Abstract
In transgenic tobacco, pea Ferredoxin-1 (Fed-1) mRNA accumulates rapidly in response to photosynthesis even when the transgene is driven by a constitutive promoter. To investigate the role of photosynthesis on Fed-1 mRNA stability, we used the tetracycline repressible Top10 promoter system to specifically shut off transcription of the Fed-1 transgene. The Fed-1 mRNA has a half-life of approximately 2.4 hr in the light and a half-life of only 1.2 hr in the dark or in the presence of the photosynthetic electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). These data indicate that cessation of photosynthesis, either by darkness or DCMU results in a destabilization of the Fed-1 mRNA. Furthermore, the Fed-1 mRNA half-life is reduced immediately upon transfer to darkness, suggesting that Fed-1 mRNA destabilization is a primary response to photosynthesis rather than a secondary response to long-term dark adaptation. Finally, the two different methods for efficient tetracycline delivery reported here generally should be useful for half-life measurements of other mRNAs in whole plants.
The pea Ferredoxin-1 (Fed-1) gene encodes plastid-localized photosystem I ferredoxin. Its mRNA is perhaps the best-characterized nuclear-encoded transcript in plants that exhibits promoter-independent regulation of mRNA abundance (e.g., refs. 1 and 2). Fed-1 initially was identified in a cDNA screen for light-regulated mRNAs in pea (3) and was chosen for further study because Fed-1 mRNA accumulated faster than several other light-regulated mRNAs in etiolated pea seedlings exposed to light (4). Analysis of transgenic tobacco plants containing the transcribed sequence of the pea Fed-1 gene under the transcriptional control of the constitutive cauliflower mosaic virus 35S promoter (P35S) revealed that the light-regulated accumulation of Fed-1 mRNA was conferred by the mRNA sequence itself rather than by the promoter (1). Furthermore, the light effect on accumulation of Fed-1 mRNA transcribed from this chimeric gene was greater in green plants than in etiolated seedlings exposed to light for the first time (5). Deletion analysis determined that 95 nt of the 5′ UTR plus the first one-third of the Fed-1 coding region (≈143 nt) are sufficient for light regulation in such constructs. This region is referred to as the internal light regulatory element (iLRE) (6). Further deletion of the Fed-1 iLRE results in a gradual loss of light regulation (7).
Translation appears to be involved in the Fed-1 mRNA light response. Mutation of the Fed-1 initiation codon to either a missense or nonsense codon abolishes light regulation of the mRNA (2). Similarly, mutation of the surrounding translational initiation context also compromises light regulation of the Fed-1 mRNA (7). Finally, the Fed-1 mRNA is polyribosome-associated in the light but not in the dark (8), thus decreased Fed-1 mRNA abundance is correlated with a decrease in Fed-1 translation rates.
Fed-1 mRNA levels also decrease when light-grown plants are treated with the photosynthetic electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). During reillumination of DCMU-treated plants after a 40-hr dark treatment, Fed-1 mRNA fails to reaccumulate and remains dissociated from polyribosomes (8). These results suggest light regulation of Fed-1 mRNA accumulation and translation requires photosynthetic electron transport.
It is of interest to know whether the Fed-1 mRNA half-life is altered by changes in photosynthetic activity. One method of mRNA half-life determination is to globally inhibit transcription and monitor the decay of the mRNA of interest over time. This approach has been used successfully in plants to give half-lives that correspond to changes in mRNA steady-state abundance (9, 10). However, apparent secondary effects from global transcriptional inhibition confounded half-life studies in other cases (11, 12).
Attempts to use actinomycin D (ActD) to determine whether Fed-1 mRNA half-life is altered in the light have been unsuccessful (13). In these experiments, ActD treatment resulted in an increase rather than the expected decrease in Fed-1 mRNA in the dark, preventing a determination of Fed-1 mRNA half-life. There are at least two ways in which these results can be reconciled with our observation that the steady-state abundance of Fed-1 mRNA declines in darkness in a promoter-independent manner. First, the Fed-1 mRNA may indeed be more stable in darkness than in light, but this effect could be overridden if transcriptional elongation is inhibited in the dark. This transcriptional effect on abundance may not be detectable by transcriptional run-on assays (reviewed in ref. 14). Second, global inhibition of transcription might lead to improper Fed-1 light regulation—for example, by causing the disappearance of a labile factor required for Fed-1 degradation in the dark.
The secondary effects caused by global transcriptional inhibitors can be circumvented by making mRNA half-life measurements under conditions in which transcription is suppressed only for the gene of interest, while all other genes are transcribed normally. The tetracycline (tet)/VP16 system used in animals has been adapted for use in plants (15). The Top10 tetracycline-repressible promoter (P-Top10) is based on the constitutive P35S, but contains seven tetracycline operator sites in place of the upstream activator sequences. Plants are transformed with a construct encoding the tet repressor fused to the VP16 transcriptional activator protein and with a construct containing the P-Top10 fused to the GUS reporter gene. In the absence of tetracycline, the tet repressor binds the P-Top10 and VP16 activates GUS transcription. Upon addition of tetracycline, the tet/VP16 fusion protein no longer binds P-Top10, preventing transcription of GUS (16). Thus, this system can be used for a direct measurement of the half-life of the GUS mRNA or any other mRNA driven by P-Top10. The Top10 system has been used successfully to measure half-life of SAUR containing mRNAs in tobacco suspension culture cells (17).
We have observed that a normal Fed-1 mRNA light response requires healthy, intact green plants. Previously, in studies of the half-life of GUS mRNA using the P-Top10 system, tetracycline was introduced into detached leaves by vacuum infiltration (16). On the whole-plant level, the half-life of Gus protein, not mRNA, was examined over a period of days, not hours, using low levels of tetracycline. To study the rapid Fed-1 mRNA light response, it was essential that we develop conditions permitting rapid delivery of tetracycline to intact, healthy plants. Here we report measuring Fed-1 mRNA half-life from intact plants by using P-Top10. We have determined that the half-life of the Fed-1 mRNA is significantly shortened in the dark and in the presence of photosynthetic electron transport inhibitor DCMU. These results show that light regulates Fed-1 mRNA abundance by affecting its stability.
METHODS
Gene Construct.
The P-Top10∷Fed-1 construct was derived from the “message” construct as described in ref. 1. An XbaI and EcoRI (blunt-ended with Klenow) fragment, which included the Fed-1 transcribed region (749 bp) and 70 bp of the 3′ flanking region, was substituted for the GUS-INT/octopine synthase terminator fragment [in pTOP10 (16)], which was excised by using XbaI/HindIII (blunt-ended with Klenow). The resulting plasmid was transferred to Agrobacterium tumefaciens strain LBA4404 by triparental mating (18).
Plant Transformations.
Nicotiana tabacum (SR-1, Petite Havana) were transformed by using the leaf disc method (19). Initially, plants were transformed with the transactivator plasmid pTetVP16 (16) and selected on kanamycin (300 μg/ml). The transformant expressing the highest amount of TetVP16 mRNA was chosen for a second round of transformation with the P-Top10∷Fed-1 construct described above, and transformants were selected on hygromycin (50 μg/ml).
Growth of Transgenic Tobacco on Nylon Membrane.
Twenty-five milliliters of sterile MS medium was added to thoroughly rinsed and autoclaved magenta boxes with membrane raft units (Sigma V8380; Sigma M1917). T2 transgenic tobacco seeds (line 5010) were sterilized in 30% commercial bleach followed by five washes with sterile deionized water. Approximately 15–20 transgenic seeds were spread on each membrane, the magenta box lids were put back on the box, and the junction between the lid and box was wrapped with micropore tape (3M Co., 1530–0). Plants were grown sterilely for 3–4 weeks to at least a four-leaf stage in a 20°C growth chamber. White light (120 μmol m−2⋅s−1) was supplied by a mixture of six incandescent and six fluorescent lights operating on a 12-hr light/12-hr dark cycle.
Half-Life Measurements in Seedlings.
Two hours after the beginning of the light cycle, transgenic tobacco plants were treated either with 3 ml of 1 mM DCMU in Hoagland’s solution/1% EtOH or with Hoagland’s solution/1% EtOH (control plants). These solutions were added to roots growing across the nylon membrane, and the Magenta Box covers propped open on the top to permit air exchange and encourage uptake of the solution by transpiration. One hour later all liquid on top of the nylon membrane was removed and replaced with 3 ml of 10 mg/liter tetracycline in Hoagland’s solution with or without 1 mM DCMU. Time zero was designated as the time after 1 hr of tetracycline solution uptake; at this time, covers were replaced and boxes were either wrapped in foil for dark time points, or left in the light for light or DCMU/light time points. At appropriate time points, leaves from all 15–20 plants grown in a single box were harvested into liquid nitrogen.
Octuplet Production.
Plantlets derived from transgenic calli were successively subdivided to produce eight clonal plantlets (octuplets) expressing Fed-1. Octuplets were rooted on rooting medium, then transferred to Plant Cons (Sigma) containing approximately 20 ml of Hoagland’s solution and placed in a growth chamber illuminated at an intensity of 240 μmol m−2⋅s−1 supplied by a mixture of 6 incandescent and 12 fluorescent lights on a 12-hr light/12-hr dark cycle. Plants were supplied with fresh Hoagland’s solution every other day for a week to allow plants to grow and to adapt to the new light conditions. Half-life measurements were carried out as above except that the entire 20 ml of Hoagland’s solution in the Plant Cons was completely replaced with 20 ml of fresh Hoagland’s solution supplemented with 10 mg/liter tet and/or 1 mM DCMU. A single member of the octuplet set was harvested for each time point.
Polyribosome Profiles.
Plants were grown on nylon membranes as above, treated with the solutions and light regime indicated, and harvested into liquid nitrogen. Polyribosomes then were isolated on a 15–65% sucrose gradient as previously described, and the absorbance of the fractions at UV 254 nm was monitored and recorded during fractionation.
RESULTS
To make the P-Top10 useful for the examination of Fed-1 half-life, we needed to develop a system for rapid and efficient uptake of tetracycline in healthy plants. Thus, we developed the P-Top10 repressible system for use in two different types of plant growth systems as follows: Tobacco Petite havana was transformed sequentially with DNA encoding the VP16/Tet activator and then with the P-Top10∷Fed-1∷Fed-1 ter construct (Fig. 1). Transgenic plant lines were screened for expression of the Fed-1 mRNA, which indicates appropriate expression of both transgenes because the VP16/Tet gene product is required for activation of the P-Top10. Most lines had significant levels of expression. One line (5010) was chosen for further study, and T2 seeds were collected for the experiments with seedlings grown hydroponically on nylon membranes. Fifteen other transgenic plant lines produced from a single VP16/Tet line were subdivided in tissue culture during the plantlet stage to form clonal octuplets (see Methods) expressing Fed-1. Five independent lines then were used for each of the treatments indicated.
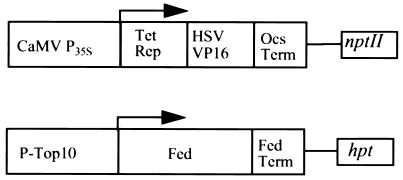
Figure 1.
Constructs used in the repressible promoter system. The chimeric transactivator Tet-VP16 is expressed under control of the cauliflower mosaic virus 35S promoter (P35S) (Upper). Progeny of plants transformed with the transactivator construct have been retransformed with Fed-1 under control of the synthetic Top10 promoter (P-Top10) (Lower). The Tet-VP16 protein binds P-Top10, allowing transcription to Fed-1. Endogenously supplied tetracycline binds Tet-VP16, making Tet-VP16 unable to bind P-Top10, thus inhibiting transcription of Fed-1.
To ensure the seedlings grown on nylon membrane exhibited a proper light response, we asked whether Fed-1 mRNA abundance decreased after transfer to darkness. The abundance of Fed-1 mRNA decreased approximately 3-fold after a 24-hr dark treatment, indicating that these conditions were appropriate for measuring Fed-1 half-life (Fig. 2A). In addition, we asked what level of tetracycline was required to shut off the P-Top10 promoter. Previously, 1 mg/liter of tetracycline has been shown to effectively repress P-Top10 during a long-term exposure (15). However, an external concentration of 1 mg/liter was ineffective in quickly repressing transcription of the P-Top10∷Fed-1 transgene. Although we eventually observed decay of the Fed-1 mRNA, the response was slow and inconsistent (ref. 20 and M.E.P., data not shown). Therefore, to rapidly deliver sufficient amounts of tetracycline to the plants, we increased the external concentration of tetracycline to 10 mg/liter. This concentration was used successfully for half-life measurements in N. tabacum suspension cultures (17) and for rapid (0.5 hr) regulation of GUS by vacuum infiltration in tobacco leaves (21).
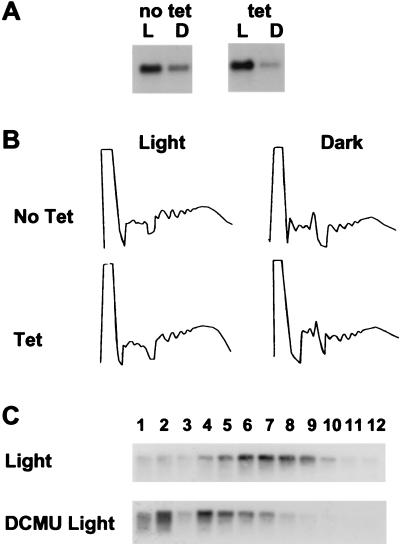
Figure 2.
Establishment of a hydroponic system to study Fed-1 mRNA half-life. (A) Decline of Fed-1 mRNA abundance in the dark. Fifteen to 20 transgenic nylon membrane grown seedlings from a transgenic line containing P-Top10∷Fed-1 treated with (tet) or without tetracycline (no tet) were either left in the light for 24 hr (L) or wrapped in foil for dark treatment for 24 hr from the time the light sample was harvested (D). Total RNA extracted and 5 μg was separated in each lane of an agarose gel, blotted, and hybridized with 32P-labeled Fed-1. (B) Lack of effect on total polyribosome loading by tetracycline. Transgenic P35S∷Fed-1 tobacco seedlings were treated with either 10 mg/liter tetracycline in Hoagland’s solution (Tet) or with Hoagland’s solution alone (No Tet). After 1 hr of uptake the plants were subjected to continued illumination (Light) or 1 hr of dark treatment (Dark). Shown are absorbance tracings at 254-nm UV of the sucrose gradients containing extracts from these plants. The direction of the sedimentation is from left to right. (C) Effective DCMU uptake. Fifteen to 20 transgenic nylon membrane-grown seedlings from a transgenic line containing P-Top10∷Fed-1 (nylon membrane grown) either were not treated (Light) or treated with 1 mM DCMU (DCMU Light) for 1 hr and then harvested into liquid nitrogen. RNAs from each fraction of a sucrose gradient were resolved by gel electrophoresis, blotted, and probed with antisense 32P-labeled RNA to either Fed-1. Light to heavy sucrose fractions were loaded from left to right. Amounts of hybridizing RNA cannot be quantitatively compared between gradients because of variations in the amount of tissue used and the efficiency of grinding.
Because 10 mg/liter tetracycline potentially could affect cytoplasmic translation and thus the light response of the Fed-1 mRNA, we monitored the polyribosome profiles of total plant mRNA in the presence and absence of tetracycline. In untreated plants we observed a significant shift from polyribosome fractions after only 1 hr of dark treatment, similar to what has been seen in agar grown plants (Eric R. Hansen, personal communication). Addition of tetracycline did not change the total polyribosome profile from that seen in untreated plants with the same light treatment (Fig. 2B), suggesting that the concentration of tetracycline delivered to the plants does not alter significantly overall translation. To further ensure that tetracycline does not alter the normal Fed-1 mRNA disappearance, we applied 10 mg/liter tetracycline to transgenic plants containing the P35S∷Fed-1 mRNA. As shown in Fig. 2A, tetracycline had no effect on the normal dark decline of the Fed-1 mRNA.
In addition to rapid delivery of tetracycline, we needed to ensure rapid delivery of DCMU so we could observe the effects of short-term inhibition of photosynthesis on Fed-1 mRNA half-life. We showed previously that Fed-1 mRNA is dissociated from polyribosomes after a 40-hr incubation with 1 mM DCMU (8). Therefore, we monitored the Fed-1 mRNA polyribosome profile 1 hr after delivery of 1 mg/ml DCMU to membrane-grown plants. Fig. 2C shows that within 1 hr after DCMU treatment, the Fed-1 mRNA is dissociated from the polyribosome fractions. Similar results were obtained for Fed-1 mRNA in plants exposed to 1 hr of dark treatment (Eric R. Hansen, personal communication). These data suggest that inhibition of photosynthesis by DCMU rapidly alters the polyribosome loading pattern of Fed-1 mRNA.
After establishing conditions that allowed rapid introduction of tetracycline and DCMU to intact, healthy plants, we asked whether light regulation of Fed-1 mRNA abundance occurs posttranscriptionally by a change in mRNA half-life. To determine the half-life of Fed-1 mRNA we used P-Top10∷Fed-1 transgenic tobacco seedlings (T2 generation) grown on nylon membranes and isolated RNA samples at the times indicated in Fig. 3. To assay plants grown under different conditions, we simultaneously performed the same experiments on clonal plantlets. In both plant systems (see Methods), identical half-lives were observed.
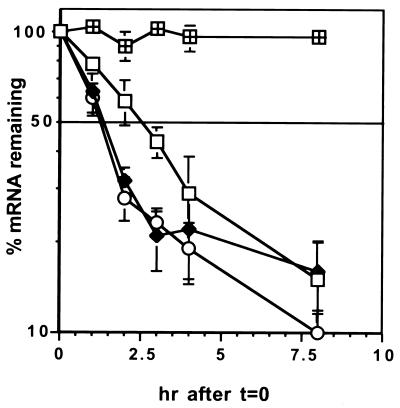
Figure 3.
The effect of photosynthesis on Fed-1 mRNA half-life. Fifteen to 20 control or DCMU-treated (1 mM) transgenic seedlings per sample from a transgenic line containing P-Top10∷Fed-1 (nylon membrane grown) or clonal octuplets were treated with 10 mg/liter tetracycline for 1 hr and then exposed to light (□), dark (♦), or DCMU and light (○) for the time indicated on the x axis. Total RNA (5 μg) was separated in each lane of an agarose gel, blotted, and hybridized with 32P-labeled Fed-1. Blots were rehybridized with 32P-labeled antisense HIS probe to detect the endogenous His HI transcript (divided square). The resulting hybridization signals were quantitated by using a PhosphorImager (Molecular Dynamics). The mean percent reduction in RNA was calculated for each time point and plotted on a semi-log plot. Each time point is derived from at least eight separate experiments, with the error bars representing SEM. A line was drawn at 50% mRNA remaining.
The half-life data from both types of growth systems are combined and summarized in Fig. 3. For all three conditions, approximately the first 80% of the decay can be fit with a single first-order component, using the equation ln(C/C0) = −kdt and ln2/kd = t1/2, where C0 is the initial mRNA concentration, C is the mRNA concentration at time t, kd is the decay constant, and t1/2 is the half-life of the mRNA (22). The half-lives estimated for this major component are approximately 2.4 hr for the light-treated plants and 1.2 hr for dark-treated plants. Correlation coefficients are 0.989 and 0.999, respectively. The remaining 20% of the mRNA appears to decay more slowly, although variation in the data prevents accurate analysis of this minor component. The apparently slower decay might indicate a subset of the Fed-1 mRNA decays more slowly than the bulk mRNA. However, measurements in this range are based on weak hybridization signals and may be influenced by background variations or a small amount of residual transcription from the P-Top10. It is therefore premature to draw any conclusions based on this minor component of the decay curve.
We also asked whether short-term inhibition of photosynthesis by DCMU treatment would show the same effect on half-life as a brief dark treatment. As shown in Fig. 3, the decay curve for Fed-1 mRNA in the presence of DCMU is similar to that for mRNA of dark-treated leaves. Fitting a first-order component to the first 80% of the decay curve yields a half-life of 1.2 hr (correlation coefficient = 0.978), a value indistinguishable from the half-life of Fed-1 mRNA in darkness. Finally, as a control, we asked whether tetracycline addition affected endogenous Histone HI mRNA levels. As expected, Histone HI RNA levels remained constant in the presence of tetracycline, indicating that tetracycline does not inhibit global transcription or turnover of other mRNAs. Taken together, our data show that photosynthetic signals control the stability of Fed-1 mRNA.
DISCUSSION
Here we present direct evidence that Fed-1 mRNA is posttranscriptionally light-regulated at the level of mRNA stability. Previously, we had only indirect evidence that did not distinguish between altered stability and altered transcriptional elongation. Furthermore, in the absence of Fed-1 transcription, a difference in Fed-1 mRNA abundance is detectable within an hour of the cessation of photosynthesis, suggesting that the change in Fed-1 mRNA stability is rapid. Previously we showed that the decrease in Fed-1 mRNA abundance is correlated with a dissociation from polyribosomes in response to cessation of photosynthesis. However, we had not been able to determine whether this response was immediate or delayed, and thus we could not answer whether the decline in mRNA abundance was because of stress from a long-term dark treatment or because of a more immediate, specific signal. The immediate decrease in Fed-1 mRNA half-life after transfer of the plants to darkness or treatment with DCMU suggests that the response to the photosynthetic state of the plant is a result of a rapid signal rather than a relatively slow change, such as increases in abscisic acid (23).
Using the P-Top10 system, we can now more easily study elements of the Fed-1 mRNA that affect its stability because half-life measurements circumvent “position effect” variation in absolute expression levels among different transgenic lines. It is difficult to firmly determine whether the stability of mutant mRNAs is altered by comparing mRNA abundance because transgene expression in plants can vary greatly. For example, although we know the Fed-1 mRNA light response is abolished when the AUG is mutated, we do not know whether this results from increasing mRNA stability in the dark or decreasing it in the light. Using the P-Top10 system, we will now be able to make those determinations.
Previously, we showed that changes in Fed-1 mRNA abundance are correlated with the ability of the mRNA to be translated (2). The data presented here support the suggestion that Fed-1 is preferentially degraded when it is not associated with polyribosomes. We propose that a signal generated by photosynthetic electron transport in the chloroplast travels to the cytoplasm and signals a change in the cytoplasmic translation rate of the Fed-1 mRNA. Furthermore, we suggest that a reduction in Fed-1 mRNA translation rate in the dark results in a faster rate of degradation of the Fed-1 mRNA. Cis analysis of the Fed-1 5′ UTR identified a region containing four tandem copies of a CATT motif that is apparently sensitive to nuclease degradation (7). When this region is mutated, Fed-1 mRNA accumulates to a similar level in both the light and the dark. However, the mutated Fed-1 mRNA that is not degraded in the dark still accumulates in the nonpolyribosomal fractions. We suggest that darkness inhibits translation at the level of initiation and that wild-type but not mutant Fed-1 mRNA is degraded in the absence of polyribosome association. The dark-induced decrease in Fed-1 mRNA half-life thus appears to be the result of a two-step process. First, Fed-1 mRNA dissociates from polyribosomes and second, the Fed-1 mRNA is degraded in the absence of polyribosome protection. It would be interesting to know whether there is in fact a “labile” factor required for Fed-1 degradation in the dark as inferred from the stabilization of Fed-1 mRNA in the presence of ActD. If so, what is the nature of this factor? For example, a labile nuclease may interact with the CATT repeat; in this case we would predict that degradation would be inhibited when expression of the nuclease is inhibited. Alternatively, it is possible that a labile factor is required to inhibit translational initiation in darkness. In either case, it will be interesting to sort out the specificity of Fed-1 degradation as well as the photosynthesis-responsive signaling pathways involved in the regulation of Fed-1 mRNA half-life.
Acknowledgments
We acknowledge excellent technical assistance from Tyrone Hughes and Tamyra Ravenel. We are also grateful to Dr. Mark Longtine for critical review of the manuscript. Controlled environment plant growth space was provided by the Southeastern Plant Environment Laboratory (Raleigh, NC). This project was supported by National Institutes of Health Postdoctoral Fellowship Grant 1F32GM15510–01 to M.P., and National Science Foundation Grant MCB-9507396 and National Institutes of Health Grant GM43108 to W.F.T. and L.F.D.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Fed-1, Ferredoxin-1; DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea; P35S, cauliflower mosaic virus 35S promoter; ActD, actinomycin D.
References
- 1.Elliott R C, Dickey L F, White M J, Thompson W F. Plant Cell. 1989;1:691–698. doi: 10.1105/tpc.1.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickey L F, Nguyen T T, Allen G C, Thompson W F. Plant Cell. 1994;6:1171–1176. doi: 10.1105/tpc.6.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson W F, Everett M, Polans N O, Jorgensen R A, Palmer J D. Planta. 1983;158:487–500. doi: 10.1007/BF00397240. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman L S, Roberts L R, Briggs W R, Thompson W F. Plant Physiol. 1986;81:1033–1038. doi: 10.1104/pp.81.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo-Meagher M, Sowinski D A, Elliott R C, Thompson W F. Plant Cell. 1992;4:389–395. doi: 10.1105/tpc.4.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickey L F, Gallo-Meagher M, Thompson W F. EMBO J. 1992;11:2311–2317. doi: 10.1002/j.1460-2075.1992.tb05290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickey L F, Petracek M E, Nguyen T T, Thompson W F. Plant Cell. 1998;10:475–484. doi: 10.1105/tpc.10.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petracek M E, Dickey L F, Huber S C, Thompson W F. Plant Cell. 1997;9:2291–2300. doi: 10.1105/tpc.9.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan M L, Green P J. RNA. 1996;2:308–315. [PMC free article] [PubMed] [Google Scholar]
- 10.Sheu J-J, Jan S-P, Lee H-T, Yu S-M. Plant J. 1994;5:655–664. [Google Scholar]
- 11.Fritz C C, Herget T, Wolter F P, Schell J, Schreier P H. Proc Natl Acad Sci USA. 1991;88:4458–4462. doi: 10.1073/pnas.88.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Sheng J, Liu L, Mehdy M C. Plant Cell. 1993;5:1089–1099. doi: 10.1105/tpc.5.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petracek M E, Dickey L F, Nguyen T, Allen G A, Sowinski D A, Hansen E R, Thompson W F. In: Regulation of Plant Growth and Development by Light. Briggs W R, Heath R L, Tobin E M, editors. Rockville, MD: Am. Soc. Plant Physiol.; 1996. pp. 80–88. [Google Scholar]
- 14.Thompson W F, White M J. Annu Rev Plant Physiol Mol Biol. 1991;42:423–466. [Google Scholar]
- 15.Gatz C. Methods Cell Biol. 1995;50:411–424. doi: 10.1016/s0091-679x(08)61047-x. [DOI] [PubMed] [Google Scholar]
- 16.Weinmann P, Gossen M, Bujard H, Hillen W, Gatz C. Plant J. 1994;5:559–569. doi: 10.1046/j.1365-313x.1994.5040559.x. [DOI] [PubMed] [Google Scholar]
- 17.Gil P, Green P. EMBO J. 1996;15:1678–1686. [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott R C, Pedersen T J, Fristensky B, White M J, Dickey L F, Thompson W F. Plant Cell. 1989;1:681–690. doi: 10.1105/tpc.1.7.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsch R B, Fry F E, Hoffman N L, Eicholtz D, Rogers S G, Fraley R T. Science. 1985;227:1229–1231. [Google Scholar]
- 20.Petracek M E, Dickey L F, Hansen E R, Sowinski D A, Nguyen T T, Allen G C, Thompson W F. In: A Look Beyond: Mechanisms Determining mRNA Stability and Translation in Plants: Proceedings of 19th Annual Riverside Symposium in Plant Physiology. Gallie D, editor. Rockville, MD: Am. Soc. Plant Physiol.; 1998. pp. 96–101. [Google Scholar]
- 21.Gatz C, Kaiser A, Wendenburg R. Mol Gen Genet. 1991;227:229–237. doi: 10.1007/BF00259675. [DOI] [PubMed] [Google Scholar]
- 22.Abler M, Green P J. Plant Mol Biol. 1996;32:63–78. doi: 10.1007/BF00039377. [DOI] [PubMed] [Google Scholar]
- 23.Weatherwax S, Ong M, Degenhardt J, Bray E, Tobin E. Plant Physiol. 1996;111:363–370. doi: 10.1104/pp.111.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]