Abstract
Epidermal changes caused by a chytridiomycete fungus (Chytridiomycota; Chytridiales) were found in sick and dead adult anurans collected from montane rain forests in Queensland (Australia) and Panama during mass mortality events associated with significant population declines. We also have found this new disease associated with morbidity and mortality in wild and captive anurans from additional locations in Australia and Central America. This is the first report of parasitism of a vertebrate by a member of the phylum Chytridiomycota. Experimental data support the conclusion that cutaneous chytridiomycosis is a fatal disease of anurans, and we hypothesize that it is the proximate cause of these recent amphibian declines.
Amphibian population declines in protected habitats are a serious global concern (1–3), and although several etiologies have been proposed, none has been substantiated (4–6). Disease has been hypothesized to be a likely cause, precipitated by an introduction of a pathogen into a naïve population (7, 8) or secondary to stress (6), but this paper presents evidence of a specific pathogen as a potential cause of amphibian declines. Previous investigations of sudden declines where adult mortality was suspected retrospectively (9, 10), or observed (6, 11, 12), have not included rigorous diagnostic examinations of large numbers of animals.
Montane riparian amphibian populations have declined markedly in Queensland (Australia) and in Central America with no evidence of environmental causes (13, 14). In these locations, documented declines have appeared to progress temporally and geographically consistent with an epidemic, and, in monitored areas, mass mortalities of adult anurans have been detected (refs. 7, 8, 14, and 15; K.R.L., unpublished data). Tadpoles were seen at these sites after adults had disappeared (ref. 7; K.R.L., unpublished data). Diagnostic investigations to detect infectious and noninfectious diseases were performed on the dead amphibians, and our results provide strong evidence that cutaneous chytridiomycosis was the cause of these mortalities.
Members of the phylum Chytridiomycota are heterotrophic fungi that are ubiquitous and cosmopolitan (16, 17). They are found primarily in soil and water where they usually act as primary degraders or saprobes, using substrates such as chitin, plant detritus, and keratin. Some genera are facultative or obligate anaerobes, and many are obligate parasites of fungi, algae, vascular plants, rotifers, nematodes, or insects. The chytrid reported here is the first member of the phylum Chytridiomycota to be recognized as a parasite of the phylum Vertebrata (18). A similar discovery of a chytrid fungus in dying captive dendrobatids in the United States made independently and contemporaneously (19) demonstrates that chytridiomycosis is widespread in amphibians in the Americas as well as Australia.
MATERIALS AND METHODS
Collection of Specimens.
Large numbers of ill and dead anurans were found during monitoring programs of amphibian populations in decline in Big Tableland, Queensland, Australia (1993–1994) (3 species) (7) and in the Fortuna Forest Reserve, western Panama (1996–1997) (10 species) (K.R.L., unpublished data). These were the last sightings of many of the affected species at these locations. Apparently healthy adults and tadpoles of Taudactylus acutirostris were collected from Big Tableland in late September 1993 for captive breeding; however, none survived. Dead or morbid wild and captive anurans from additional locations in Australia and Central America also were collected. (Table 1). The captive anurans included rain forest frogs, Mixophyes fasciolatus, and introduced toads, Bufo marinus, which had been bred in captivity in Australia and held in large, open collections that experienced high mortality (>90% of the M. fasciolatus) at 2–3 weeks postmetamorphosis. Retrospective histological examinations of the skin of formalin-fixed, ethanol-stored museum specimens were performed on riparian anurans (n = 28; 7 genera, 13 species) that had been collected during amphibian surveys in 1987–1995 in three protected sites in the montane regions of south-central Costa Rica (Las Tablas and Las Cruces, Puntarenas Province) and western Panama (Fortuna Forest Reserve), 1–10 years before population declines at those sites. Toeclips collected into formalin during survey work on healthy Litoria spenceri from southeastern Australia (Kosciuszko National Park) also were examined histologically for chytrids. Toeclips from 42 frogs had been collected during the 2 years before any observations of unusual mortality.
Table 1.
Amphibian deaths and morbidity associated with chytridiomycosis
| Species | Location | Date of death | No. with chytridiomycosis/ no. examined |
|---|---|---|---|
| Taudactylus acutirostris | Big Tableland, Australia, 15°42′S, 145°16′E, and | November 1993–January 1994 | 2/2 |
| Captive Townsville, Australia | October 1993 | 5/7* | |
| Litoria rheocola | Big Tableland, Australia, 15°42′S, 145°16′E | October–November 1993 | 1/2* |
| Litoria nannotis | Big Tableland, Australia, 15°42′S, 145°16′E | October–November 1993 | 2/4* |
| Taudactylus eungellensis | Eungella National Park, 21°04′S, 148°38′E | October 1995 | 1/1 |
| Litoria lesueuri | Mary River, Australia, 26°37′S, 152°41′E | May–June 1996 | 5/5 |
| Limnodynastes dumerilii | Goomburra, Australia, 27°58′S, 152°20′E | December 1996 | 1/1 |
| Litoria caerulea | Brisbane, Australia, 27°38′S, 152°23′E, | July–September 1996 | 7/8 |
| Casino, Australia, 28°50′S, 153°02′E; and | July 1996 | 3/4 | |
| Brisbane, Australia, 27°37′S, 152°38′E | June–July 1997 | 5/5 | |
| Limno dynastes tasmaniensis | Adelaide, Australia, 34°46′S, 138°32′E | May–June 1996 | 4/4 |
| Mixophyes fleayi | Cunningham’s Gap, Australia, 28°03′S, 152°22′E | August–October 1996 | 4/5 |
| Bufo marinus | Captive Geelong, Australia | June–August 1996 | 18/18 |
| Mixophyes fasciolatus | Captive Melbourne, Australia | December 1996–February 1997 | 35/35 |
| Litoria spenceri | Central Highlands, Australia, 37°22′S, 146°02′E | February 1998 | 4/4 |
| Atelopus chiriquiensis | Captive, Cerro Pando, Panama | February 1994 | 4/5 |
| Atelopus varius | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 3/3 |
| Bufo haematiticus | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 2/2 |
| Cochranella prosoblepon | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 2/2 |
| Cochranella albomaculata | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 2/2 |
| Eleutherodactylus emcelae | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 8/8 |
| Eleutherodactylus cruentus | Fortuna, Panama, 8°43′N, 82°14′W | January 1997 | 2/2 |
Histological examination of the skin was limited to the dorsum.
Pathology and Microbiology.
Frogs were fixed whole in neutral buffered 10% formalin after opening the body cavity, or necropsied in a sterile manner when fresh or after being stored frozen. Skin scrapings were collected from fresh specimens by using sterile, disposable scalpel blades and examined unstained with a light microscope or preserved in 100% ethanol for DNA analysis. Giemsa-stained blood smears were prepared and examined from 24 frogs received live. Formalin-fixed tissues were paraffin-embedded, sectioned at 6-μm thick, and stained with hematoxylin and eosin by using routine methods for histology. Samples were collected for bacteriology and mycology by using alginate swabs and immediately plated onto horse blood agar, chocolate agar, MacConkey agar, thiosulfate-citrate-bile-sucrose (TCBS) agar, and Sabauraud’s agar. Bacterial isolates were identified by Gram stain, Microbact 24 NE (Medvet Science, Adelaide, Australia), and API 20E (BioMerieux, Charbonnier les Bains, France) systems. For virological testing, frozen samples of liver, lung, kidney, muscle, gonad, and brain were macerated, passed through a 0.45-μm filter, and adsorbed to a monolayer of fat head minnow [FHM; American Type Culture Collection (ATCC), Manassas, VA; CCL 442), African clawed toad kidney (A6; ATCC CCL 102), bullfrog tongue (FT; ATCC CCL 41), or bluegill fry (BF-2; ATCC CCL 91) cells for Australian samples, and rainbow trout gonad (RTG-2) (20), chinook salmon embryo (CHSE) (21), Rana pipiens liver (RPL; KS Tweedell, University of Notre Dame, IN), and Terrapene carolina heart (TH; ATCC CCL 50) cells for Panamanian samples. Cells were observed for two passages of at least 7 days each.
Electron Microscopy.
For scanning electron microscopy, skin was fixed in 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated, critical point-dried, sputter-coated with gold, and examined by using a JEOL JSM 840 scanning electron microscope at 5 kV. For transmission electron microscopy (TEM), skin was fixed in neutral-buffered 10% formalin, postfixed in 2.5% glutaraldehyde then 1% osmium tetroxide, stained en-bloc with 2% uranyl acetate, dehydrated, embedded in Araldite epoxy resin, sectioned at 70 nm (serial sectioning was not performed), stained with Reynold’s lead citrate, and examined on either a Phillips 301 or a Hitachi 600 TEM. In addition, internal organs were retrieved from paraffin blocks, dewaxed, and prepared for TEM.
DNA Sequencing of Chytrid.
DNA was extracted from ethanol-fixed skin scrapings from two wild frogs, Litoria caerulea, from Queensland that were naturally diseased with cutaneous chytridiomycosis. Ethanol was removed and tissues were crushed in extraction buffer (50 mM Tris, pH 8.0/0.7 M NaCl/10 mM EDTA/1% hexadecyltrimethylammonium bromide/0.1% 2-mercaptoethanol) before incubation at 65°C for 1 hr with 100 μg/ml proteinase-k added. DNA was extracted by using phenol/chloroform and precipitated in ethanol. The nuclear gene encoding small-subunit ribosomal RNA (ssu-rDNA) (≈1,800 bp) was amplified by using universal primers A (5′-CCAACCTGGTTGATCCTGCCAGT-3′) and B (5′-GATCCTTCTGCAGGTTCACCTAC-3′) modified from Medlin et al (22). DNA was purified by using QiaQuick spin columns (Qiagen, Chatsworth, CA) following the protocol recommended by the manufacturer. Products were sequenced by using the dye terminator sequencing reaction (Perkin–Elmer) and run on an acrylamide gel on an automated ABI Prism 377 DNA sequencer (Applied Biosystems). A total of 1,726 nt (GenBank accession no. AF051932) were collected and aligned with the ssu-rDNA of 44 other eukaryotes (23). Ambiguously aligned regions were removed to yield a 45 × 1,607 matrix that corresponds closely to matrix B (23). Neighbor-joining, parsimony, and maximum likelihood trees were inferred by using phylip 3.53 (24). All inferences used random-addition order; parsimony and likelihood analyses used global (43-level) optimization, and neighbor-joining and parsimony analyses were bootstrapped (n = 1,000).
Transmission Experiment.
Experimental transmission of cutaneous chytridiomycosis was conducted by using captive-bred sibling frogs, M. fasciolatus, which were housed individually at 24°C in plastic tubs with gravel and 800 ml aged tap water. Before experimental infection, 10 frogs were tested for the presence of chytrids by histological examination of clipped toes and all were negative for the fungus. For the experiment 14 frogs (6 exposed, 8 controls) from the previously sampled cohort were used. Each of six frogs was exposed to 800 ml of an aqueous suspension of approximately 3 × 103 sporangia in fresh skin scrapings taken from a dead frog of the same species that had naturally acquired cutaneous chytridiomycosis. Four frogs were exposed to water that had contained infected skin scrapings and had been passed through a 0.45-μm filter, and four were kept as untreated controls.
RESULTS
At necropsy, no consistent gross lesions were found in the sick and dying anurans. However, areas of abnormal epidermal sloughing were seen in a number of amphibians from both Australia and Central America. Examinations of fresh, unstained wet mounts of this dysecdic skin consistently revealed large numbers of spherical to subspherical sporangia of a nonhyphal, previously unreported fungus. Histologically, significant abnormalities were restricted to the skin, in which sporangia were found in the stratum corneum and stratum granulosum (Fig. 1) principally of the digits and ventral body, especially the hypervascularized pelvic patch (“drink patch”). Epidermal changes associated with these parasites consisted of irregular cell loss, erosions, and segments of marked thickening of the stratum corneum (parakeratotic hyperkeratosis). Deeper epidermal changes consisted of moderate hyperplasia of the stratum intermedium (acanthosis), but there were negligible inflammatory cell infiltrates. Sporangia were also seen on histological examination of the keratinized mouth parts of wild Panamian tadpoles (three of seven) (three genera) and in killed, otherwise-healthy, captive M. fasciolatus (three of four) and B. marinus (three of eight) tadpoles from the spawning groups that had high mortality rates after metamorphosis. In these tadpoles, the fungus was found only in the mouth parts and not in the unkeratinized skin of the body and tail.
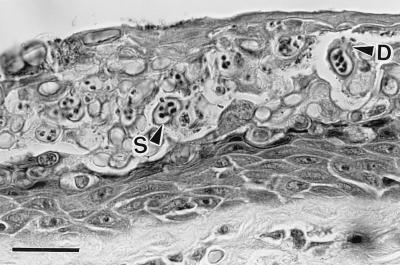
Figure 1.
Histological section of severely infected digital skin of a wild frog, Litoria caerulea, from Queensland, Australia. The stratum corneum is markedly thickened because of a massive infection by a chytrid parasite. Thickness of normal stratum corneum is 2–5 μm, but here it is about 60 μm. This section contains a mass of intracellular sporangia (S) and developing sporangia. The mature sporangia are 12–20 μm (n = 25) in diameter, have refractile walls (0.5- to 2.0-μm thick), and contain zoospores. Many sporangia have discharged all zoospores. Zoospores are released through discharge tubes (D). Note the absence of an inflammatory cell reaction in the dermis and epidermis. (Bar = 30 μm.)
There was no evidence of epidermal viral infection in adult anurans based on histological, ultrastructural, and culture findings, including culture attempts from 23 wild and captive frogs originating from Big Tableland (three species) and 26 wild anurans from Fortuna (8 species). A range of bacteria was isolated from these specimens, including Aeromonas hydrophila from the Australian frogs and Flavobacterium indologenes from the Panamanian amphibians. No species of bacteria was isolated from more than 44% of sick and dead amphibians in any wild group, and the histological findings were not consistent with primary bacterial disease. Aeromonas spp. were not isolated from any of 26 frozen anurans collected from the mass mortality incident in Panama. Infectious organisms were not detected on light microscopic examination of Giemsastained blood smears from 24 diseased Australian frogs received alive. No epidermal myxozoa or protozoa were detected histologically or ultrastructurally in tissues from either sick or dead amphibians. Mycological cultures of frozen tissue failed to detect chytrids.
Chytrid fungi were not found on histologic examination of the 28 archived Panamanian and Costa Rican specimens collected from the wild before population declines, or the 42 archived toeclips from healthy Australian frogs.
Scanning electron microscopical examination of the surface of digital skin from infected Australian frogs showed marked roughening and sloughing of the skin surface compared with the smooth and intact appearance of uninfected epidermis from the control frogs. Infection of the superficial keratinized cells was evident by the presence of prominent discharge tubes of the fungus protruding through the bulging cells (Fig. 2). Transmission electron microscopical examination of wild and captive Australian and wild Panamanian anurans identified this parasite as a chytrid fungus (Chytridiomycota; Chytridiales) primarily based on zoospore ultrastructure (17). The fungus in the epidermis of the anurans had a thallus bearing a network of rhizoids and smooth-walled, spherical to subspherical, inoperculate sporangia. Each sporangium produced a single discharge tube. Zoospores (Fig. 3) had an elongate–ovoidal body and a single, posterior flagellum and possessed a core area of ribosomes often with membrane-bound spheres of ribosomes within the main ribosomal mass. A small spur was located at the posterior of the cell body, adjacent to the flagellum. It is unknown whether this represents an artifact in these formalin-fixed specimens. The core area of ribosomes was surrounded by a single cisterna of endoplasmic reticulum, two to three mitochondria, and an extensive microbody–lipid globule complex. The microbody closely apposed and almost surrounded four to six lipid globules (three anterior and one to three laterally), some of which appeared bound by a cisterna. Some zoospores appeared to contain more lipid globules; however, this may have been a result of a plane-of-sectioning effect, because the globules were often lobed in the zoospores examined (Fig. 4). A rumposome was not observed. A nonfunctioning centriole lay adjacent to the kinetosome. Nine interconnected props attached the kinetosome to the plasmalemma, and a terminal plate was present in the transitional zone (Fig. 4). An inner ring-like structure attached to the tubules of the flagellar doublets within the transitional zone was observed in transverse section. No roots associated with the kinetosome were observed. In many zoospores, the nucleus lay partially within the aggregation of ribosomes and was invariably situated laterally. Small vacuoles and a Golgi body with stacked cisternae occurred within the cytoplasm outside the ribosomal area. Mitochondria, which often contained a small number of ribosomes, were densely staining with discoidal cristae.
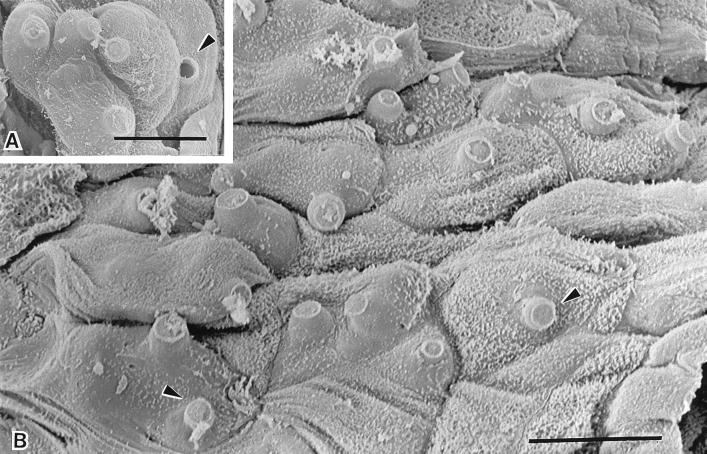
Figure 2.
Scanning electron micrographs of infected digital skin of a wild frog, Litoria lesueuri, from Queensland, Australia, which died with cutaneous chytridiomycosis. (A) A cluster of mature sporangia are evident within the cells of the epidermis. The discharge tubes of the sporangia are visible projecting outwards from the cell surface. The plug of material within the discharge tube has been released in one of the sporangia (arrowhead). (Bar = 10 μm.) (B) Most of the cells in this field of the superficial layer of the epidermis contain sporangia, as evident by the bulging surfaces and the protrusion of unopened discharge tubes through infected cells (arrowheads). (Bar = 10 μm.)
Figure 3.
Transmission electron micrographs of the zoospores found within fungal sporangia in the epidermis of naturally infected amphibians from Panama (Eleutherodactylus emcelae, A and C) and from Australia (Litoria caerulea, B and D). (A) Longitudinal section through a zoospore. The ribosomal area (Rb) is surrounded by a single cisterna of endoplasmic reticulum and bounded by a nucleus (N), mitochondria (Mi), and a microbody–lipid globule complex (asterisk). A single flagellum (Fl) connects to the posterior of the zoospore. (Bar = 1 μm.) (B) A transverse–oblique section through the anterior aspect of a zoospore demonstrates the unusual microbody–lipid globule complex. The microbody (asterisk) lies adjacent to four lipid globules (L) in this section. There is no evidence of a cisterna bounding these lipid globules. Rb, ribosomal area. (Bar = 1 μm.) (C) Transverse section through the anterior aspect of a zoospore. Note the discoidal cristae within mitochondria (Mi). The ribosomal area is bounded by the microbody–lipid globule complex (asterisk) and the mitochondria. No cisterna bound the lipid globules in this section. (Bar = 0.5 μm.) (D) A longitudinal–oblique section through a zoospore in the epidermis of an Australian amphibian for comparison with A. (Bar = 0.75 μm.)
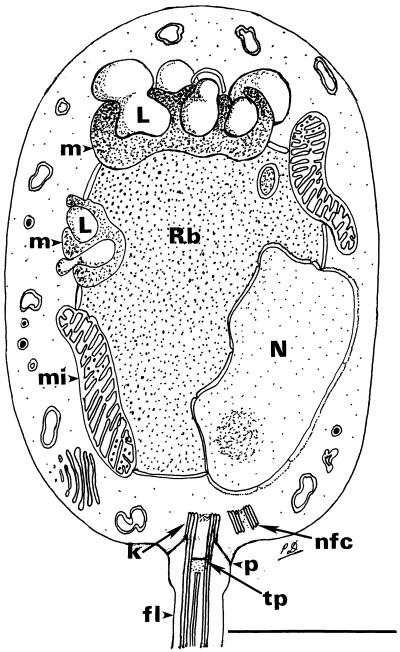
Figure 4.
A composite line drawing of a longitudinal section through the zoospore of the chytridialean parasite found in the epidermis of Australian and Panamanian amphibians. The drawing is based on examination of thin sections of zoospores within the sporangium, not of serially sectioned, cultured, glutaraldehyde-fixed specimens. For these reasons, some details of the kinetosomal region were poorly preserved or may not have been visible. The unusual microbody–lipid globule complex and some details of the flagellar attachment are shown. In some sections, it appears that two microbody–lipid globule complexes are present, but it is unknown whether this represents a single complex wrapped around the ribosomal region. The flagellum in longitudinal and transverse section is drawn slightly larger than scale to adequately demonstrate the aspects of the flagellar attachment. Rb, ribosomal area; N, nucleus; L, lipid globule; m, microbody; mi, mitochondrion; k, kinetosome; p, prop attaching kinetosome to plasmalemma; tp, terminal plate; nfc, nonfunctioning centriole; fl, flagellum. (Bar = 1 μm.)
DNA analyses showed that the amphibian parasite ssu-rDNA groups were among those of the chytrid fungi, with the closest match to Chytridium confervae (Fig. 5).
Figure 5.
Maximum-likelihood tree inferred from ssu-rDNA sequences of the amphibian parasite and 44 other eukaryotes, based on 1,607 stably aligned positions at a transition/transversion ratio of 2.0. GenBank accession numbers of ssu-rDNAs other than the amphibian parasite in this tree have been published (23). Bootstrap values (percent of 1,000 replicates) are taken from the topologically identical parsimony tree. Distance matrices (corrected for superimposed substitutions by a generalized Kimura two-parameter model) were calculated by using dnadist from the phylip package, and neighbor-joining trees were inferred by using neighbor (24). Unweighted parsimony trees were inferred by using dnapars with 1,000 iterations (jumbles). Neighbor-joining and parsimony analyses were bootstrapped (n = 1000) by sequential use of seqboot, the inference program(s) above, and consense (24, 25). A maximum-likelihood tree was inferred by using dnaml. All inferences used random-addition order and, for parsimony and likelihood, global (43-level) optimization. The position of Apusomonas proboscidea ssu-rDNA was unstable, and in likelihood inference depended on the transition/transversion ratio.
Between 10 and 18 days postexperimental exposure, all six frogs exposed to unfiltered skin scrapings became moribund; one died and the rest were euthanized with 0.2% tricaine methanesulfonate (Ruth Consolidated Industries, Annandale, Australia). Cutaneous chytridiomycosis was diagnosed cytologically, histologically, and ultrastructurally. The eight control frogs remained healthy and were free of chytrids when killed after 22 days and examined histologically.
DISCUSSION
The extensive pathological investigations reported in this paper provide evidence for a pathogen causing amphibian mortality in areas where declines are marked and well documented. Furthermore, the similarity between pathological findings from Australian and Central American amphibians is remarkable, and it appears that a similar fungal pathogen is present in these two widely separated regions.
The ultrastructural studies and DNA analyses firmly place the epidermal fungus within the phylum Chytridiomycota, class Chytridiomycetes, order Chytridiales (18, 26). It was not possible to further classify the organism by using ultrastructural characters because cultured, glutaraldehyde-fixed specimens were not available and serial sectioning was not performed. However, the occurrence of several lipid globules in the microbody–lipid globule complex with some bounded by a cisterna, is unusual within the Chytridiales (27, 28). These and other ultrastructural characters, together with parasitism of the amphibian epidermis, suggest that this parasite represents a new chytridialean genus. Despite extensive ultrastructural examination of zoospores and other developmental stages, no significant differences could be found between chytrids from wild and captive Australian amphibians and chytrids from wild Panamanian amphibians, suggesting that animals from both continents were infected by the same fungus.
Chytrids were observed to infect tadpoles, but the localized distribution in the only keratinized body region, the mouth parts, may explain the lack of mortality. We assume that only after metamorphosis, when the skin becomes keratinized (29), does the chytrid cause a widespread, fatal epidermal infection. The absence of infection in nonkeratinized epithelial surfaces of tadpoles (body, limbs, tail, mouth, gills) and postmetamorphic anurans (conjunctiva, nasal cavity, mouth, tongue, intestines) emphasizes the strictly keratinophilic affinity of this newly recognized pathogen.
Although the transmission experiment was a preliminary trial that used unpurified material, it demonstrates that chytrids are associated with a transmissible fatal disease of anurans and supports our diagnostic findings that cutaneous chytridiomycosis was the cause of the mortality events in wild amphibians. Furthermore, the failure to identify alternative causes of death after examination of the wild amphibians and their environment (refs. 13 and 30; K.R.L., unpublished data) supports our theory that cutaneous chytridiomycosis was the cause of the riparian amphibian population declines in the montane rain forests of Queensland and Panama.
The mechanism(s) by which cutaneous chytridiomycosis becomes a fatal infection in postmetamorphic anurans is not clear. The epidermal hyperplasia may seriously impair cutaneous respiration and osmoregulation, particularly as chytrids consistently infect the pelvic patch, a major site of water absorption in some anurans (31). Alternatively, death may be a result of absorption of a toxic product released by the fungus.
An analysis of declining amphibian species at high altitudes in eastern Australia (J.-M. Hero, unpublished data) found that affected species have low clutch sizes and occupy restricted geographic ranges. This may be consistent with the impact of a disease affecting a range of amphibian species, but with recovery of more robust populations. There are many examples of infectious disease affecting wildlife populations (32), including examples where introduction of pathogens has decimated populations (33–35). There may be several explanations as to why chytridiomycosis has emerged as a disease of frogs in Australia and Panama. The chytrid may be an introduced pathogen spreading through naïve populations (7, 8), or it may be a widespread organism that has emerged as a pathogen to frogs because of either an increase in virulence or an increased host susceptibility caused by other factors, such as environmental changes or as yet undetected coinfections.
Acknowledgments
For collection of frogs and technical and other assistance we thank, in Australia, G. Russell, M. Braun, T. Wise, F. Fillipi, T. Stephens, J. Humphrey, P. Hooper, M. Williamson, G. Rowe, J. Muschialli, D. Carlson, T. Chamberlain, S. Larkin, and S. Daglas (Australian Animal Health Laboratory); K. Field and R. Ritallick (James Cook University); J.-M. Hero (Griffith University); G. Gillespie (Arthur Rylah Institute); N. Murphy (Commonwealth Scientific and Industrial Research Organization, Marine Research); J. Koehler (Townsville General Hospital); H. McCracken (Royal Melbourne Zoo); M. Tyler, R. Short, and C. Williams (University of Adelaide); L. Skerratt (University of Melbourne); D. Charley (NSW, National Parks and Wildlife Service); R Natrass and B. Dadds (Queensland Department of Environment); P. O’Donoghue (University of Queensland); M. Mahony (University of Newcastle); and members of the public who found sick frogs. In the United States we gratefully acknowledge many contributions of R. Hess, V. Beasley, B. Jakstys, L. Miller, J. Cochran, J. Cheney, and B. Ujhelyi (University of Illinois at Urbana–Champaign); G. Rabb and T. Meehan (Brookfield Zoo, Chicago), R. Mast (Conservation International); and J. Savage (CRE Collections). This work was supported by the Australian Nature Conservation Agency, Biodiversity Australia, Wet Tropics Authority, IUCN/SSC/Declining Amphibian Populations Task Force, the University of Illinois at Urbana–Champaign, and American Airlines.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: ssu-rDNA, small-subunit rRNA genes.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF051932).
References
- 1.Blaustein A R, Wake D B. Trends Ecol Evol. 1990;5:203–204. [Google Scholar]
- 2.Wake D B. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- 3.Drost C A, Fellers G M. Conserv Biol. 1996;10:414–425. [Google Scholar]
- 4.Blaustein A R, Hoffman P D, Hokit DG, Kiesecker J M, Walls S C, Hays J B. Proc Natl Acad Sci USA. 1994;91:1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiesecker J M, Blaustein A R. Proc Natl Acad Sci USA. 1995;92:11049–11052. doi: 10.1073/pnas.92.24.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey C. Conserv Biol. 1993;7:355–362. [Google Scholar]
- 7.Laurance W F, McDonald K R, Speare R. Conserv Biol. 1996;10:406–413. [Google Scholar]
- 8.Laurance W F, McDonald K R, Speare R. Conserv Biol. 1997;11:1030–1034. [Google Scholar]
- 9.Pounds J A, Crump M L. Conserv Biol. 1994;8:72–85. [Google Scholar]
- 10.Heyer W R, Rand A S, Goncalvez da Cruz C A, Peixoto O L. Biotropica. 1988;20:230–235. [Google Scholar]
- 11.Bradford D F. J Herpetol. 1991;25:174–177. [Google Scholar]
- 12.Sherman C K, Morton M L. J Herpetol. 1993;27:186–198. [Google Scholar]
- 13.Richards S R, McDonald K R, Alford R A. Pac Conserv Biol. 1993;1:66–77. [Google Scholar]
- 14.Lips K R. Conserv Biol. 1998;12:106–117. [Google Scholar]
- 15.Trenerry M P, Laurance W F, McDonald K R. Pac Conserv Biol. 1994;1:150–153. [Google Scholar]
- 16.Sparrow F K. Aquatic Phycomycetes. Ann Arbor, MI: The Univ. of Michigan Press; 1960. pp. 16–18. [Google Scholar]
- 17.Karling J S. Chytridiomycetarum Iconographia: An Illustrated and Brief Descriptive Guide to the Chytridomycetous Genera with a Supplement of the Hyphochytriomycetes. Monticello, NY: Lubrecht and Cramer; 1977. [Google Scholar]
- 18.Barr D J S. In: Handbook of Protoctista. Margulis L, Corliss J O, Melkonian M, Chapman D J, editors. Boston: Jones and Bartlett; 1990. pp. 454–466. [Google Scholar]
- 19.Pessier, A. P., Nichols, D. K., Longcore, J. E. & Fuller, M. S. (1998) J. Vet. Diag. Invest., in press. [DOI] [PubMed]
- 20.Wolf K, Quimby M L. Science. 1962;135:1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- 21.Lannan C N, Winton J R, Fryer J L. In Vitro. 1984;20:671–676. doi: 10.1007/BF02618871. [DOI] [PubMed] [Google Scholar]
- 22.Medlin L, Elwood H J, Stickel S, Sogin M L. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 23.Ragan M A, Goggin C L, Cawthorn R J, Cerenius L, Jamieson A V C, Plourde S M, Rand T G, Soderhall K, Gutell R R. Proc Natl Acad Sci USA. 1996;93:11907–11912. doi: 10.1073/pnas.93.21.11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felsenstein J. Cladistics. 1989;5:164–166. [Google Scholar]
- 25.Felsenstein J. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 26.Barr D J S. Can J Bot. 1980;58:2380–2394. [Google Scholar]
- 27.Powell M J, Roychoudhury S. Can J Bot. 1992;70:750–761. [Google Scholar]
- 28.Longcore J E. Can J Bot. 1993;71:414–425. [Google Scholar]
- 29.Warburg M R, Lewinson D, Rosenberg M. In: Amphibian Biology, The Integument. Heatwole H, Barthalmus G T, editors. Vol. 1. Chipping Norton, Australia: Surrey Beatty & Sons; 1994. pp. 33–63. [Google Scholar]
- 30.Laurance W F. Biol Conserv. 1996;77:203–212. [Google Scholar]
- 31.Parsons R H. In: Amphibian Biology, The Integument. Heatwole H, Barthalmus G T, editors. Vol. 1. Chipping Norton, Australia: Surrey Beatty & Sons; 1994. pp. 132–146. [Google Scholar]
- 32.May R M. Conserv Biol. 1988;2:28–30. [Google Scholar]
- 33.Hess G. Ecology. 1996;77:1617–1632. [Google Scholar]
- 34.Warner R E. The Condor. 1968;70:101–120. [Google Scholar]
- 35.Leberg P L, Vrijenhoek R C. Conserv Biol. 1994;8:419–424. [Google Scholar]