Abstract
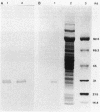
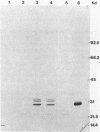
Eubacterium sp. strain VPI 12708 is a human intestinal bacterium which contains an inducible bile acid 7-dehydroxylase. Two-dimensional polyacrylamide gel electrophoresis showed that at least four new polypeptides were synthesized after exposure of growing cells to sodium cholate. One of these, of molecular weight 27,000 (PP-27), was implicated in 7-dehydroxylase catalysis. PP-27 was purified to greater than 95% homogeneity by DEAE-cellulose chromatography, high-pressure liquid chromatographic gel filtration, high-pressure liquid chromatography-DEAE chromatography, and preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The first 33 amino acid residues of the N terminus of PP-27 were determined with a gas-phase sequencer, and a corresponding mixed oligonucleotide (17-mer) was synthesized. Southern blot analysis of EcoRI total digests of chromosomal DNA showed a 2.2-kilobase fragment which hybridized to the 32P-labeled 17-mer. This fragment was enriched for by size fractionation of an EcoRI total digest of genomic DNA, ligated into the bacterial plasmid pUC8, and used to transform Escherichia coli HB101. Transformants containing the putative 7-dehydroxylase gene were detected with the 32P-labeled 17-mer by colony hybridization techniques. The insert was 2.2 kilobases in length and contained the first 290 bases of the PP-27 gene. Preliminary nucleic acid sequence data correlate with the amino acid sequence. The entire gene was cloned on a 1,150-base-pair TaqI fragment. Western blot analysis of E. coli strains containing these plasmids indicated that PP-27 is expressed in E. coli but is not regulated by bile acids under the conditions used.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagheri S. A., Bolt M. G., Boyer J. L., Palmer R. H. Stimulation of thymidine incorporation in mouse liver and biliary tract epithelium by lithocholate and deoxycholate. Gastroenterology. 1978 Feb;74(2 Pt 1):188–192. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kelsey M. I., Pienta R. J. Transformation of hamster embryo cells by cholesterol-alpha-epoxide and lithocholic acid. Cancer Lett. 1979 Mar;6(3):143–149. doi: 10.1016/s0304-3835(79)80025-7. [DOI] [PubMed] [Google Scholar]
- Kulkarni M. S., Heidepriem P. M., Yielding K. L. Production by lithocholic acid of DNA strand breaks in L1210 cells. Cancer Res. 1980 Aug;40(8 Pt 1):2666–2669. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Lipsky R. H., Hylemon P. B. Characterization of a NADH:flavin oxidoreductase induced by cholic acid in a 7 alpha-dehydroxylating intestinal Eubacterium species. Biochim Biophys Acta. 1980 Apr 11;612(2):328–336. doi: 10.1016/0005-2744(80)90115-1. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Nutter S. Colonic bacterial activity, biliary cholesterol saturation, and pathogenesis of gallstones. Lancet. 1978 Nov 18;2(8099):1063–1065. doi: 10.1016/s0140-6736(78)91800-7. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Paone D. A., Hylemon P. B. HPLC purification and preparation of antibodies to cholic acid-inducible polypeptides from Eubacterium sp. V.P.I. 12708. J Lipid Res. 1984 Dec 1;25(12):1343–1349. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- White B. A., Cacciapuoti A. F., Fricke R. J., Whitehead T. R., Mosbach E. H., Hylemon P. B. Cofactor requiremets for 7 alpha-dehydroxylation of cholic and chenodeoxycholic acid in cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 13708. J Lipid Res. 1981 Aug;22(6):891–898. [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]