selective activator, 8-para-chlorophenylthio-cGMP (8-pCPT-cGMP), consistently inhibited forskolin-stimulated renin release. Furthermore, overexpression of cGK II and I in JG cells by using adenoviral vectors enhanced cGMP-mediated inhibition of renin release.
MATERIALS AND METHODS
Isolated Perfused Rat Kidney.
Kidney perfusion was performed in a recycling system as described in detail (15). The time between 20 and 30 min after start of perfusion was monitored as a control (baseline) period for each kidney. Subsequently, test agents dissolved in freshly prepared perfusate were infused into the arterial limb of the perfusion circuit, directly proximal to the kidney, at a rate equal to 1% of the perfusate flow rate. For determination of renin activity, perfusate aliquots were withdrawn from both the arterial and venous sides of the kidney. Samples were centrifuged at 1,500 × g for 15 min and the resulting supernatants were stored at −20°C before renin activity assay. Renin secretion rates were calculated by using the perfusate flow rate and the arterio-veneous difference in renin activity.
Microdissected Glomeruli Containing Afferent Arterioles.
Kidney perfusion and digestion with collagenase (Type 1, Sigma) for microdissection of glomeruli-afferent arterioles (AA) (from superficial nephrons only) was performed as described (13). Dissection of 150 glomeruli for each experiment was completed within 1 h. After microdissection, glomeruli were transferred to small plastic cylinders closed on one side with 54-μm nylon mesh, and preincubated 30 min in 500 μl of oxygenated (95% O2/5% CO2) Medium 199 (Life Technologies, Karlsruhe, Germany) with 0.1% BSA. Subsequently, the cylinders were rinsed, blotted, and transferred to fresh medium in which the glomeruli were incubated with various agents for 1 h and then removed from the media, which was frozen for later measurement of released renin. The glomeruli were transferred to new microtubes, solubilized with the same medium containing 0.1% Triton X-100, and frozen before measurement of unreleased renin. In all experiments, 10 individual samples (each containing five microdissected glomeruli) were averaged for each condition, and the radioimmunoassay (RIA) analysis of each sample was performed in triplicate. Both 8-pCPT-cGMP and Rp-8-pCPT-cGMPS were obtained from Biolog (Bremen, Germany).
Isolated JG Cells.
Mouse kidneys were digested with collagenase A (Boehringer Mannheim), and JG cells were isolated as described (16, 17). JG cells were recovered after centrifugation in isosmotic Percoll (Pharmacia) gradients, and the cellular layer (density = 1.07 g/ml) with the highest specific renin activity was used for cell culture. JG cells resuspended in RPMI 1640 medium (Biochrom, Berlin) containing 0.66 units/ml insulin, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2% fetal calf serum were distributed in 100 μl aliquots into 96-well plates. After 20 h of primary culture (37°C, 95% O2/5% CO2), the culture medium was removed, and cultures were washed once with medium containing 2% fetal calf serum and subsequently incubated with medium containing test substances. At the end of the experiment, medium was removed, clarified by centrifugation, and frozen for analysis of secreted renin. Cells were lysed by incubation with PBS containing 0.1% Triton X-100 as described (17) and frozen for later determination of unreleased renin remaining in the cells.
Infection of JG Cells with Adenoviral Vectors Expressing cGK II and cGK Iβ.
Construction of the adenoviral vectors for expression of cGK II and cGK Iβ was described (18). After JG cells were cultured for 20 h and washed as described above, they were either infected, or not infected, with adenoviral vectors (1011 particles/ml) expressing cGK II or Iβ to test the effect of 8-pCPT-cGMP on forskolin-stimulated renin release. Control and infected cells were then incubated at 37°C (95% O2/5% CO2) for a further 24 h to allow time for cGK expression. Then the JG cells were incubated −/+ 250 μM 8-pCPT-cGMP for 1 h, and subsequently −/+ 10 μM forskolin (Sigma) for a further 24 h, before JG cells and the media containing released renin were harvested separately as described above for renin activity assay. In all experiments, six individual JG cell samples were averaged for each condition. The RIA analysis of each sample was performed in triplicate.
Renin Activity Assay.
Renin was measured by its activity for converting angiotensinogen (in plasma of bilaterally nephrectomized rats) to angiotensin I (AT I) as described (17). AT I was measured by RIA (Sorin Biomedica, Düsseldorf, Germany), and renin release was calculated as nanograms of AT I generated/h of renin assay. To minimize variation among different glomeruli or JG cell culture preparations, renin secretion was expressed as percentage of renin release, i.e., the ratio of released renin/total renin (total sum of released renin plus that remaining in the cells) as described (17).
Western Blot Analysis.
The level of endogenous and expressed cGK in JG cells was determined by Western blot analysis. Control (uninfected) JG cells and cells infected with adenoviral vectors expressing cGK II and cGK Iβ were harvested after 48 h by using hot SDS/PAGE stop solution as described (18) and analyzed by Western blot by using antibodies made against recombinant cGK II or cGK Iβ (19), diluted 1:200 each, and 125I-protein A (Amersham).
Immunogold Electron Microscopy.
For postembedding immunogold staining, small pieces of perfusion-fixed kidneys, or glomeruli-AA, were fixed by immersion in 4% paraformaldehyde in PBS and embedded in LR White resin (London Resin, Basingstoke, U.K.). Thin sections were cut and labeled with cGK II (19) or renin (16) antibody (diluted 1:200 and 1:2000, respectively), and goat anti-rabbit antibody-coupled gold particles (Amersham Buchler, Braunschweig, Germany) diluted 1:40. Thereafter the sections were treated with 1% glutaraldehyde, double stained with uranyl acetate and lead citrate, and analyzed by using a Philips EM 300 electron microscope.
Statistics.
Student’s paired t test was used to calculate levels of significance and P < 0.05 was considered significant.
RESULTS
cGMP-Dependent Inhibition of Isoproterenol-Stimulated Renin Release in the Isolated Perfused Kidney.
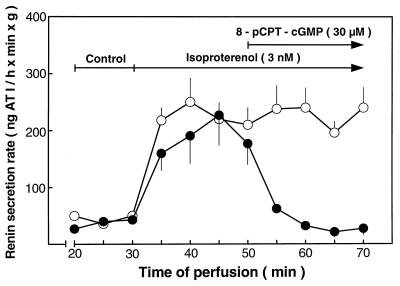
The left kidney of Sprague–Dawley CD rats was perfused by using a constant pressure of 100 mm Hg, which corresponds to the normal arterial pressure of rats. The renin secretion rate during a control period (shown for 20–30 min after perfusion begin, Fig. 1) before each experiment was constant at 41 ± 6 ng of AT I/h/min/g tissue. Addition of isoproterenol (3nM) to the perfusate at 30 min strongly increased the average renin secretion rate 5-fold to 209 ± 16 ng of AT I/h/min/g tissue, which remained relatively constant until the end of experiment at 70 min (Fig. 1). Addition of 8-pCPT-cGMP (30 μM), a membrane-permeable, PDE resistant, selective activator of cGMP kinases (20), 20 min after stimulation by isoproterenol reversed the renin secretion rate to the control level. In the absence of isoproterenol, 8-pCPT-cGMP alone did not affect basal renin secretion (data not shown). Under conditions of constant pressure, the perfusion flow rate (12.6 ± 0.1 ml/min/g kidney weight during the control period) was unaltered after stimulation of renin release by isoproterenol (12.8 ± 0.1), but was slightly increased (15.2 ± 0.2, P < 0.05, vs. control and isoproterenol) after addition of 8-pCPT-cGMP to the perfusate.
Figure 1.
Inhibition of isoproterenol-stimulated renin release by 8-pCPT-cGMP in the isolated perfused rat kidney. Baseline renin release was measured between 20 and 30 min after perfusion begin. Subsequently, isoproterenol (3 nM) was added to the perfusate, and at the indicated time, 8-pCPT-cGMP (30 μM) was either added (•) or not added (○) to the perfusate. Perfusate samples withdrawn from both the arterial and venous sides of the kidney at the times indicated were used to calculate the renin secretion rate expressed as nanogram of AT I formed from angiotensinogen/h of assay conversion time/min of perfusion/g of kidney weight. Shown are the means ± SEM of results from five perfused kidneys.
cGMP-Dependent Inhibition of Forskolin-Stimulated Renin Release in Microdissected Glomeruli-AA.
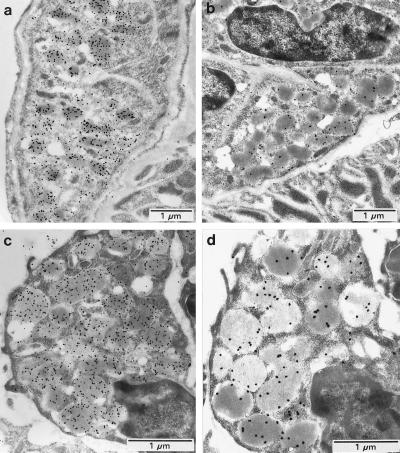
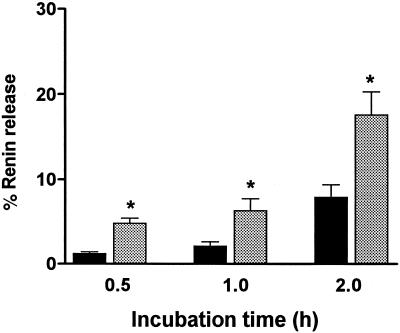
The 8-pCPT-cGMP inhibition of isoproterenol-stimulated renin release in whole kidney perfusion may represent the overall result of different possible mechanisms. To exclude indirect effects on renal hemodynamics, the macula densa, renal nerves, etc., glomeruli with attached AA (21) were microdissected from rat kidneys for examining direct effects of 8-pCPT-cGMP on renin release. The suitability of this preparation was investigated by electron microscopy with immunogold staining for renin and cGK II. Microdissected glomeruli-AA were removed after the renin release studies for subsequent fixation and electron microscopy. In Fig. 2, the ultrastructural features of the microdissected glomeruli-AA (Fig. 2 c and d Bottom) was observed to be unchanged from the same structures in sections of perfusion-fixed kidney (Fig. 2 a and b Top ). Also in both preparations, the JG cells contained well preserved renin granules that were strongly labeled with antibodies against renin (Fig. 2 a and c) and cGK II (Fig. 2 b and d). In further studies, a linear relationship was observed between numbers of glomeruli-AA assayed and total renin activity (data not shown). In three different experiments, 10 tubes containing different numbers of microdissected glomeruli-AA (from 1 to 10) were shown to contain renin activities ranging from 120 to 1,200 ng of AT I/h (indicating an average of 120 per glomerulus-AA). However, glomeruli cannot be kept for prolonged periods of time after dissection, and therefore the optimal time of incubation for studying renin release was determined (Fig. 3). The time of 1 h was chosen for further studies because basal renin release remained small in comparison to forskolin-stimulated release. Renin release was shown to be maximally stimulated (≈2.5–3.5-fold in four experiments, P < 0.01, compared with control) by 10 μM forskolin or 100 μM isoproterenol incubation for 1 h (data not shown).
Figure 2.
Immunogold localization of renin and cGK II in rat kidney JG cells (a and b) and in JG cells of microdissected rat glomeruli containing afferent arterioles (c and d). Colocalization of renin (a and c) and cGK II (b and d) was observed in renin secretory granules in both types of preparations. Control sections treated with preimmune serum exhibited no specific labeling (not shown). These results are representative of several experiments.
Figure 3.
Time course of renin release from microdissected glomeruli-AA. The glomeruli-AA were incubated in Medium 199 with 0.1% BSA for 0.5, 1, and 2 h (control, black bars) or for the same times in medium containing 10 μM forskolin (grey bars). Renin release data are expressed as percentage of renin release, i.e., the ratio of released renin/total renin (total sum of released renin plus that remaining in the cells) as described (17). Data represent the mean ± SEM from three different experiments. Forskolin samples were significantly different (∗, P < 0.01) from their respective controls at each time point. From these data, 1 h was chosen as the incubation time for all other experiments with glomeruli-AA.
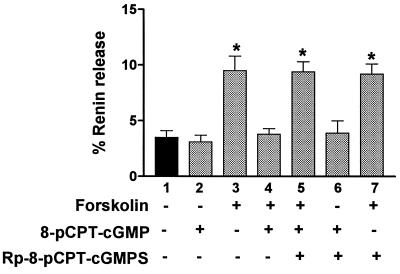
Forskolin-stimulation of renin release (Fig. 4, lane 3) was almost completely inhibitable to basal levels of release (lane 1) by preincubation with 2.5 μM, or higher concentrations, of 8-pCPT-cGMP (lane 4). Also, the inhibition by 8-pCPT-cGMP could be prevented by prior treatment with a stereo-specific inhibitor of the cGMP kinases, Rp-8-pCPT-cGMPS (lane 5) (22). The specificity of Rp-8-pCPT-cGMPS is demonstrated by lane 7 because this compound has no effect on the forskolin effect, which is mediated by cAMP and most likely cAMP kinase (23). The cGMP analogs themselves had no significant effects on basal renin release (lanes 2 and 6).
Figure 4.
Inhibition of forskolin-stimulated renin release from microdissected glomeruli-AA by a selective activator of cGK. Glomeruli were incubated 1 h in Medium 199 with 0.1% BSA either in the absence or presence of 10 μM forskolin for 1 h as shown. Preincubation of some samples for 5 min with the cGK-selective activator 8-pCPT-cGMP (2.5 μM) before forskolin was added caused a major inhibition of forskolin-stimulated renin release (lane 4). In other samples, a cGK-selective inhibitor, Rp-8-pCPT-cGMPS (25μM) was added 15 min before the addition of 8-pCPT-cGMP (lanes 5 and 6) or forskolin (lane 7). To minimize variation among different glomeruli preparations, renin secretion was expressed as percentage of renin release, as described in Fig. 3. Data represent the mean ± SEM from three different experiments. Designated samples (∗) are significantly different (P < 0.01) from untreated controls (lane 1), cGMP analogs which alone had no significant effect on renin release in the absence of forskolin (lanes 2 and 6), and the forskolin-stimulated renin release, which was inhibited by 8-pCPT-cGMP (lane 4).
Inhibition of Forskolin-Stimulated Renin Release by NO Donors in Microdissected Glomeruli-AA.
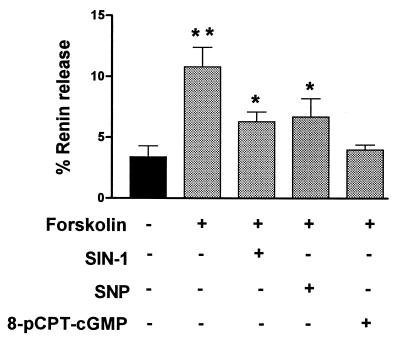
The effect of NO donors, some of which are used clinically to mimic the effects of endogenous endothelium derived relaxing factor (EDRF), were tested because certain, but not all, NO effects are mediated by cGMP (5). The shown concentrations of sodium nitroprusside (SNP; 50 μM) and 3-morpholinosydnonimine hydrochloride (SIN-1; 10 μM) caused the maximal inhibitions of forskolin-stimulated renin release that were possible to achieve with these agents (Fig. 5). In contrast to the effects observed with 8-pCPT-cGMP, inhibition caused by these agents was only partial.
Figure 5.
Inhibition of forskolin-stimulated renin release from microdissected glomeruli-AA by NO donors. Glomeruli were incubated 1 h in Medium 199 with 0.1% BSA either in the absence or presence of 10 μM forskolin as shown. Preincubation of glomeruli with either SIN-1 (10 μM) or SNP (50 μM) 5 min before forskolin was added resulted in a partial inhibition of forskolin-stimulated renin release, which was less than that observed with 8-pCPT-cGMP. Results are presented as percentage of renin release as described in Fig. 3. Data represent the mean ± SEM from three different experiments. All designated (∗ or ∗∗) samples were significantly different (P < 0.01) from control, and forskolin-treated glomeruli (∗∗) were significantly different from those pretreated with SIN-1 or SNP (P < 0.05).
cGMP-Dependent Protein Kinase Mediated Inhibition of Forskolin-Stimulated Renin Release in Primary Cultures of JG Cells.
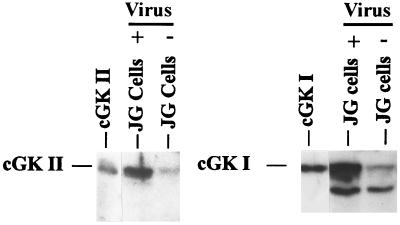
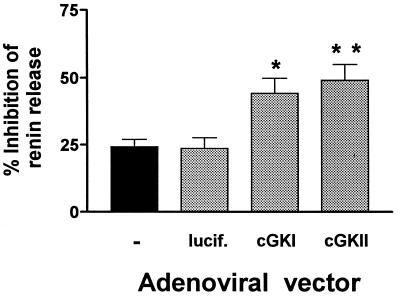
JG cells were isolated and found to contain small amounts of endogenous cGK I and cGK II on Western blots (Fig. 6). Although renin release from JG cells could be inhibited by 8-pCPT-cGMP, the inhibition (24%) was much less than that observed in the experiments with isolated glomeruli and perfused kidney (≈95% inhibition) (see Figs. 1 and 4). Therefore, we studied whether it was possible to increase cGK I and cGK II in these JG cells and to identify whether one or both kinases can mediate the inhibitory effects of cGMP on renin release. Adenoviral vectors used to express either cGK Iβ or cGK II increased the level of the kinases several fold in infected JG cells in comparison with control (uninfected) cells (Fig. 6). Whereas 8-pCPT-cGMP (250 μM) inhibited only 24% of the forskolin-stimulated renin release in uninfected JG cells, or in cells infected with a control, luciferase-expressing adenoviral vector, it inhibited 44% and 49% of the forskolin-stimulated renin release in cells expressing cGK Iβ and cGK II from adenoviral vectors, respectively (Fig. 7).
Figure 6.
Western blot of isolated mouse JG cells containing endogenous cGK II and cGK I (- virus), or additional cGK II and cGK I expressed from adenoviral vectors (+ virus). cGK II and cGK I were identified by their migration like recombinant kinases expressed in Sf9 cell lysates (86 and 76 kDa, respectively, shown at the left side of each panel) and were detected by using specific antisera and 125I- labeled protein A. The band migrating faster than cGK I is most likely a well known cGK I tryptic degradation product also sometimes observed in the cGK I standard. JG cell samples are 30 μg of protein each. The blots shown are representative of results from three different experiments.
Figure 7.
Inhibition of forskolin-stimulated renin release by 8-pCPT-cGMP in primary cultures of mouse JG cells. Shown are control (-) JG cells containing endogenous cGK (both I and II, see Fig. 6) and JG cells after infection with adenoviral vectors expressing either luciferase (lucif., control vector), cGK I, or cGK II. Control and infected cells were incubated for 24 h to allow expression of cGK. Then the JG cells were incubated −/+ 250 μM 8-pCPT-cGMP for 1 h and subsequently with −/+ 10 μM forskolin for a further 24 h before JG cells and media containing released renin were harvested separately for renin activity assay. Basal release of renin for untreated controls (-) averaged 0.9 μg AT I/h/100 μg cellular protein/20 h, corresponding to a fractional release of 24.3 ± 2.8% (mean ± SEM, n = 4) of total renin activity. Results are expressed as percentage of inhibition of renin release stimulated by forskolin. In all experiments, forskolin-stimulated renin release averaged 2.1 ± 0.4 (SD) -fold. Data shown are expressed as the mean ± SEM from four to six different experiments. ∗, P < 0.05 and ∗∗, P < 0.01, compared with control and luciferase vectors.
DISCUSSION
Renin controls blood pressure via the production of both angiotensin II, an extremely potent vasoconstrictor, and aldosterone, responsible for salt homeostasis. Because these agents and their effects are major determinants of blood pressure, it is not surprising that renin itself is highly regulated and subject to feedback control. Renin is released from JG, which are modified myoepithelial cells located in the walls of the afferent arterioles of kidney glomeruli. Renin release responds to the tone of the afferent arteriole, variation in sodium levels in the distal tubule (tubuloglomerular feedback via the macula densa), sympathetic nerve activity, and humoral factors (23, 24). Agents which increase cAMP or lower Ca2+ stimulate renin release, whereas agents which inhibit renin release either raise Ca2+, may increase cGMP, or decrease cAMP (23). The inhibitory effect of Ca2+ is paradoxical with respect to most other mechanisms of secretion which are enhanced by Ca2+.
Analysis of the effect of cGMP on renin release are complicated by the use of systems of varying complexity (isolated perfused rat kidney, renal cortex slices or cells, isolated JG cells or JG apparatus, and cocultures of endothelial cells with JG cells), and by the analysis of EDRF/NO or NO donors, which can have effects independent of cGMP (5, 25). Furthermore, cGMP has several targets of action including ion channels, phosphodiesterases, and kinases. Therefore, we restricted our examination to the effects of 8-pCPT-cGMP, a selective activator of cGMP kinases, on renin release.
Our use of 8-pCPT-cGMP caused inhibition of isoproterenol- and forskolin-stimulated renin release in preparations of isolated perfused kidney, microdissected glomeruli-AA, and isolated JG cells. The effect of the cGMP analog and cGK was constant despite the use of systems of varying complexity with regard to renin regulatory mechanisms. Even in the most complex system, that of the isolated perfused kidney, which comes closest to the in vivo situation, the role of cGK appears to be inhibition of renin release. In contrast to the inhibition of renin release by 8-pCPT-cGMP, EDRF has been shown to stimulate basal renin release at low kidney perfusion pressure (40 mm Hg, compared with 100 mm Hg used here) (26). In our microdissected glomeruli-AA, maximal effects of SNP and SIN-1 did not cause as complete an inhibition of forskolin-stimulated renin-release as did 8-pCPT-cGMP. Also, these agents alone had a significant stimulatory effect on basal renin release. In comparison to forskolin (10 μM) stimulation (2.7 ± 0.3 fold), 50 μM SNP, 10 μM SIN-1, and 1 μM ANP stimulated renin release 1.5 ± 0.2, 1.6 ± 0.2, and 1.6 ± 0.3 fold, respectively, although 2.5 μM 8-pCPT-cGMP alone caused no stimulation (n = 3–4 experiments). Whereas 8-pCPT-cGMP has no effects on phosphodiesterases, the cGMP generated by EDRF or NO donors also may activate cGMP-inhibited phosphodiesterase (cGI-PDE, PDE-3), which would decrease cAMP breakdown and enhance cAMP-dependent stimulation of renin release. Inhibition of NO synthase has been shown to decrease cGMP, thus decreasing cAMP by disinhibition of cGI-PDE, which hydrolyzes cAMP, and suppressing the renin response to isoproterenol (reviewed in 27). Thus, renin secretion regulation by opposing forces may depend on which forces predominate under various conditions and determine net release. In heart, cGMP inhibition of cAMP-stimulated L-type Ca2+ channels was shown to be mediated either by cGK or a cGMP-stimulated phosphodiesterase that hydrolyzed cAMP, although the mediator which predominated varied among different species (28, 29). Thus, agents which activate EDRF or NO donors used as medication may have other effects superimposed on those of cGK, and these may become most obvious in certain pathological conditions. For example, in congestive heart failure, cAMP-PDE and cGMP-PDE activities are enhanced and the cGMP response to both ANP and SNP is blunted (30).
The fact that the inhibitory effect of 8-pCPT-cGMP on renin release observed in the isolated perfused kidney was preserved in microdissected glomeruli-AA and isolated JG cells suggested that cGK may have a direct effect on the renin release mechanism because these preparations do not contain baroreceptor, macula densa, and sympathetic nerve pathways, which could serve as intermediate targets. In microdissected glomeruli-AA, the cGMP kinase inhibitor Rp-8-pCPT-cGMPS prevented the response to 8-pCPT-cGMP attributed to cGK, whereas it had no effect on the forskolin response, which is mediated by cAMP and most likely the cAMP kinase.
cGK I and cGK II could not be distinguished as mediators of 8-pCPT-cGMP inhibition of renin release. 8-pCPT-cGMP can activate both kinases and Rp-8-pCPT-cGMPS also can inhibit both (9), and no other specific agents are available. Western blot analysis demonstrated evidence of both kinases endogenously in cultured JG cells. Immunogold electron microscopy demonstrated cGK II labeling in JG cell secretory granules, whereas the localization of cGK I was diffuse and not particularly associated with granules. Adenoviral vector expression of either cGK II or cGK Iβ in isolated JG cells enhanced the inhibition observed for 8-pCPT-cGMP on forskolin-stimulated renin release. However, appropriate conditions for preserving both JG cell structural morphology and antibody antigenicity was not achieved; therefore we cannot say in which locations the kinases were expressed. In intestinal mucosa, endogenous cGK II and cGK I have distinct locations and functions. cGK I is in vascular smooth muscle cells of the lamina propria in the villus center, whereas cGK II is in the epithelial cell brush border where its location overlaps with that of the CFTR Cl− channel, which it regulates (19). However, in contrast to the results reported here, adenoviral vector expression of cGK I and cGK II in an intestinal cell line lacking endogenous cGKs, demonstrated that only cGK II and not cGK Iβ could phosphorylate and activate CFTR (18).
In general, however, the effects of 8-pCPT-cGMP were greater in the isolated perfused kidney and microdissected glomeruli-AA than in the cultured JG cells. This result could have many reasons; however, one might be the instability of the cGK itself. Both the isolated perfused kidney and microdissected glomeruli are used within a few hours of isolation, plus the incubation times are relatively short. In contrast, the isolated JG cells are cultured for almost a day before use. Because of an initial phase of cell leakiness, the forskolin incubation is carried out over almost another day to achieve a reasonable accumulation of forskolin-dependent renin release (16, 17). Also, cells preincubated with 8-pCPT-cGMP before forskolin are exposed to it for almost a day. In primary smooth muscle cells, elevation of cAMP or cGMP for 24 h was reported recently to partially down-regulate endogenous cGK I levels (31).
The mechanism of cGK-mediated inhibition of forskolin-stimulated renin release requires further study, especially because the renin release mechanism itself is not entirely clear. cGK I, however, would normally not be a good candidate for inhibiting renin release because in most cells it lowers Ca2+ (9), and in JG cells this would stimulate rather than inhibit renin release. Calcium has been suggested to inhibit renin release by activation of Cl− channels followed by cell shrinkage, by influencing the contractile apparatus of the cell, and by direct effects on secretory granules (reviewed in ref. 32). Several reports suggest (23, 32–34) that Ca2+ activates Cl− channels resulting in Cl− efflux. This effect leads to other ion and osmotic changes that result in secretory granule shrinkage and inability to fuse with the JG cell plasma membrane for exocytosis. The one known mechanism of action of cGK II is to stimulate Cl− secretion via the CFTR channel into the intestinal lumen, an effect which cannot be performed by cGK I or cAMP kinase (9, 11, 18).
In summary, our data suggest that endogenous cGK II in JG cells mediates certain inhibitory effects of cGMP on cAMP-stimulated renin release. The rather pure effect that can be obtained with 8-pCPT-cGMP, a selective activator of cGK, may be more complicated in the intact organism in which cGMP is produced and can regulate phosphodiesterases which also affect renin release. Our data do not exclude that cGK I also could participate in renin regulation because either cGK II or cGK I overexpressed in JG cells with adenoviral vectors could inhibit renin release. Thus, cGK II (unclear if cGK I) may be involved in the counterregulatory effects of the ANP and EDRF/NO on the RAAS for control of hypertension and salt and volume overload. Further investigations into the mechanisms of cGMP and cGK effects on the RAAS in not only healthy but also pathological states is particularly important for the clarification of the syndrome of ANP resistance and NO tolerance observed in heart failure (6, 30).
Acknowledgments
We thank Andrea Jahn and Susanne Stumpf for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AA, afferent arteriole; ANP, atrial natriuretic peptide; AT I, angiotensin I; cAK, cAMP-dependent protein kinase; CFTR, cystic fibrosis transmembrane conductance regulator; cGK, cGMP-dependent protein kinase; EDRF, endothelium derived relaxing factor; JG, juxtaglomerular; PDE, phosphodiesterase; RAAS, renin-angiotensin-aldosterone system; SNP, sodium nitroprusside; SIN-1, 3-morpholinosydnonimine hydrochloride; 8-pCPT, 8-para-chlorophenylthio.
References
- 1.Crozier I, Ikram H, Awan N, Cleland J, Stephen N, Dickstein K, Frey M, Young J, Klinger G, Makris L, et al. Circulation. 1995;91:691–697. doi: 10.1161/01.cir.91.3.691. [DOI] [PubMed] [Google Scholar]
- 2.Bohlender J, Fukamizu A, Lippoldt A, Nomura T, Dietz R, Menard J, Murakami K, Luft F C, Ganten D. Hypertension. 1997;29:428–434. doi: 10.1161/01.hyp.29.1.428. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto M, Egashira K, Usui M, Numaguchi K, Tomita H, Tsutsui H, Shimokawa H, Sueishi K, Takeshita A. J Clin Invest. 1997;99:278–287. doi: 10.1172/JCI119156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shesely E G, Maeda N, Kim H-S, Desai K M, Krege J H, Laubach V E, Sherman P A, Sessa W C, Smithies O. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt H H H W, Lohmann S M, Walter U. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 6.Middlekauff H R. Curr Opin Cardiol. 1997;12:265–275. doi: 10.1097/00001573-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Oliver P M, Fox J E, Kim R, Rockman H A, Kim H-S, Reddick R L, Pandey K N, Milgram S L, Smithies O, Maeda N. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orstavik S, Natarajan V, Tasken K, Jahnsen T, Sandberg M. Genomics. 1997;42:311–318. doi: 10.1006/geno.1997.4743. [DOI] [PubMed] [Google Scholar]
- 9.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge H R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 10.Joyce N C, DeCamilli P, Lohmann S M, Walter U. J Cyclic Nucleotide Protein Phosphor Res. 1986;11:191–198. [PubMed] [Google Scholar]
- 11.Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 12.Jarchau T, Häusler C, Markert T, Pöhler D, Vandekerckhove J, De Jonge H R, Lohmann S M, Walter U. Proc Natl Acad Sci USA. 1994;91:9426–9430. doi: 10.1073/pnas.91.20.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambaryan S, Häusler C, Markert T, Pöhler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann S M. J Clin Invest. 1996;98:662–670. doi: 10.1172/JCI118837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballermann B J, Zeidel M L. In: The Kidney: Physiology and Pathophysiology. Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. 1843–1884. [Google Scholar]
- 15.Scholz H, Kaissling B, Inagami T, Kurtz A. J Physiol. 1991;441:453–468. doi: 10.1113/jphysiol.1991.sp018761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Bruna R, Pinet F, Corvol P, Kurtz A. Cell Physiol Biochem. 1991;1:98–110. [Google Scholar]
- 17.Della Bruna R, Pinet F, Corvol P, Kurtz A. Am J Physiol. 1992;262:F397–F402. doi: 10.1152/ajprenal.1992.262.3.F397. [DOI] [PubMed] [Google Scholar]
- 18.Vaandrager A B, Tilly B C, Smolenski A, Schneider-Rasp S, Bot A G M, Edixhoven M, Scholte B J, Jarchau T, Walter U, Lohmann S M, et al. J Biol Chem. 1997;272:4195–4200. doi: 10.1074/jbc.272.7.4195. [DOI] [PubMed] [Google Scholar]
- 19.Markert T, Vaandrager A B, Gambaryan S, Pöhler D, Häusler C, Walter U, De Jonge H R, Jarchau T, Lohmann S M. J Clin Invest. 1995;96:822–830. doi: 10.1172/JCI118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt E, Nolte C, Schulz S, Beltman J, Beavo J A, Jastorff B, Walter U. Biochem Pharmacol. 1992;43:2591–2600. doi: 10.1016/0006-2952(92)90148-c. [DOI] [PubMed] [Google Scholar]
- 21.Skott O, Baumbach L. Pflügers Arch. 1985;404:232–237. doi: 10.1007/BF00581244. [DOI] [PubMed] [Google Scholar]
- 22.Butt E, Eigenthaler M, Genieser H-G. Eur J Pharmacol. 1994;269:265–268. doi: 10.1016/0922-4106(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz A. Rev Physiol Biochem Pharmacol. 1989;113:1–40. doi: 10.1007/BFb0032674. [DOI] [PubMed] [Google Scholar]
- 24.Schricker K, Hamann M, Kurtz A. Am J Physiol. 1995;269:F825–F830. doi: 10.1152/ajprenal.1995.269.6.F825. [DOI] [PubMed] [Google Scholar]
- 25.Hill-Kapturczak N, Kapturczak M H, Malinski T, Gross P. Endothelium. 1995;3:253–299. [Google Scholar]
- 26.Scholz H, Kurtz A. J Clin Invest. 1993;91:1088–1094. doi: 10.1172/JCI116266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu T, Reid I A. J Pharmacol Exp Ther. 1996;278:793–799. [PubMed] [Google Scholar]
- 28.Mery P-F, Lohmann S M, Walter U, Fischmeister R. Proc Natl Acad Sci USA. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hove-Madsen L, Mery P-F, Jurevicius J, Skeberdis A V, Fischmeister R. Basic Res Cardiol. 1996;91:1–8. doi: 10.1007/BF00795355. [DOI] [PubMed] [Google Scholar]
- 30.Supaporn T, Sandberg S M, Borgeson D D, Heublein D M, Luchner A, Wei C-M, Dousa T P, Burnett J J. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- 31.Soff G A, Cornwell T L, Cundiff D L, Gately S, Lincoln T M. J Clin Invest. 1997;100:2580–2587. doi: 10.1172/JCI119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen B L, Skott O. Am J Physiol. 1994;266:F604–F611. doi: 10.1152/ajprenal.1994.266.4.F604. [DOI] [PubMed] [Google Scholar]
- 33.Jensen B L, Skott O. Am J Physiol. 1996;270:F718–F727. doi: 10.1152/ajprenal.1996.270.5.F718. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz A, Penner R. Proc Natl Acad Sci USA. 1989;86:3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]